Mutualistic Fungal Endophyte Colletotrichum tofieldiae Ct0861 Colonizes and Increases Growth and Yield of Maize and Tomato Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Inoculum
2.2. In vitro Experiments
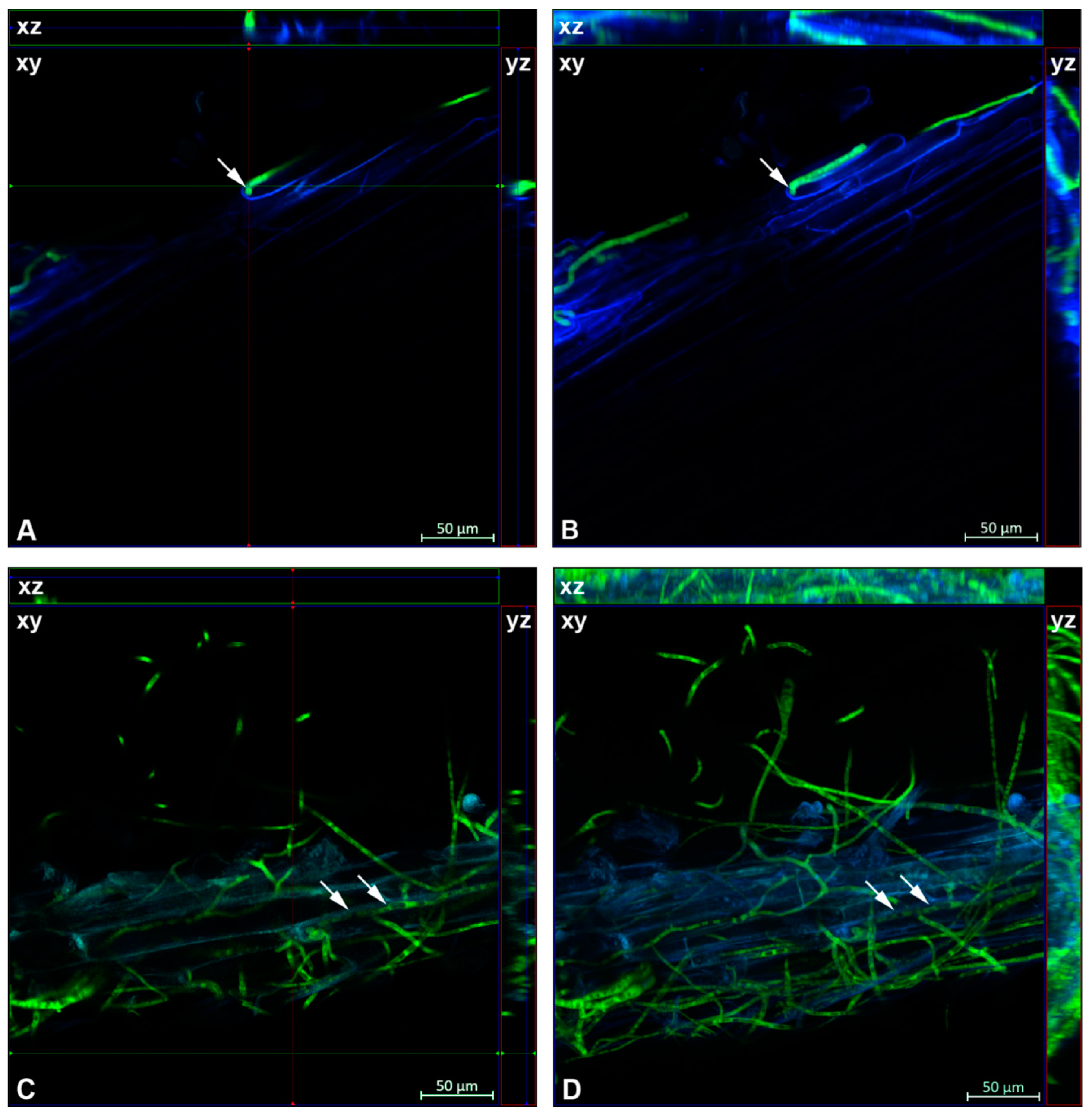
2.3. Confocal Microscopy
2.4. Maize Field Trial
2.4.1. Site Description, Irrigation, and Fertilization
2.4.2. Plant Material and Inoculation
2.4.3. Trial Design
2.4.4. Measurements
2.5. Tomato Greenhouse Trial
2.5.1. Site Description, Irrigation, and Fertilization
2.5.2. Plant Material and Inoculation
2.5.3. Trial Design
2.5.4. Measurements
2.6. Detection of Fungal Colonization
2.7. Statistical Analyses
3. Results
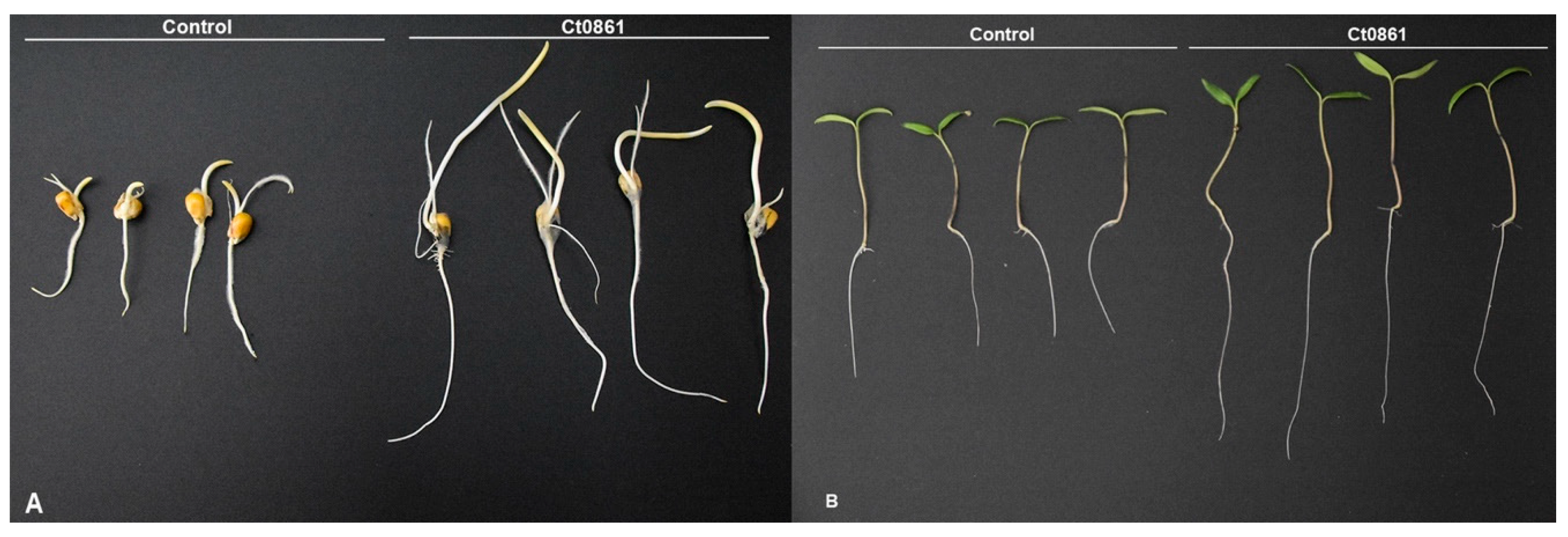
3.1. Ct0861 Colonizes Maize and Tomato Plants and Increases Maize and Tomato Seedlings Growth In Vitro
3.2. Ct0861 Increases Maize and Tomato Growth and Yield in Grower-Close Conditions
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, D.; Ansari, M.; Sahoo, R.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howden, S.M.; Soussana, J.F.; Tubiello, F.N.; Chhetri, N.; Dunlop, M.; Meinke, H. Adapting agriculture to climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19691–19696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingali, P.L. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Organic Materials Review Institute OMRI. Products List. Available online: https://www.omri.org/sites/default/files/opl_pdf/CropByProduct-NOP.pdf (accessed on 25 October 2019).
