Fertigation Management and Growth-Promoting Treatments Affect Tomato Transplant Production and Plant Growth after Transplant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Transplant Production
2.2. Transplanting and Tomato Plant Growth
2.3. Statistical Analysis
3. Results
3.1. Tomato Transplant Production
3.2. Tomato Plants Soilless Cultivation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Herrera, F.; Castillo, J.E.; Chica, A.F.; López Bellido, L. Use of municipal solid waste compost (MSWC) as a growing medium in the nursery production of tomato plants. Bioresour. Technol. 2008, 99, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.M. Biological amendment, fertilizer rate, and irrigation frequency for organic Bll pepper transplant production. HortScience 2006, 41, 1402–1407. [Google Scholar] [CrossRef]
- McCall, D. Effect of supplementary light on tomato transplant growth, and the after-effects on yield. Sci. Hortic. 1992, 51, 65–70. [Google Scholar] [CrossRef]
- Masson, J.; Tremblay, N.; Gosselin, A. Effects of nitrogen fertilization and HPS supplementary lighting on vegetable transplant production. II. Yield. J. Am. Soc. Hortic. Sci. 1991, 116, 599–602. [Google Scholar] [CrossRef] [Green Version]
- Nicola, S.; Cantliffe, D.J. Increasing cell size and reducing medium compression enhance lettuce transplant quality and field production. HortScience 1996, 31, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Huang, Y.; Caldwell, R.D. Best management practices for minimizing nitrate leaching from container-grown nurseries. Sci. World J. 2001, 1, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Vetrano, F.; Iapichino, G.; Poma, M.A.; Fascella, S.; Incalcaterra, G. Use of organic fertilizers for lettuce plug plant production. Acta Hortic. 2009, 607–612. [Google Scholar] [CrossRef]
- Lee, I.-J. Practical application of plant growth regulator on horticultural crops. J. Hortic. Sci. 2003, 10, 211–217. [Google Scholar]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Miceli, A.; Vetrano, F.; Sabatino, L.; D’Anna, F.; Moncada, A. Influence of preharvest gibberellic acid treatments on postharvest quality of minimally processed leaf lettuce and rocket. Horticulturae 2019, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Vetrano, F.; Moncada, A.; Miceli, A. Use of Gibberellic Acid to Increase the Salt Tolerance of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2020, 10, 505. [Google Scholar] [CrossRef] [Green Version]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Basra, A. Plant. Growth Regulators in Agriculture and Horticulture: Their Role and Commercial Uses; CRC Press: Boca Raton, FL, USA, 2000; ISBN 1560228911. [Google Scholar]
- Choudhury, S.; Islam, N.; Ali, M. Growth and Yield of Summer Tomato as Influenced by Plant Growth Regulators. Int. J. Agric. Sustain. 2013, 5, 25–28. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Brock, T.G. Combined effects of hormones and light during growth promotion in primary leaves of Phaseolus vulgaris. Can. J. Bot. 1993, 71, 501–505. [Google Scholar] [CrossRef]
- Emongor, V.E. Effect of benzyladenine and gibberellins on growth, yield and yield components of common bean (Phaseolus vulgaris). UNISWA. Res. J. Agric. Sci. Technol. 2002, 6, 65–72. [Google Scholar] [CrossRef]
- Khan, N.A. Comparative effect of modes of gibberellic acid application on photosynthetic biomass distribution and productivity of rapeseed-mustard. Physiol. Mol. Biol. Plants 2003, 9, 141–145. [Google Scholar]
