Yeasts Associated with the Olive Fruit Fly Bactrocera oleae (Rossi) (Diptera: Tephritidae) Lead to New Attractants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment in 2015
2.1.1. Preparation of 12 Insect-Associated Yeasts
2.1.2. Field Testing
2.2. Experiment in 2016
2.2.1. Preparation of the Five Most Attractive Insect-Associated Yeasts
2.2.2. Preparation of Dry Yeast
2.2.3. Field Testing
2.3. Statistical Analysis
3. Results
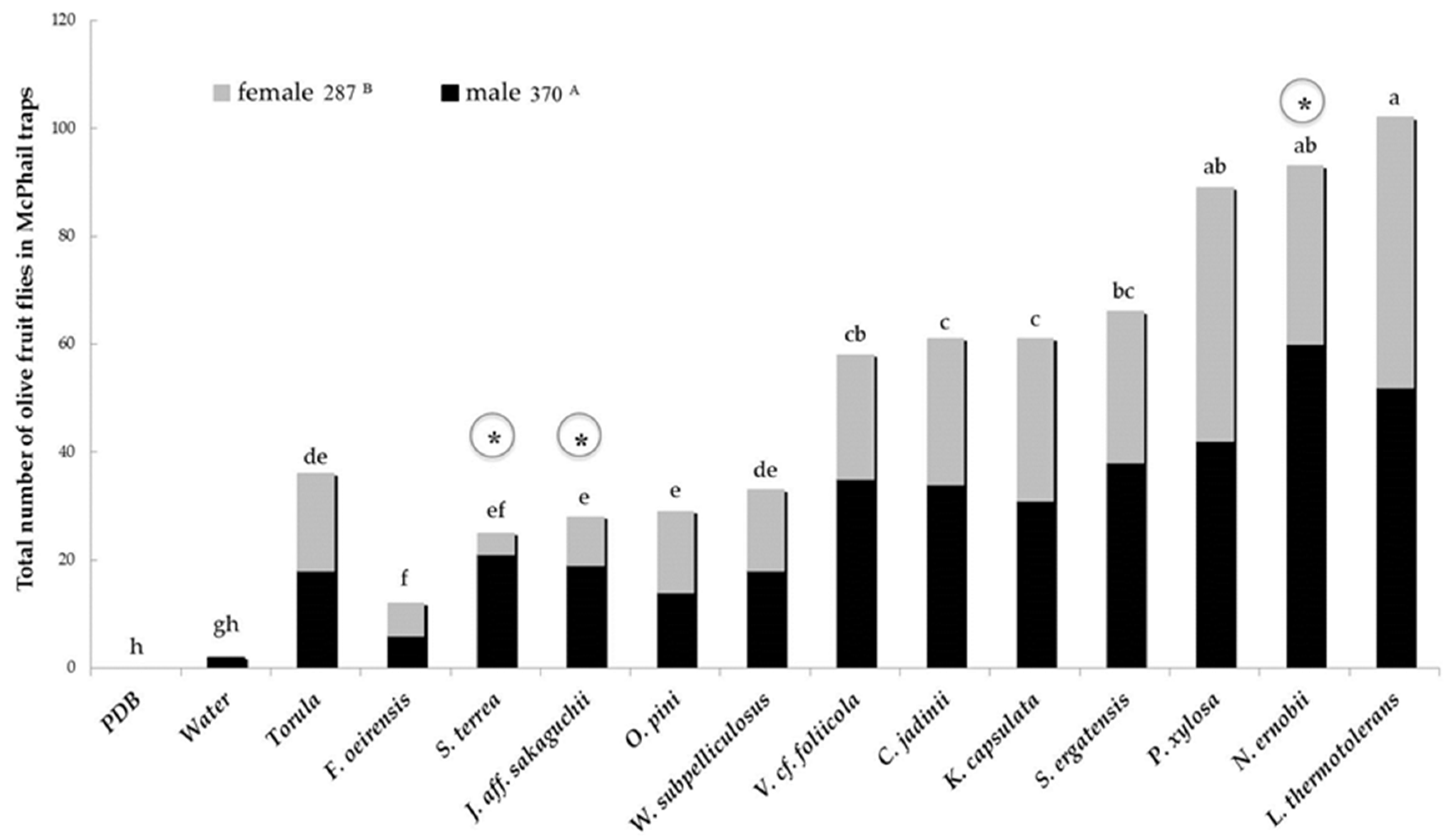
3.1. Experiment in 2015
Attraction of B. oleae to 12 Insect-Associated Yeasts
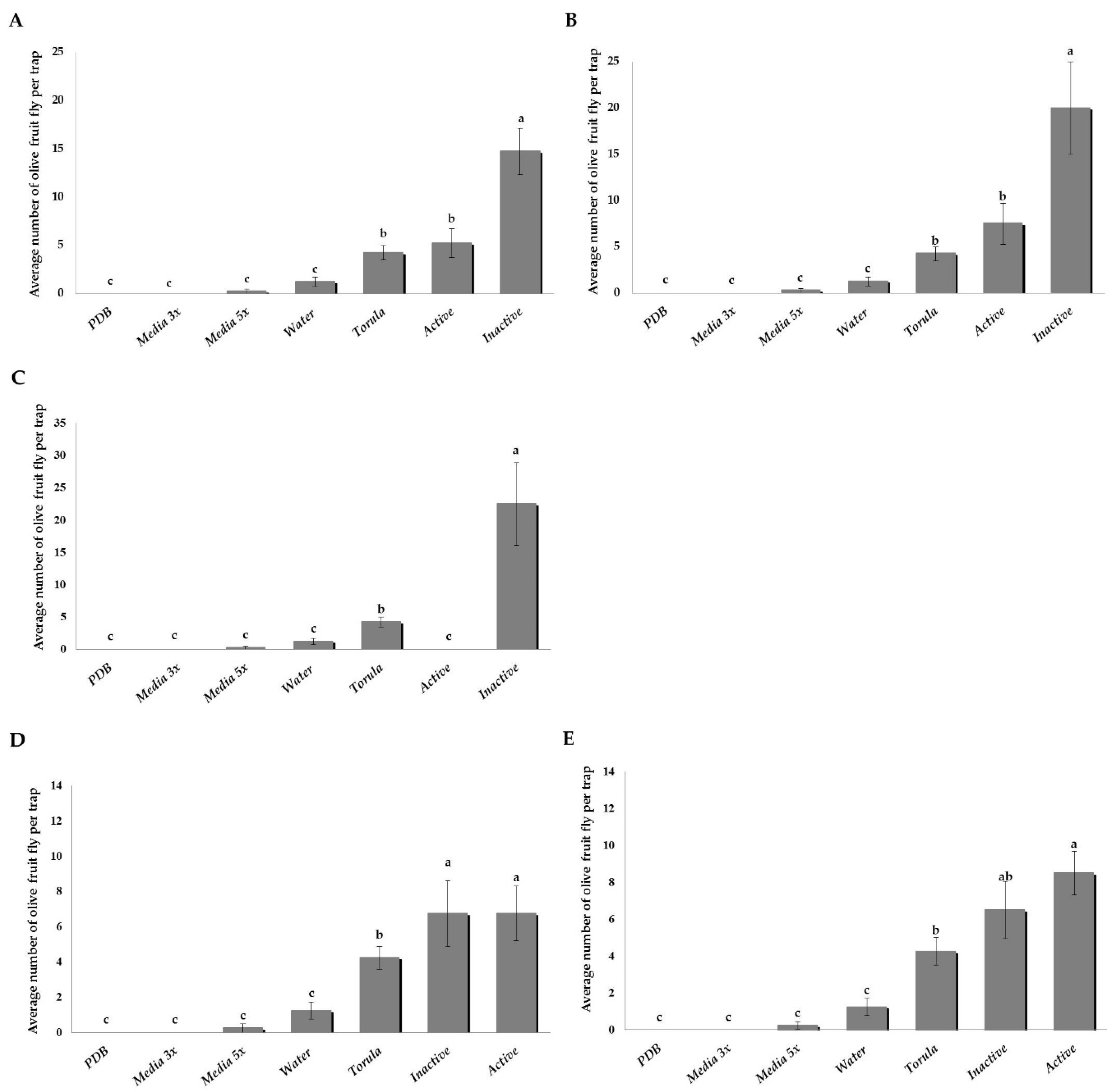
3.2. Experiments in 2016
3.2.1. Attraction of B. oleae to the Five Most Active and Inactive Yeasts
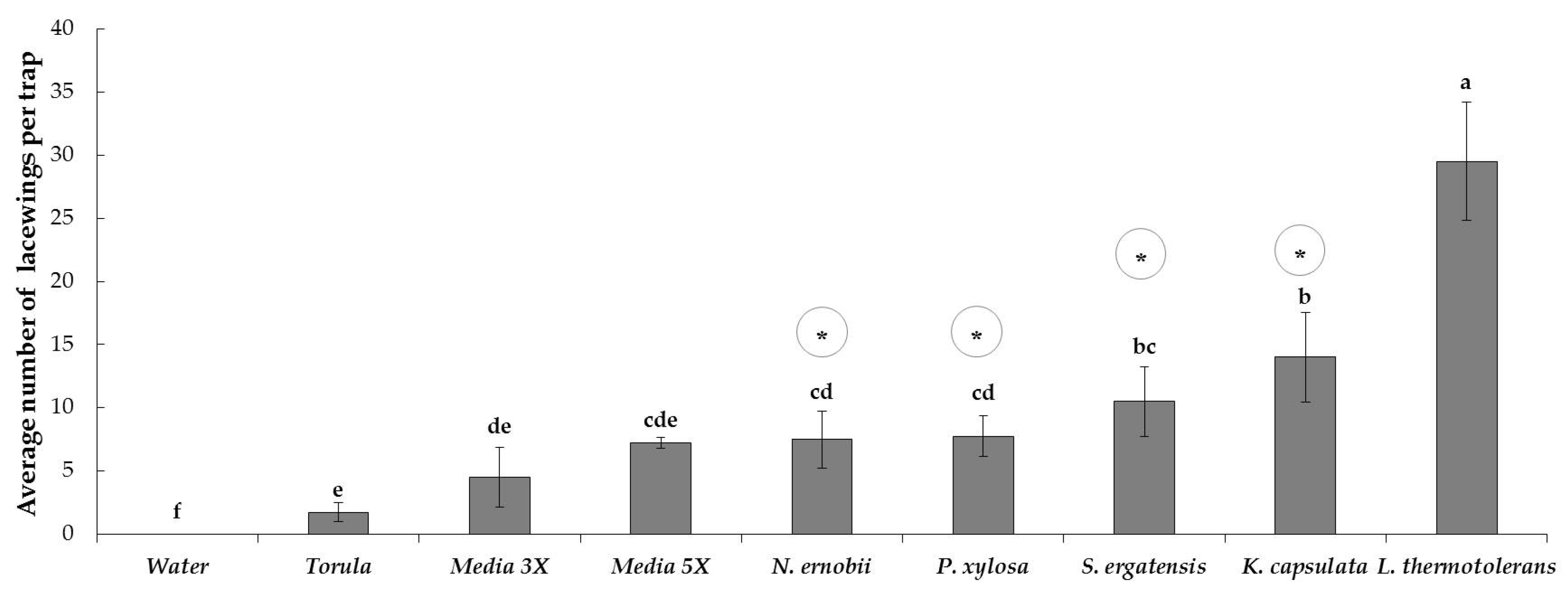
3.2.2. Attraction of a green lacewing to the five most active and inactive yeasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montiel Bueno, A.; Jones, O. Alternative methods for controlling the olive fly, Bactrocera oleae, involving semiochemicals. IOBC wprs Bull. 2002, 25, 1–11. [Google Scholar]
- Collier, T.R.; Van Steenwyk, R.A. Prospects for integrated control of olive fruit fly are promising in California. Calif. Agric. 2003, 57, 28–31. [Google Scholar] [CrossRef]
- Cirio, U. Agrochemicals and environmental impact in olive farming. Olivae 1997, 65, 32–39. [Google Scholar]
- Civantos, M. Control de plagas y enfermedades del olivar; Consejo Oleicola International: Madrid, Spain, 1999; pp. 1–207. [Google Scholar]
- Heim, G. Effect of insecticidal sprays on predators and indifferent arthropods found in olives trees on the north of Lebanon. In Integrated Pest Control in Olive Groves; Cavalloro, R., Crovetti, A., Eds.; CEC/FAO/ IOBC/IJMP: Pisa, Italy, 1985; pp. 456–465. [Google Scholar]
- Vitanovic, E.; Ivezić, M.; Kačić, S.; Katalinić, M.; Durbesić, P.; Barčić, J.I. Arthropod communities within the olive canopy as bioindicators of different management systems. Span. J. Agric. Res. 2018, 16, e0301. [Google Scholar] [CrossRef] [Green Version]