- Richards, D.E.; King, K.E.; Ait-ali, T.; Harberd, N.P. How gibberellin regulates plant growth and development: A molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef] [Green Version]
- Balaguera-López, H.E.; Cárdenas-Hernández, J.F.; Álvarez-Herrera, J.G. Effect of gibberellic acid (GA3) on seed germination and growth of tomato (Solanum lycopersicum L.). Acta Hortic. 2009, 141–148. [Google Scholar] [CrossRef]
- Cleland, R.E. Introduction: Nature, occurrence and functioning of plant hormones. In Biochemistry and Molecular Biology of Plant Hormones; Hooykaas, P.J.J., Hall, M.A., Libbenga, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 33, pp. 3–22. [Google Scholar]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- European Union. Regulation No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. [Google Scholar]
- European Union. No 1069/2009, Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 177. Off. J. Eur. Union 2009, 300, 1–33. [Google Scholar]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- García de Salamone, I.E.; Hynes, R.K.; Nelson, L.M. Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can. J. Microbiol. 2001, 47, 404–411. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants—A review. Plant Soil Environ. 2008, 54, 89–99. [Google Scholar] [CrossRef]
- Widnyana, I.K.; Javandira, C. Activities Pseudomonas spp. and Bacillus sp. to Stimulate Germination and Seedling Growth of Tomato Plants. Agric. Agric. Sci. Procedia 2016, 9, 419–423. [Google Scholar] [CrossRef] [Green Version]
- Oancea, F.; Răut, I.; Zamfiropol-Cristea, V. Influence of soil treatment with microbial plant biostimulant on tomato yield and quality. J. Int. Sci. Publ. Agric. Food 2017, 5, 156–165. [Google Scholar]
- Joo, G.-J.; Kim, Y.-M.; Lee, I.-J.; Song, K.-S.; Rhee, I.-K. Growth promotion of red pepper plug seedlings and the production of gibberellins by Bacillus cereus, Bacillus macroides and Bacillus pumilus. Biotechnol. Lett. 2004, 26, 487–491. [Google Scholar] [CrossRef]
- Gholami, A.; Shahsavani, S.; Nezarat, S. The Effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. Int. Sch. Sci. Res. Innov. 2009, 3, 9–14. [Google Scholar]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; van den Boom, T.; Weber, E. Phanologische Entwicklungsstadien von Gemusepflanzen II. Fruchtgemuse und Hulsenfruchte. Nachr. Des Dtsch. Pflanzenschutzd. 1995, 47, 217–232. [Google Scholar]
- Maggio, A.; Barbieri, G.; Raimondi, G.; de Pascale, S. Contrasting Effects of GA 3 Treatments on Tomato Plants Exposed to Increasing Salinity. J. Plant Growth Regul. 2010, 29, 63–72. [Google Scholar] [CrossRef]
- Kazemi, M. Effect of gibberellic acid and potassium nitrate spray on vegetative growth and reproductive characteristics of tomato. J. Biol. Environ. Sci. 2014, 8, 1–9. [Google Scholar]
- Khan, M.M.A.; Gautam, C.; Mohammad, F.; Siddiqui, M.H.; Naeem, M.; Khan, M.N. Effect of gibberellic acid spray on performance of tomato. Turk. J. Biol. 2006, 30, 11–16. [Google Scholar]
- Gelmesa, D.; Abebie, B.; Desalegn, L. Effects of gibberellic acid and 2, 4-dichlorophenoxyacetic acid spray on fruit yield and quality of tomato (Lycopersicon esculentum Mill.). J. Plant Breed. Crop Sci. 2010, 2, 316–324. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of nutrient efficient plants in improving crop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Masson, J.; Tremblay, N.; Gosselin, A. Nitrogen fertilization and HPS supplementary lighting influence vegetable transplant production. I. Transplant growth. J. Am. Soc. Hortic. Sci. 1991, 116, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Schrader, W.L. Using Transplants in Vegetable Production; UCANR Publications: Davis, CA, USA, 2000; ISBN 1601071930. [Google Scholar]
- Liptay, A.; Nicholls, S. Nitrogen Supply during Greenhouse Transplant Production Affects Subsequent Tomato Root Growth in the Field. J. Am. Soc. Hortic. Sci. 1993, 118, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Kloepper, J.W.; Reddy, M.S.; Rodriguez-Kabana, R.; Kenney, D.S.; Kokalis-Burelle, N.; Martinez-Ochoa, N.; Vavrina, C.S. Application for rhizobacteria in transplant production and yield enhancement. Acta Hortic. 2004, 631, 217–229. [Google Scholar] [CrossRef]
- Latimer, J.G. Mechanical conditioning to control height. Horttechnology 1998, 8, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Dufault, R.J. Vegetable transplant nutrition. Horttechnology 1998, 8, 515–523. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Contribution of indole-3-acetic acid in the plant growth promotion by the rhizospheric strain Bacillus amyloliquefaciens SQR9. Biol. Fertil. Soils 2015, 51, 321–330. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Ekin, Z.; Faruk, O.; Erman, M.; Erdal, Ö. The effect of Bacillus sp. OSU-142 inoculation at various levels of nitrogen fertilization on growth, tuber distribution and yield of potato (Solanum tuberosum L.). Afr. J. Biotechnol. 2009, 8, 4418–4424. [Google Scholar]
- Welbaum, G.E.; Sturz, A.V.; Dong, Z.; Nowak, J. Managing soil microorganisms to improve productivity of agro-ecosystems. CRC Crit. Rev. Plant Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Jingguo, W.; Bakken, L.R. Competition for nitrogen during mineralization of plant residues in soil: Microbial response to C and N availability. Soil Biol. Biochem. 1997, 29, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Rovira, A.D.; Davey, C.B. Biology of the rhizosphere. In The Plant Root and its Environment; Carson, E., Ed.; University of Virginia Press: Charlottesville, VA, USA, 1974; ISBN 978-0813904115. [Google Scholar]
- Oliveira, A.L.M.; Urquiaga, S.; Döbereiner, J.; Baldani, J.I. The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 2002, 242, 205–215. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]
- Achard, P.; Gusti, A.; Cheminant, S.; Alioua, M.; Dhondt, S.; Coppens, F.; Beemster, G.T.S.; Genschik, P. Gibberellin Signaling Controls Cell Proliferation Rate in Arabidopsis. Curr. Biol. 2009, 19, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Ubeda-Tomás, S.; Federici, F.; Casimiro, I.; Beemster, G.T.S.; Bhalerao, R.; Swarup, R.; Doerner, P.; Haseloff, J.; Bennett, M.J. Gibberellin Signaling in the Endodermis Controls Arabidopsis Root Meristem Size. Curr. Biol. 2009, 19, 1194–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.N.; Datta, S.K. Influence of gibberellic acid (GA3) on growth and flowering in chrysanthemum (Chrysanthemummorifolium, Ramat) cv. Jayanti. Indian J. Plant Physiol. 2001, 6, 420–422. [Google Scholar]
- Huttly, A.K.; Phillips, A.L. Gibberellin-regulated plant genes. Physiol. Plant. 1995, 95, 310–317. [Google Scholar] [CrossRef]
- van Huizen, R.; Ozga, J.A.; Reinecke, D.M. Influence of auxin and gibberellin on in vivo protein synthesis during early pea fruit growth. Plant. Physiol. 1996, 112, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Cohn, N.S.; Zhang, L.; Mitchell, J.P.; Vierheller, C.-Z.J. Gibberellin-stimulated changes in abundance of two mRNAs in the developing shoot of dwarf peas (Pisum sativum L.). Int. J. Plant Sci. 1994, 155, 498–505. [Google Scholar] [CrossRef]