- Andreadis, S.S.; Witzgall, P.; Becher, P.G. Survey of arthropod assemblages responding to live yeasts in an organic apple orchard. Front. Ecol. Evol. 2015, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Biasazin, T.D.; Karlsson, M.F.; Hillbur, Y.; Seyoum, E.; Dekker, T. Identification of Host Blends that Attract the African Invasive Fruit Fly, Bactrocera invadens. J. Chem. Ecol. 2014, 40, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Brevault, T.; Quilici, S. Flower and fruit volatiles assist host-plant location in the Tomato fruit flyNeoceratitis cyanescens. Physiol. Èntomol. 2010, 35, 9–18. [Google Scholar] [CrossRef]
- Dalby-Ball, G.; Meats, A. Influence of the odour of fruit, yeast and cue-lure on the flight activity of the Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Aust. J. Èntomol. 2000, 39, 195–200. [Google Scholar] [CrossRef]
- Kleiber, J.R.; Unelius, R.; Lee, J.C.; Suckling, D.M.; Qian, M.C.; Bruck, D.J. Attractiveness of Fermentation and Related Products to Spotted Wing Drosophila (Diptera: Drosophilidae). Environ. Èntomol. 2014, 43, 439–447. [Google Scholar] [CrossRef]
- Batista, M.R.; Uno, F.; Chaves, R.D.; Tidon, R.; Rosa, C.A.; Klaczko, L.B. Differential attraction of drosophilids to banana baits inoculated with Saccharomyces cerevisiae and Hanseniaspora uvarum within a Neotropical forest remnant. PeerJ 2017, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Dweck, H.K.M.; Ebrahim, S.A.; Farhan, A.; Hansson, B.S.; Stensmyr, M.C. Olfactory Proxy Detection of Dietary Antioxidants in Drosophila. Curr. Boil. 2015, 25, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, B.A.; Whitener, A.B.; Leinweber, Y.; Revadi, S.; Beers, E.H.; Witzgall, P.; Becher, P.G. Enhanced yeast feeding following mating facilitates control of the invasive fruit pestDrosophila suzukii. J. Appl. Ecol. 2016, 54, 170–177. [Google Scholar] [CrossRef]
- Murgier, J.; Everaerts, C.; Farine, J.F.; Ferveur, J.F. Live yeasts in juvenile diet induces species-specific effects on Drosophila adult behavior and fitness. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- K Koerte, S.; Keesey, I.W.; Easson, M.L.A.E.; Gershenzon, J.; Hansson, B.S.; Knaden, M. Variable dependency on associated yeast communities influences host range in Drosophila species. Oikos 2020, 00, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Scheidler, N.H.; Liu, C.; Hamby, K.A.; Zalom, F.G.; Syed, Z. Volatile codes: Correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci. Rep. 2015, 5, 14059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamby, K.A.; Becher, P.G. Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. J. Pest Sci. 2016, 89, 621–630. [Google Scholar] [CrossRef]
- Becher, P.G.; Hagman, A.; Verschut, V.; Chakraborty, A.; Rozpędowska, E.; Lebreton, S.; Bengtsson, M.; Flick, G.; Witzgall, P.; Piškur, J. Chemical signaling and insect attraction is a conserved trait in yeasts. Ecol. Evol. 2018, 8, 2962–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piper, A.M.; Farnier, K.; Linder, T.; Speight, R.; Cunningham, J.P. Two Gut-Associated Yeasts in a Tephritid Fruit Fly have Contrasting Effects on Adult Attraction and Larval Survival. J. Chem. Ecol. 2017, 43, 891–901. [Google Scholar] [CrossRef]
- Deutscher, A.T.; Reynolds, O.L.; A Chapman, T. Yeast: An Overlooked Component of Bactrocera tryoni (Diptera: Tephritidae) Larval Gut Microbiota. J. Econ. Èntomol. 2016, 110, 298–300. [Google Scholar] [CrossRef]
- Malacrinò, A.; Schena, L.; Campolo, O.; Laudani, F.; Mosca, S.; Giunti, G.; Strano, C.P.; Palmeri, V. A Metabarcoding survey on the fungal microbiota associated to the olive fruit fly. Microb. Ecol. 2017, 73, 677–684. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Tsiamis, G.; Caceres, C.; Abd-Alla, A.M.M.; Bourtzis, K. Taxonomy, diet, and developmental stage contribute to the structuring of gut-associated bacterial communities in thephritid pest species. Front. Microb. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blow, F.; Gioti, A.; Goodhead, I.B.; Kalyva, M.; Kampouraki, A.; Vontas, J.; Darby, A.C. Functional Genomics of a Symbiotic Community: Shared Traits in the Olive Fruit Fly Gut Microbiota. Genome Boil. Evol. 2019, 12, 3778–3791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koskinioti, P.; Ras, E.; Augustinos, A.A.; Tsiamis, G.; Beukeboom, L.W.; Caceres, C.; Bourtzis, K. The effects of geographic origin and antibiotic treatment on the gut symbiotic communities of Bactrocera oleae populations. Èntomol. Exp. et Appl. 2019, 167, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Scalzo, R.L.; Scarpati, M.L.; Verzegnassi, B.; Vita, G. Olea europaea chemicals repellent toDacus oleae females. J. Chem. Ecol. 1994, 20, 1813–1823. [Google Scholar] [CrossRef]