- Shiri, Y.; Solouki, M.; Ebrahimie, E.; Emamjomeh, A.; Zahiri, J. Gibberellin causes wide transcriptional modifications in the early stage of grape cluster development. Genomics 2020, 112, 820–830. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Khan, M.N.; Mohammad, F.; Khan, M.M.A. Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop. Sci. 2008, 194, 214–224. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Jiang, C.-Z.; Mitcham, E.J. Mechanisms involved in calcium deficiency development in tomato fruit in response to gibberellins. J. Plant Growth Regul. 2012, 31, 221–234. [Google Scholar] [CrossRef]
- Khan, N.A.; Ansari, H.R. Effect of gibberellic acid spray during ontogeny of mustard on growth, nutrient uptake and yield characteristics. J. Agron. Crop. Sci. 1998, 181, 61–63. [Google Scholar] [CrossRef]
- Al-Wakeel, S.A.M.; Dadoura, S.S.; Hamed, A.A. Interactive effects of water stress and gibberellic acid on mineral composition of fenugreek plant. Egypt. J. Rhysiological Sci. 1994, 18, 269–272. [Google Scholar]
- Ansari, H. Effect of Some Phytohormones and NPK on Growth and Metabolism of Mustard. Ph.D. Thesis, Aligarh Muslim University, Aligarh, India, 1996. [Google Scholar]
- Cramer, M.D.; Nagel, O.W.; Lips, S.H.; Lambers, H. Reduction, assimilation and transport of N in normal and gibberellin-deficient tomato plants. Physiol. Plant. 1995, 95, 347–354. [Google Scholar] [CrossRef]
- Estruch, J.J.; Peretó, J.G.; Vercher, Y.; Beltrán, J.P. Sucrose loading in isolated veins of Pisum sativum: Regulation by abscisic acid, gibberellic acid, and cell turgor. Plant. Physiol. 1989, 91, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Wang, Q.-M.; Guo, Y.-P.; Guo, D.-P.; Shah, G.A.; Liu, H.-L.; Mao, A. Stem-swelling and photosynthate partitioning in stem mustard are regulated by photoperiod and plant hormones. Environ. Exp. Bot. 2008, 62, 160–167. [Google Scholar] [CrossRef]
- Sugiura, D.; Sawakami, K.; Kojima, M.; Sakakibara, H.; Terashima, I.; Tateno, M. Roles of gibberellins and cytokinins in regulation of morphological and physiological traits in Polygonum cuspidatum responding to light and nitrogen availabilities. Funct. Plant Biol. 2015, 42, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, N.; Senecal, M. Nitrogen and potassium in nutrient solution influence seedling growth of four vegetable species. HortScience 1988, 23, 1018–1020. [Google Scholar]
- Tesi, R.; Tallarico, R. L’indurimento delle piantine di pomodoro in vivaio e loro resistenza al freddo. Colt. Prolette 1984, 11, 49–54. [Google Scholar]
- Kratky, B.A.; Mishima, H.Y. Foliar Fertilization during Transplant Production. J. Am. Soc. Hortic. Sci 1981, 106, 3–7. [Google Scholar]
- Vavrina, C.S.; Hochmuth, G.J.; Cornell, J.A.; Olson, S.M. Nitrogen fertilization of Florida-grown tomato transplants: Seasonal variation in greenhouse and field performance. HortScience 1998, 33, 251–254. [Google Scholar] [CrossRef] [Green Version]
- Melton, R.R.; Dufault, R.J. Nitrogen, phosphorus, and potassium fertility regimes affect tomato transplant growth. HortScience 1991, 26, 141–142. [Google Scholar] [CrossRef] [Green Version]
- Nicola, S.; Basoccu, L. Nitrogen and n,p,k relation affect tomato seedling growth, yield and earliness. Acta Hortic. 1994, 95–102. [Google Scholar] [CrossRef]
- Khan, N.A.; Mir, R.; Khan, M.; Javid, S. Effects of gibberellic acid spray on nitrogen yield efficiency of mustard grown with different nitrogen levels. Plant Growth Regul. 2002, 38, 243–247. [Google Scholar] [CrossRef]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M. Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom. Biotechnol. Lett. 2013, 18, 8132–8143. [Google Scholar]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M.; Hritcu, L. Seed inoculation with plant growth promoting rhizobacteria enhances photosynthesis and yield of runner bean (Phaseolus coccineus L.). Sci. Hortic. 2013, 151, 22–29. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, K.D. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric. Biol. Sci. 2005, 1, 210–215. [Google Scholar]
- Miceli, A.; Miceli, C. Effect of nitrogen fertilization on the quality of swiss chard at harvest and during storage as minimally processed produce. J. Food Qual. 2014, 37, 125–134. [Google Scholar] [CrossRef]
- Ihl, M.; Shene, C.; Scheuermann, E.; Bifani, V. Correlation for pigment content through colour determination using tristimulus values in a green leafy vegetable, swiss chard. J. Sci. Food Agric. 1994, 66, 527–531. [Google Scholar] [CrossRef]
- Madeira, A.C.; Ferreira, A.; de Varennes, A.; Vieira, M.I. SPAD Meter Versus Tristimulus Colorimeter to Estimate Chlorophyll Content and Leaf Color in Sweet Pepper. Commun. Soil Sci. Plant Anal. 2003, 34, 2461–2470. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Sabatino, L.; Iapichino, G.; D’Anna, F.; Vetrano, F. Effect of Molybdenum Rate on Yield and Quality of Lettuce, Escarole, and Curly Endive Grown in a Floating System. Agronomy 2018, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Ciardi, J.A.; Vavrina, C.S.; Orzolek, M.D. Evaluation of tomato transplant production methods for improving establishment rates. HortScience 1998, 33, 229–232. [Google Scholar]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Das, N.; Deb, P.; Suresh, C.P. Effect of NAA and GA3 on yield and quality of tomato (Lycopersicon esculentum Mill.). Environ. Ecol. 2009, 27, 1048–1050. [Google Scholar]
- Prasad, R.N.; Singh, S.K.; Yadava, R.B.; Chaurasia, S.N.S. Effect of GA3 and NAA on growth and yield of tomato. Veg. Sci. 2013, 40, 195–197. [Google Scholar]
- Iqbal, N.; Nazar, R.; Khan, M.I.R.; Masood, A.; Khan, N.A. Role of gibberellins in regulation of source–sink relations under optimal and limiting environmental conditions. Curr. Sci. 2011, 100, 998–1007. [Google Scholar]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shin, J.-H.; Ahmad, B.; Shin, D.-H.; Lee, I.-J. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem. 2010, 58, 7226–7232. [Google Scholar] [CrossRef]
- Gonai, T.; Kawahara, S.; Tougou, M.; Satoh, S.; Hashiba, T.; Hirai, N.; Kawaide, H.; Kamiya, Y.; Yoshioka, T. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J. Exp. Bot. 2004, 55, 111–118. [Google Scholar] [CrossRef]
- Akram, W.; Anjum, T.; Ali, B. Co-cultivation of tomato with two Bacillus strains: Effects on growth and yield. J. Anim. Plant Sci. 2015, 25, 1644–1651. [Google Scholar]
- Gul, A.; Kidoglu, F.; Tüzel, Y.; Tüzel, I.H. Effects of nutrition and “Bacillus amyloliquefaciens” on tomato (“Solanum lycopersicum L.”) growing in perlite. Span. J. Agric. Res. 2008, 6, 422–429. [Google Scholar] [CrossRef]
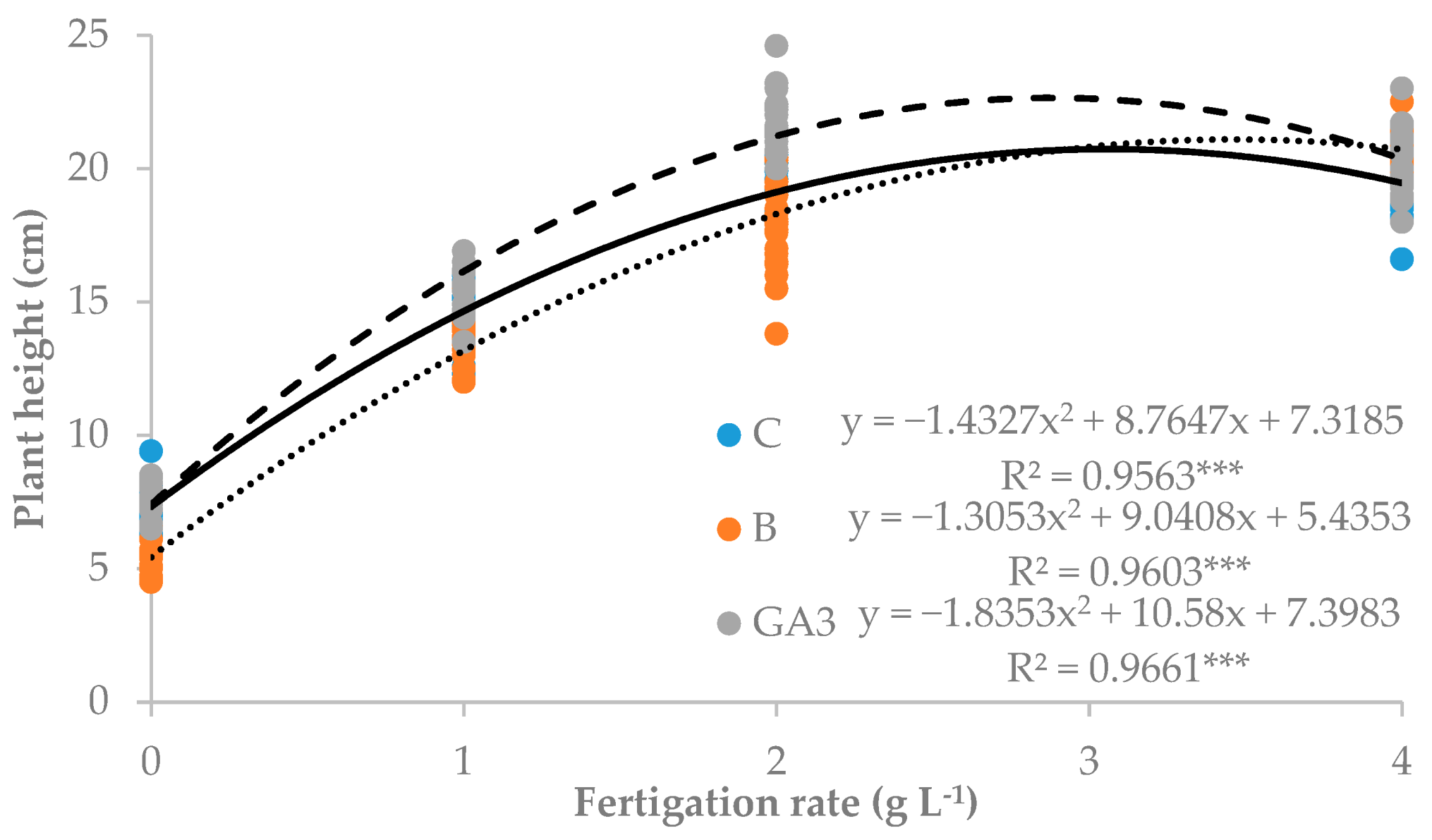
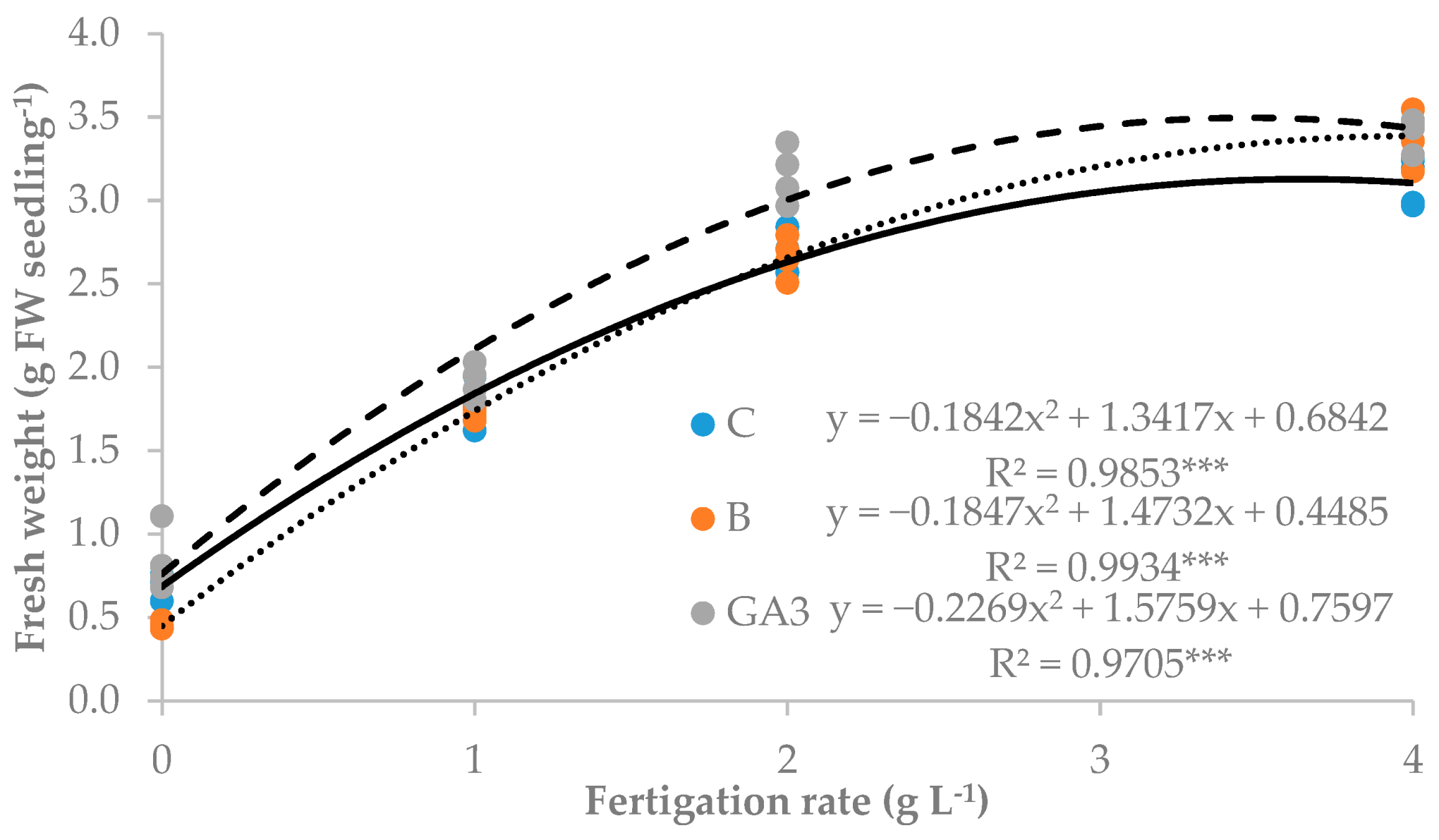


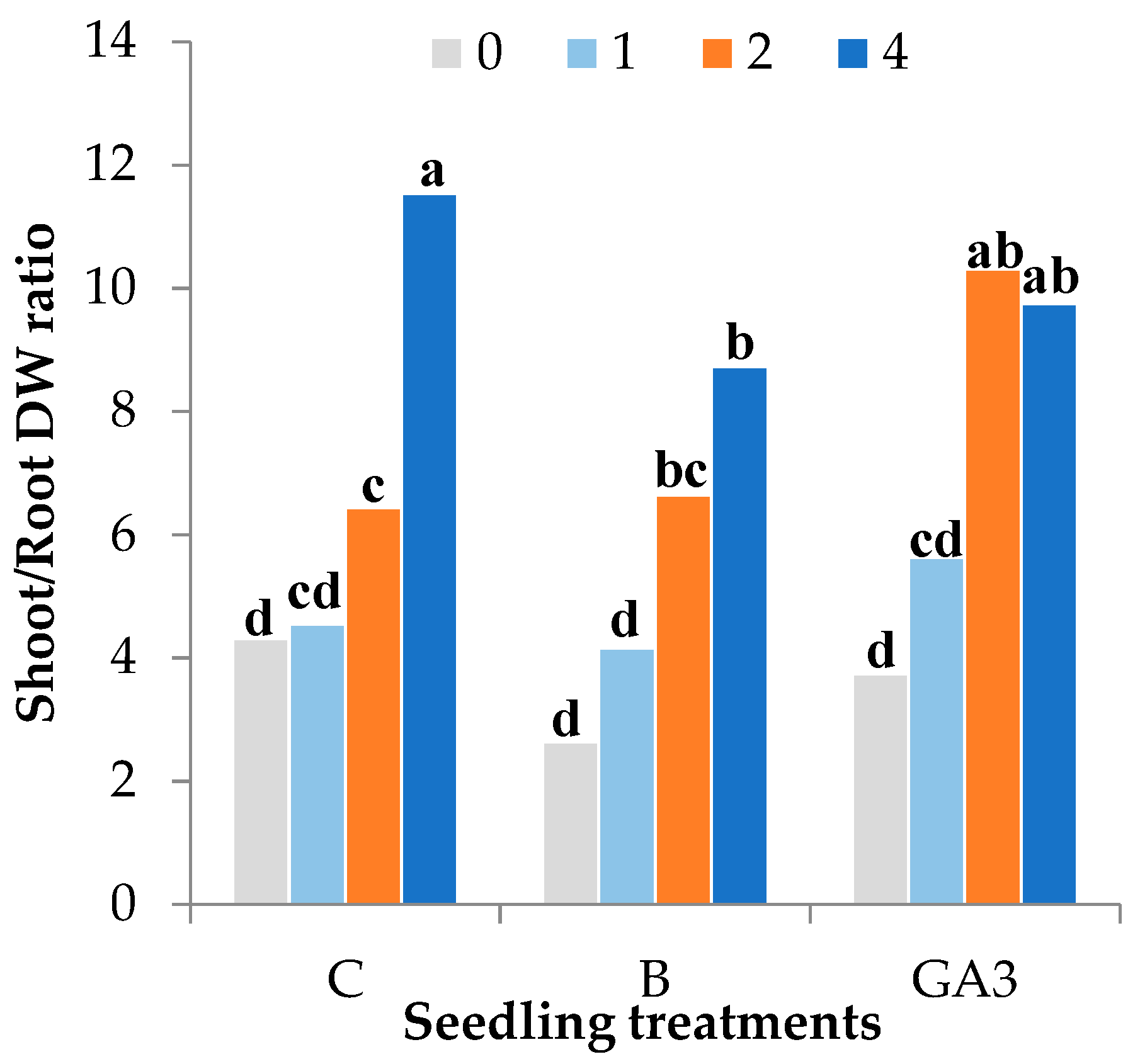
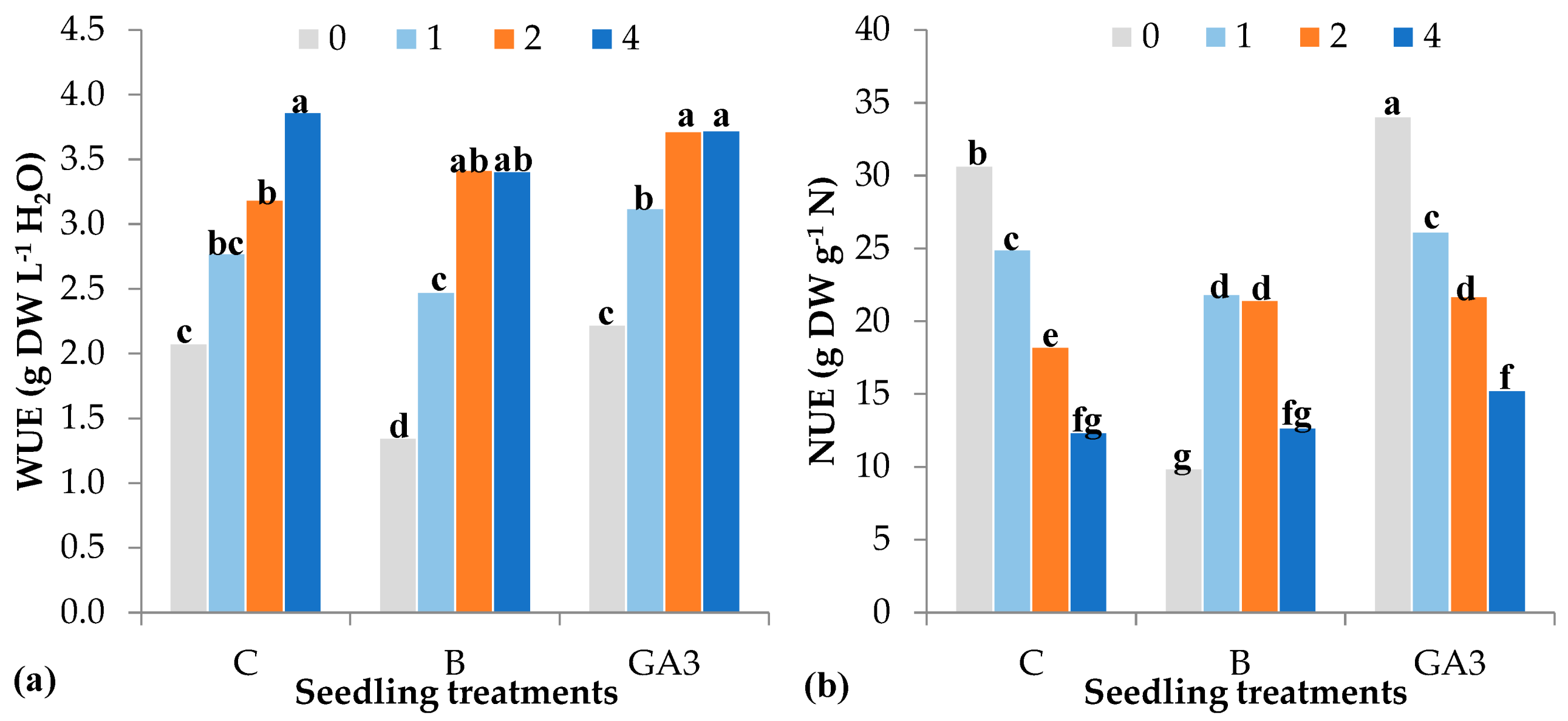




| Source of Variance | Seedling Height (cm) | Stem Diameter (mm) | Seedling Fresh Weight (g FW) | Seedling Dry Weight (mg DW) | Dry Matter (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Roots | Stem | Leaves | Total | Roots | Stem | Leaves | |||||