- Malheiro, R.; Ortiz, A.; Casal, S.; Baptista, P.; Pereira, J.A. Electrophysiological response of Bactrocera oleae (Rossi) (Diptera: Tephritidae) adults to olive leaves essential oils from different cultivars and olive tree volatiles. Ind. Crop. Prod. 2015, 77, 81–88. [Google Scholar] [CrossRef]
- Scarpati, M.L.; Scalzo, R.L.; Vita, G. Olea europaea Volatiles attractive and repellent to the olive fruit fly (Dacus oleae, Gmelin). J. Chem. Ecol. 1993, 19, 881–891. [Google Scholar] [CrossRef]
- Scarpati, M.L.; Scalzo, R.L.; Vita, G.; Gambacorta, A. Chemotropic behavior of female olive fly (Bactrocera oleae, GMEL.) on Olea eruopaea L. J. Chem. Ecol. 1996, 22, 1027–1036. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Salvatore, G. Behavioral and electrophysiological responses to overlooked female pheromone components in the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae ). Chemoecology 2015, 25, 147–157. [Google Scholar] [CrossRef]
- Carpita, A.; Canale, A.; Raffaelli, A.; Saba, A.; Benelli, G.; Raspi, A. (Z)-9-tricosene identified in rectal gland extracts of Bactrocera oleae males: First evidence of a male-produced female attractant in olive fruit fly. Naturwissenschaften 2011, 99, 77–81. [Google Scholar] [CrossRef]
- Liscia, A.; Angioni, P.; Sacchetti, P.; Poddighe, S.; Granchietti, A.; Setzu, M.; Belcari, A. Characterization of olfactory sensilla of the olive fly: Behavioral and electrophysiological responses to volatile organic compounds from the host plant and bacterial filtrate. J. Insect Physiol. 2013, 59, 705–716. [Google Scholar] [CrossRef]
- Vitanović, E.; Aldrich, J.R.; Boundy-Mills, K.; Čagalj, M.; E Ebeler, S.; Burrack, H.; Zalom, F.G. Olive Fruit Fly, Bactrocera oleae (Diptera: Tephritidae), Attraction to Volatile Compounds Produced by Host and Insect-Associated Yeast Strains. J. Econ. Èntomol. 2019, 113, 752–759. [Google Scholar] [CrossRef]