| Treatment | ||||||||||||
| C | z 15.1 | 2.3 | 2.07 | 0.24 | 1.12 | 0.70b | 137.3 | 19.0 | 57.3 | 61.0 | 7.8 | |
| B | 14.4 | 2.3 | 2.06 | 0.25 | 1.11 | 0.69b | 126.8 | 19.0 | 52.3 | 55.8 | 7.5 | |
| GA3 | 16.3 | 2.3 | 2.33 | 0.24 | 1.24 | 0.85a | 160.0 | 19.5 | 66.0 | 74.5 | 7.8 | |
| NPK (g L−1) | ||||||||||||
| 0 | 6.7 | 2.0d | 0.66 | 0.18 | 0.30 | 0.18d | 75.7 | 16.7c | 31.3 | 28.0 | 12.4a | |
| 1 | 14.6 | 2.1c | 1.81 | 0.28 | 0.90 | 0.64c | 128.7 | 22.3a | 50.7 | 55.7 | 6.9b | |
| 2 | 19.6 | 2.4b | 2.83 | 0.26 | 1.56 | 1.01b | 171.7 | 20.0ab | 71.7 | 80.0 | 5.9c | |
| 4 | 20.2 | 2.6a | 3.30 | 0.25 | 1.88 | 1.17a | 189.3 | 17.7bc | 80.3 | 91.3 | 5.7c | |
| Treatment × NPK | ||||||||||||
| C | 0 | 7.3g | 2.0 | 0.70e | 0.19bc | 0.35e | 0.17 | 83.0d | 15.0 | 37.0ef | 30.0de | 12.9 |
| 1 | 14.7ef | 2.2 | 1.79d | 0.29a | 0.89d | 0.61 | 130.0c | 21.0 | 51.0de | 55.0c | 7.0 | |
| 2 | 19.1c | 2.4 | 2.67c | 0.25ab | 1.51c | 0.92 | 153.0bc | 21.0 | 63.0dc | 69.0bc | 5.4 | |
| 4 | 19.5c | 2.7 | 3.10b | 0.22b | 1.76b | 1.12 | 183.0ab | 19.0 | 78.0b | 90.0ab | 5.9 | |
| B | 0 | 5.2h | 1.8 | 0.45f | 0.15c | 0.21f | 0.09 | 53.0e | 16.0 | 22.0g | 17.0e | 12.8 |
| 1 | 13.7f | 2.1 | 1.73d | 0.29a | 0.86d | 0.59 | 110.0cd | 24.0 | 44.0e | 45.0cd | 6.2 | |
| 2 | 17.9d | 2.5 | 2.66c | 0.28a | 1.44c | 0.94 | 163.0b | 21.0 | 68.0c | 74.0b | 5.9 | |
| 4 | 20.8b | 2.6 | 3.39a | 0.28ab | 1.95a | 1.16 | 181.0ab | 15.0 | 75.0bc | 87.0ab | 5.2 | |
| GA3 | 0 | 7.7g | 2.1 | 0.83e | 0.20bc | 0.35e | 0.28 | 91.0d | 19.0 | 35.0f | 37.0d | 11.6 |
| 1 | 15.4e | 2.1 | 1.91d | 0.25ab | 0.95d | 0.71 | 146.0bc | 22.0 | 57.0d | 67.0bc | 7.4 | |
| 2 | 21.7a | 2.4 | 3.15b | 0.25ab | 1.75b | 1.16 | 199.0a | 18.0 | 84.0ab | 97.0a | 6.3 | |
| 4 | 20.3bc | 2.6 | 3.41a | 0.26ab | 1.92a | 1.23 | 204.0a | 19.0 | 88.0a | 97.0a | 6.0 | |
| Significance x | ||||||||||||
| Treatment | *** | ns | *** | ns | *** | *** | *** | ns | *** | *** | ns | |
| NPK | *** | *** | *** | *** | *** | *** | *** | ** | *** | *** | *** | |
| Treatment × NPK | *** | ns | *** | * | *** | ns | ** | ns | *** | * | ns | |
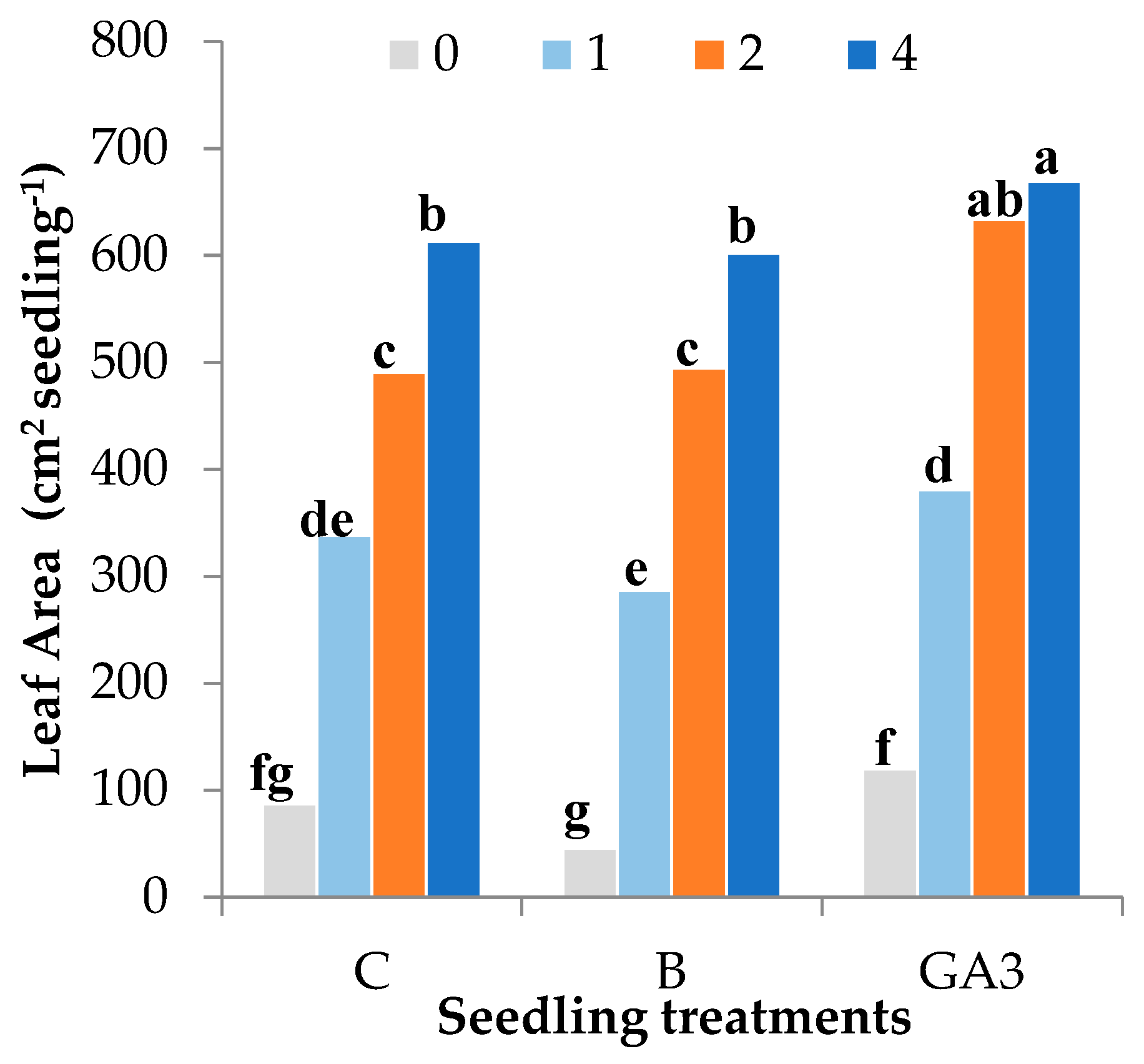
| Source of Variance | Number of Leaves | Leaf Area (cm2 Seedling−1) | Leaf Area (cm2 Leaf−1) | SLA y (cm2 g DW−1) | Stomatal Conductance (mmol m2 s−1) | L* | Chroma | Hue° | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment | |||||||||
| C | z 4.0b | 38.3 | 7.3b | 485.6 | 507.6 | 47.9 | 34.3 | 136.8b | |
| B | 4.0b | 35.7 | 7.8ab | 573.1 | 569.3 | 48.2 | 33.6 | 139.1a | |
| GA3 | 4.6a | 45.1 | 9.1a | 560.4 | 368.3 | 49.2 | 35.6 | 136.7b | |
| NPK (g L−1) | |||||||||
| 0 | 2.2c | 8.4 | 3.7c | 301.1c | 275.9 | 52.6 | 39.5 | 136.2d | |
| 1 | 4.3b | 33.6 | 7.1b | 552.0b | 592.0 | 49.9 | 36.5 | 137.1c | |
| 2 | 5.1a | 54.0 | 10.5a | 681.0a | 506.2 | 47.2 | 32.6 | 137.9b | |
| 4 | 5.2a | 62.8 | 10.9a | 624.6ab | 552.8 | 44.0 | 29.4 | 138.9a | |
| Treatment × NPK | |||||||||
| C | 0 | 2.1 | 8.7fg | 4.2 | 297.4 | 260.1c | 52.2b | 40.4a | 134.7c |
| 1 | 4.2 | 33.9de | 6.2 | 433.3 | 714.3a | 48.7d | 36.3b | 135.9c | |
| 2 | 4.9 | 49.1c | 10.0 | 718.1 | 600.0ab | 47.0e | 31.1d | 137.8b | |
| 4 | 5.0 | 61.4b | 9.0 | 493.6 | 456.1b | 43.5f | 29.4de | 138.8ab | |
| B | 0 | 2.0 | 4.6g | 2.3 | 278.3 | 286.2c | 51.1c | 36.1b | 139.0ab |
| 1 | 4.0 | 28.7e | 7.2 | 648.7 | 595.9ab | 50.0cd | 36.2b | 139.9a | |
| 2 | 4.9 | 49.5c | 10.2 | 673.3 | 607.1ab | 47.9de | 33.4c | 138.8ab | |