- Lopez-D, F.; Steiner, L.F.; Holbrook, F.R. A New Yeast Hydrolysate-Borax Bait for Trapping the Caribbean Fruit Fly. J. Econ. Èntomol. 1971, 64, 1541–1543. [Google Scholar] [CrossRef]
- Bortoli, L.C.; Machota, R.; Garcia, F.R.M.; Botton, M. Evaluation of Food Lures for Fruit Flies (Diptera: Tephritidae) Captured in a Citrus Orchard of the Serra Gaúcha. Fla. Èntomol. 2016, 99, 381–384. [Google Scholar] [CrossRef] [Green Version]
- Burrack, H.; Connell, J.H.; Zalom, F.G. Comparison of olive fruit fly (Bactrocera oleae (Gmelin)) (Diptera: Tephritidae) captures in several commercial traps in California. Int. J. Pest Manag. 2008, 54, 227–234. [Google Scholar] [CrossRef]
- Vitanović, E.; Aldrich, J.R.; Winterton, S.L.; Boundy-Mills, K.; Lopez, J.M.; Zalom, F.G. Attraction of the Green Lacewing Chrysoperla comanche (Neuroptera: Chrysopidae) to Yeast. J. Chem. Ecol. 2019, 45, 388–391. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Selby, T.; Lin, T.; Zhu, S.; Boundy-Mills, K.L. Carbon source utilization and inhibitor tolerance of 45 oleaginous yeast species. J. Ind. Microbiol. Biotechnol. 2014, 41, 1061–1070. [Google Scholar] [CrossRef] [Green Version]
- Sitepu, I.R.; Sestric, R.; Ignatia, L.; Levin, D.; German, J.B.; Gillies, L.A.; Almada, L.A.; Boundy-Mills, K.L. Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour. Technol. 2013, 144, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Kendall, M.; Stuart, A. The advanced theory of statistics: Inference and relationship, 4th ed.; Charles Griffin: London, UK, 1979; Volume 2, pp. 1–723. [Google Scholar]
- Rhoades, H.M.; Overall, J.E. A sample size correction for Pearson chi-square in 2 × 2 contingency tables. Psychol. Bull. 1982, 91, 418–423. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Rice, W.R. Analyzing tables of statistical tests. Am. Sociol. Rev. 1989, 78, 1–3. [Google Scholar] [CrossRef]
- Mangan, R.L.; Thomas, D.B. Comparison of torula yeast and various grape juice products as attractants for Mexican fruit fly (Diptera: Tephritidae). J. Econ. Èntomol. 2014, 107, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Flick, G.; Rozpędowska, E.; Schmidt, A.; Hagman, A.; Lebreton, S.; Larsson, M.C.; Hansson, B.S.; Piškur, J.; Witzgall, P.; et al. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct. Ecol. 2012, 26, 822–828. [Google Scholar] [CrossRef]