| 4 | 5.2 | 60.2b | 11.6 | 692.0 | 787.8a | 43.7f | 28.7e | 138.8ab | |
| GA3 | 0 | 2.6 | 12.0f | 4.6 | 327.5 | 281.4c | 54.6a | 42.0a | 135.0c |
| 1 | 4.8 | 38.1d | 8.0 | 574.1 | 465.8b | 51.0c | 37.1b | 135.5c | |
| 2 | 5.6 | 63.4ab | 11.4 | 651.7 | 311.6c | 46.6e | 33.2c | 137.1bc | |
| 4 | 5.5 | 66.9a | 12.3 | 688.2 | 414.6bc | 44.6f | 30.1de | 139.2ab | |
| Significance x | |||||||||
| Treatment | *** | *** | * | ns | ** | *** | *** | *** | |
| NPK | *** | *** | *** | *** | *** | *** | *** | *** | |
| Treatment × NPK | ns | * | ns | ns | * | *** | *** | *** | |
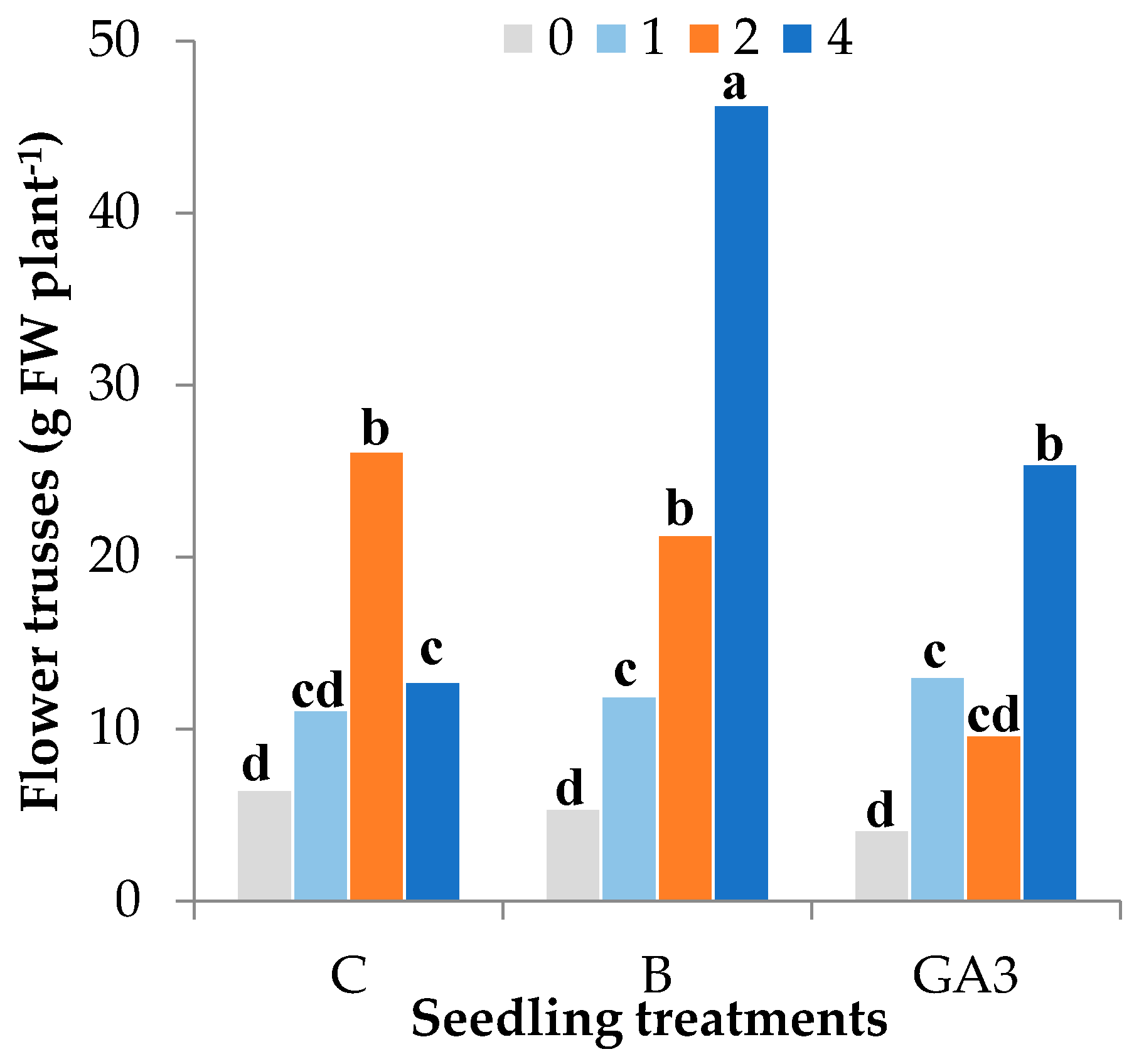
| Source of Variance | Plant Height (cm) | Stem Diameter (mm) | Plant Fresh Weight (g FW) | Plant Dry Weight (g DW) | Dry Matter (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Roots | Stem | Leaves | Trusses | Total | Roots | Stem | Leaves | Trusses | |||||
| Treatment | ||||||||||||||
| C | z 77.6 | 9.7 | 229.1 | 20.0 | 60.1 | 134.9 | 14.1 | 26.1 | 2.0 | 7.7 | 14.6 | 1.9 | 11.3 | |
| B | 76.1 | 9.7 | 230.4 | 18.8 | 58.5 | 131.8 | 21.2 | 26.3 | 2.0 | 7.5 | 14.5 | 2.3 | 11.4 | |
| GA3 | 78.8 | 9.7 | 225.9 | 18.5 | 60.0 | 134.2 | 13.1 | 25.6 | 1.8 | 7.7 | 14.6 | 1.5 | 11.3 | |
| NPK (g L−1) | ||||||||||||||
| 0 | 61.4 | 9.3b | 171.1c | 14.1b | 49.2b | 102.5b | 5.3c | 18.2c | 1.4b | 5.3c | 10.8b | 0.6c | 10.6b | |
| 1 | 76.3 | 9.9a | 234.2b | 20.7a | 61.0a | 140.4a | 12.0bc | 27.2b | 2.2a | 8.0b | 15.6a | 1.5bc | 11.6a | |
| 2 | 86.9 | 9.6ab | 250.4a | 21.1a | 65.5a | 144.8a | 19.0ab | 29.2a | 2.1a | 8.7a | 15.9a | 2.4ab | 11.6a | |
| 4 | 85.3 | 10.0a | 258.0a | 20.5a | 62.5a | 146.9a | 28.2a | 29.5a | 2.1a | 8.5a | 15.9a | 3.0a | 11.4a | |
| Treatment × NPK | ||||||||||||||
| C | 0 | 65.4c | 8.89 | 179.9 | 18.5 | 53.0 | 102.0 | 6.5d | 19.0 | 1.7 | 5.8 | 10.7 | 0.8cd | 10.6 |
| 1 | 73.4bc | 10.2 | 236.1 | 20.5 | 60.3 | 144.1 | 11.1cd | 26.8 | 2.3 | 7.7 | 15.3 | 1.5c | 11.3 | |
| 2 | 84.8ab | 9.71 | 250.7 | 20.2 | 61.4 | 143.0 | 26.2b | 30.0 | 2.1 | 8.4 | 15.9 | 3.7ab | 12.0 | |
| 4 | 86.8ab | 9.92 | 249.6 | 20.7 | 65.8 | 150.4 | 12.8c | 28.7 | 2.1 | 8.8 | 16.4 | 1.5c | 11.5 | |
| B | 0 | 56.0d | 9.23 | 162.4 | 11.8 | 44.2 | 101.0 | 5.4d | 18.2 | 1.3 | 4.9 | 11.4 | 0.6d | 11.2 |
| 1 | 75.0b | 9.80 | 235.2 | 20.8 | 61.1 | 141.3 | 11.9c | 27.7 | 2.2 | 8.0 | 15.9 | 1.6c | 11.8 | |
| 2 | 89.4a | 9.62 | 255.2 | 22.2 | 68.8 | 142.9 | 21.3b | 28.9 | 2.2 | 8.9 | 15.4 | 2.3bc | 11.3 | |
| 4 | 83.8ab | 10.2 | 268.8 | 20.4 | 60.1 | 142.0 | 46.3a | 30.3 | 2.1 | 8.2 | 15.2 | 4.8a | 11.3 | |
| GA3 | 0 | 62.8dc | 9.70 | 170.9 | 12.0 | 50.3 | 104.4 | 4.2d | 17.3 | 1.3 | 5.1 | 10.4 | 0.5d | 10.1 |
| 1 | 80.4b | 9.87 | 231.4 | 20.8 | 61.7 | 135.8 | 13.1c | 27.2 | 2.0 | 8.2 | 15.4 | 1.5c | 11.7 | |
| 2 | 86.6ab | 9.50 | 245.5 | 20.9 | 66.4 | 148.4 | 9.7cd | 28.5 | 2.0 | 8.7 | 16.5 | 1.3c | 11.6 | |
| 4 | 85.2ab | 9.91 | 255.7 | 20.3 | 61.6 | 148.3 | 25.4b | 29.6 | 2.0 | 8.7 | 16.2 | 2.7b | 11.6 | |
| Significance x | ||||||||||||||
| Treatment | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| NPK | *** | * | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Treatment × NPK | * | ns | ns | ns | ns | ns | * | ns | ns | ns | ns | * | ns | |
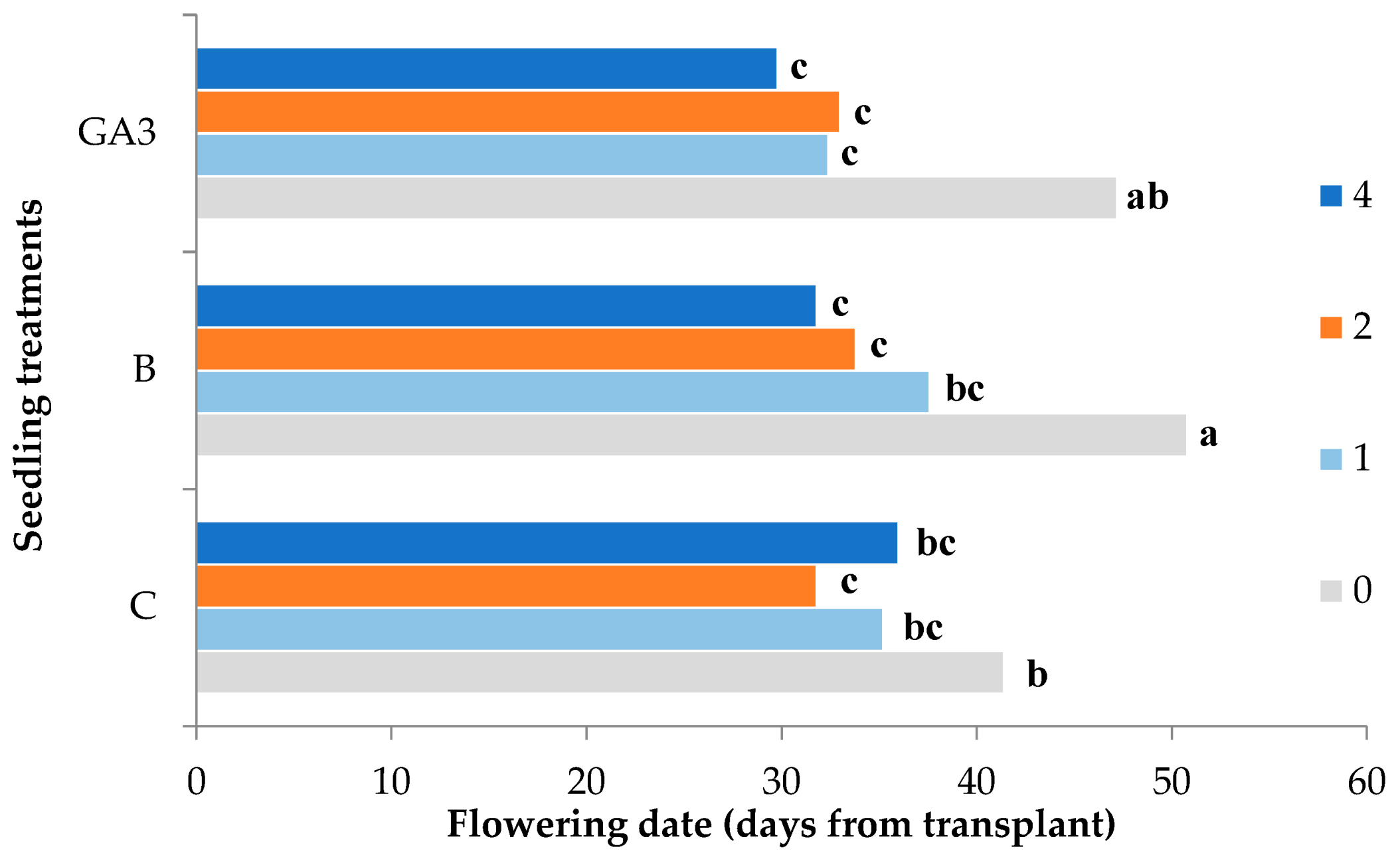
| Source of Variance | Flowering Date (d from Transplant) | Height of First Flower Truss (cm) | Nodes of First Flower Truss (n.) | Internode Length (cm) | |
|---|---|---|---|---|---|
| Treatment | |||||
| C | z 36.1 | 38.4ab | 9.7 | 4.4 | |
| B | 38.5 | 39.8a | 10.2 | 4.4 | |
| GA3 | 35.6 | 36.8b | 9.7 | 4.2 | |
| NPK (g L-1) | |||||