- Ganter, P. Yeast and invertebrate associations. In The Yeast Handbook: Biodiversity and Ecophysiology of Yeasts; Peter, G., Rosa, C., Eds.; Springer: Berlin, Germany, 2006; pp. 303–369. [Google Scholar]
- Hamby, K.A.; Hernández, A.; Boundy-Mills, K.L.; Zalom, F.G. Associations of Yeasts with Spotted-Wing Drosophila (Drosophila suzukii; Diptera: Drosophilidae) in Cherries and Raspberries. Appl. Environ. Microbiol. 2012, 78, 4869–4873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janson, E.M.; Stireman, J.O.; Singer, M.S.; Abbot, P. Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution 2008, 62, 997–1012. [Google Scholar] [CrossRef] [Green Version]
- Phaff, H.J.; Starmer, W.T. Yeasts associated with plants, insects, and soil. In The Yeasts, 2nd ed.; Rose, A.H., Harrison, J.S., Eds.; Academic Press: Cambridge, MA, USA, 1987; pp. 123–180. [Google Scholar]
- Brysch-Herzberg, M. Ecology of yeasts in plant-bumblebee mutualism in Central Europe. FEMS Microbiol. Ecol. 2004, 50, 87–100. [Google Scholar] [CrossRef]
- Davis, T.S.; Boundy-Mills, K.; Landolt, P.J. Volatile Emissions from an Epiphytic Fungus are Semiochemicals for Eusocial Wasps. Microb. Ecol. 2012, 64, 1056–1063. [Google Scholar] [CrossRef]
- Hagen, K.S.; Tassan, R.L.; Sawall, E.F., Jr. Some ecophysiological relationships between certain Chrysopa, honeydews and yeasts. Boll. Lab. Entomol. Agrar. Portici. 1970, 28, 113–134. [Google Scholar]
- Gibson, C.M.; Hunter, M.S. Reconsideration of the role of yeasts associated with Chrysoperla green lacewings. Boil. Control. 2005, 32, 57–64. [Google Scholar] [CrossRef]
- Daanel, K.M.; Yokota, G.Y. Release Strategies Affect Survival and Distribution of Green Lacewings (Neuroptera: Chrysopidae) in Augmentation Programs. Environ. Èntomol. 1997, 26, 455–464. [Google Scholar] [CrossRef]

| Yeast Strain and UCDFST ID Number | Geographic Source | Habitat Source |
|---|---|---|
| Kuraishia capsulata UCDFST 68-897.1 | British Columbia, Canada | Beetle frass in Pinus contorta |
| Peterozyma xylosa UCDFST 52-162 | California, USA | Drosophila miranda |