| 0 | 46.5 | 33.2b | 10.1 | 3.7c | |
| 1 | 35.1 | 35.2b | 9.5 | 4.2b | |
| 2 | 32.9 | 41.3a | 9.6 | 4.8a | |
| 4 | 32.5 | 43.6a | 10.2 | 4.8a | |
| Treatment × NPK | |||||
| C | 0 | 41.4b | 32.0 | 9.4 | 3.8 |
| 1 | 35.2cd | 33.7 | 9.4 | 4.0 | |
| 2 | 31.8de | 40.6 | 9.2 | 5.0 | |
| 4 | 36.0cd | 47.2 | 10.8 | 4.9 | |
| B | 0 | 50.8a | 37.4 | 11.6 | 3.6 |
| 1 | 37.6c | 35.9 | 9.4 | 4.3 | |
| 2 | 33.8d | 43.2 | 10.0 | 4.8 | |
| 4 | 31.8de | 42.8 | 9.6 | 5.0 | |
| GA3 | 0 | 47.2a | 30.2 | 9.4 | 3.6 |
| 1 | 32.4de | 36.0 | 9.6 | 4.2 | |
| 2 | 33.0de | 40.2 | 9.6 | 4.7 | |
| 4 | 29.8e | 40.9 | 10.2 | 4.5 | |
| Significance x | |||||
| Treatment | * | * | ns | ns | |
| NPK | *** | *** | ns | *** | |
| Treatment × NPK | ** | ns | ns | ns | |
| Source of Variance | Number of Leaves | Leaf Area (cm2 Plant−1) | Leaf Area (cm2 Leaf−1) | SLA y (cm2 g DW−1) | Stomatal Conductance (mmol m2 s−1) | L* | Chroma | Hue° | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment | |||||||||
| C | z 18.7 | 3164.7a | 171.3 | 223.0a | 319.5 | 38.0a | 24.3 | 125.6a | |
| B | 18.5 | 2834.7b | 149.9 | 197.8b | 379.2 | 37.3b | 21.1 | 124.8b | |
| GA3 | 19.1 | 3103.8a | 161.2 | 206.7b | 406.2 | 38.3a | 24.2 | 124.4b | |
| NPK (g L−1) | |||||||||
| 0 | 16.7b | 2426.7c | 145.5 | 228.3a | 417.8 | 38.3a | 25.2 | 121.2d | |
| 1 | 19.2a | 3105.2b | 160.9 | 197.0b | 380.0 | 38.1ab | 23.7 | 124.0c | |
| 2 | 19.6a | 3212.6ab | 164.5 | 203.0b | 308.0 | 37.7ab | 22.6 | 126.4b | |
| 4 | 19.5a | 3393.0a | 172.4 | 208.4b | 367.4 | 37.4b | 21.2 | 128.3a | |
| Treatment × NPK | |||||||||
| C | 0 | 16.6 | 2408.3 | 144.4c | 241.5a | 396.2 | 38.6 | 27.2a | 122.2 |
| 1 | 19.8 | 3234.7 | 167.3b | 221.1ab | 245.2 | 37.9 | 25.4ab | 124.6 | |
| 2 | 19.2 | 3305.3 | 174.3b | 204.9b | 258.6 | 37.8 | 23.0bc | 127.1 | |
| 4 | 19.2 | 3710.3 | 199.0a | 224.5ab | 377.8 | 37.7 | 21.6bc | 128.7 | |
| B | 0 | 17.0 | 2247.7 | 134.9c | 200.9b | 467.4 | 37.5 | 21.5bc | 121.2 |
| 1 | 18.0 | 2894.7 | 155.1bc | 184.3b | 395.4 | 37.3 | 20.1c | 123.9 | |
| 2 | 19.6 | 3151.7 | 157.4bc | 214.3ab | 251.6 | 37.1 | 21.4bc | 125.8 | |
| 4 | 19.2 | 3044.7 | 152.3bc | 191.9b | 402.4 | 37.3 | 21.5bc | 128.5 | |
| GA3 | 0 | 16.4 | 2624.0 | 157.2bc | 242.6a | 389.8 | 38.8 | 27.0a | 120.4 |
| 1 | 19.8 | 3186.3 | 160.3bc | 185.5b | 499.3 | 38.9 | 25.7ab | 123.4 | |
| 2 | 20.0 | 3180.7 | 161.7bc | 189.9b | 413.8 | 38.1 | 23.5b | 126.2 | |
| 4 | 20.2 | 3424.0 | 165.7bc | 208.8b | 322.0 | 37.2 | 20.63bc | 127.6 | |
| Significance x | |||||||||
| Treatment | ns | ** | *** | *** | ns | *** | *** | *** | |
| NPK | *** | *** | *** | *** | ns | * | *** | *** | |
| Treatment × NPK | ns | ns | ** | ** | ns | ns | *** | ns | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncada, A.; Vetrano, F.; Esposito, A.; Miceli, A. Fertigation Management and Growth-Promoting Treatments Affect Tomato Transplant Production and Plant Growth after Transplant. Agronomy 2020, 10, 1504. https://doi.org/10.3390/agronomy10101504
Moncada A, Vetrano F, Esposito A, Miceli A. Fertigation Management and Growth-Promoting Treatments Affect Tomato Transplant Production and Plant Growth after Transplant. Agronomy. 2020; 10(10):1504. https://doi.org/10.3390/agronomy10101504
Chicago/Turabian StyleMoncada, Alessandra, Filippo Vetrano, Alessandro Esposito, and Alessandro Miceli. 2020. "Fertigation Management and Growth-Promoting Treatments Affect Tomato Transplant Production and Plant Growth after Transplant" Agronomy 10, no. 10: 1504. https://doi.org/10.3390/agronomy10101504