| Ogataea pini UCDFST 52-128 | California, USA | Frass of bark beetle Ips oregoni in Pinus contorta |
| Solicoccozyma terrea UCDFST 05-757 | California, USA | Bactrocera oleae (olive fruit fly) adult male |
| Nakazawaea ernobii UCDFST 51-28 | Unknown | Caeca of larva of Ernobius mollis beetle |
| Lachancea thermotolerans UCDFST 04-833 | California, USA | Bactrocera oleae (olive fruit fly) adult male |
| Vishniacozyma cf. foliicola UCDFST 05-508 | California, USA | Bactrocera oleae (olive fruit fly) adult female |
| Scheffersomyces ergatensis UCDFST 80-53 | Spain | Ergastes faber beetle |
| Filobasidium oeirensis UCDFST 05-501 | California, USA | Bactrocera oleae (olive fruit fly) adult male |
| Wickerhamomyces subpelliculosus UCDFST 76-85 | USA | Fermenting cucumbers |
| Jianyunia aff. sakaguchii UCDFST 73-1015 | California, USA | Bark beetle frass in Abies magnifica |
| Cyberlindnera jadinii (syn. Candida utilis, Torula yeast) UCDFST 75-33 | USA | Torula yeast cultivation tank |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitanović, E.; Lopez, J.M.; Aldrich, J.R.; Jukić Špika, M.; Boundy-Mills, K.; Zalom, F.G. Yeasts Associated with the Olive Fruit Fly Bactrocera oleae (Rossi) (Diptera: Tephritidae) Lead to New Attractants. Agronomy 2020, 10, 1501. https://doi.org/10.3390/agronomy10101501
Vitanović E, Lopez JM, Aldrich JR, Jukić Špika M, Boundy-Mills K, Zalom FG. Yeasts Associated with the Olive Fruit Fly Bactrocera oleae (Rossi) (Diptera: Tephritidae) Lead to New Attractants. Agronomy. 2020; 10(10):1501. https://doi.org/10.3390/agronomy10101501
Chicago/Turabian StyleVitanović, Elda, Julian M. Lopez, Jeffrey R. Aldrich, Maja Jukić Špika, Kyria Boundy-Mills, and Frank G. Zalom. 2020. "Yeasts Associated with the Olive Fruit Fly Bactrocera oleae (Rossi) (Diptera: Tephritidae) Lead to New Attractants" Agronomy 10, no. 10: 1501. https://doi.org/10.3390/agronomy10101501
APA StyleVitanović, E., Lopez, J. M., Aldrich, J. R., Jukić Špika, M., Boundy-Mills, K., & Zalom, F. G. (2020). Yeasts Associated with the Olive Fruit Fly Bactrocera oleae (Rossi) (Diptera: Tephritidae) Lead to New Attractants. Agronomy, 10(10), 1501. https://doi.org/10.3390/agronomy10101501