- Le Cocq, K.; Gurr, S.J.; Hirsch, P.R.; Mauchline, T.H. Exploitation of endophytes for sustainable agricultural intensification. Mol. Plant Pathol. 2017, 18, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Sessitsch, A.; Mitter, B. 21st century agriculture: Integration of plant microbiomes for improved crop production and food security. Microb. Biotechnol. 2015, 8, 32–33. [Google Scholar] [CrossRef]
- Sergaki, C.; Lagunas, B.; Lidbury, I.; Gifford, M.L.; Schäfer, P. Challenges and approaches in microbiome research: From fundamental to applied. Front. Plant Sci. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Bender, S.F.; Schlaeppi, K.; Held, A.; Van der Heijden, M.G.A. Establishment success and crop growth effects of an arbuscular mycorrhizal fungus inoculated into Swiss corn fields. Agric. Ecosyst. Environ. 2019, 273, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.G.; Griffin, E.A. The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: How far have we come and where do we go from here? Environ. Microbiol. 2020, 22, 2107–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugtenberg, B.J.J.; Caradus, J.R.; Johnson, L.J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ashraf, N.; Mohiuddin, T.; Riyaz-Ul-Hassan, S. Plant-endophyte symbiosis, an ecological perspective. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Gopalan, V.; Shaanker, R.U.; Sengupta, A.; Ravikanth, G. Translating endophyte research to applications: Prospects and challenges. In Diversity and Benefits of Microorganisms from the Tropics; de Azevedo, J., Quecine, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 343–365. ISBN 9783319558042. [Google Scholar]
- Griffin, E.A.; Carson, W.P. Tree endophytes: Cryptic drivers of tropical forest diversity. In Endophytes of Forest Trees; Pirttilä, A., Frank, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 63–103. ISBN 9783319898339. [Google Scholar]
- García, E.; Alonso, Á.; Platas, G.; Sacristán, S. The endophytic mycobiota of Arabidopsis thaliana. Fungal Divers. 2013, 60, 71–89. [Google Scholar] [CrossRef]
- Hiruma, K.; Gerlach, N.; Sacristán, S.; Nakano, R.T.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramírez, D.; Bucher, M.; O’Connell, R.J.; et al. Root endophyte colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Hacquard, S.; Kracher, B.; Hiruma, K.; Münch, P.C.; Garrido-Oter, R.; Thon, M.R.; Weimann, A.; Damm, U.; Dallery, J.F.; Hainaut, M.; et al. Survival trade-offs in plant roots during colonization by closely related beneficial and pathogenic fungi. Nat. Commun. 2016, 7, 11362. [Google Scholar] [CrossRef] [Green Version]
- Damm, U.; Woudenberg, J.H.C.; Cannon, P.F.; Crous, P.W. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 2009, 39, 45–87. [Google Scholar]
- Tao, G.; Liu, Z.Y.; Liu, F.; Gao, Y.H.; Cai, L. Endophytic Colletotrichum species from Bletilla ochracea (Orchidaceae), with descriptions of seven new speices. Fungal Divers. 2013, 61, 139–164. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Damm, U.; Cai, L.; Liu, M.; Li, X.H.; Zhang, W.; Zhao, W.S.; Yan, J.Y. Notes on currently accepted species of Colletotrichum. Mycosphere 2016, 7, 1192–1260. [Google Scholar] [CrossRef]
- Shivas, R.G.; Tan, Y.P.; Edwards, J.; Dinh, Q.; Maxwell, A.; Andjic, V.; Liberato, J.R.; Anderson, C.; Beasley, D.R.; Bransgrove, K.; et al. Colletotrichum species in Australia. Australas. Plant Pathol. 2016, 45, 447–464. [Google Scholar] [CrossRef]
- FAOSTAT Database. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 25 October 2019).
- Ministerio de Agricultura, Pesca y Alimentación. Superficies y Producciones Anuales de Cultivos. Datos Avances de Cereales. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 25 October 2019).
- Roca, A.; Pizarro-Tobías, P.; Udaondo, Z.; Fernández, M.; Matilla, M.A.; Molina-Henares, M.A.; Molina, L.; Segura, A.; Duque, E.; Ramos, J.-L. Analysis of the plant growth-promoting properties encoded by the genome of the rhizobacterium Pseudomonas putida BIRD-1. Environ. Microbiol. 2013, 15, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Agencia Estatal de Meteorología (Ministerio de Medio Ambiente y Medio Rural y Marino de España) and Instituto de Meteorologia de Portugal. Iberian Climate Atlas. Available online: http://www.aemet.es/documentos/es/conocermas/recursos_en_linea/publicaciones_y_estudios/publicaciones/Atlas-climatologico/Atlas.pdf (accessed on 25 October 2019).
- Agencia Estatal de Meteorología (Ministerio de Medio Ambiente y Medio Rural y Marino). Servicios Climáticos: Datos Climatológicos Normales de la Estación de San Javier Aeropuerto (Murcia). Available online: http://www.aemet.es/es/serviciosclimaticos/datosclimatologicos/valoresclimatologicos?l=7031&k=undefined (accessed on 25 October 2019).
- Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario (IMIDA). Data from: Sistema de Información Agrometeorológica de la Región de Murcia (SIAM). Available online: http://siam.imida.es/apex/f?p=101:46:1821222586473642 (accessed on 20 August 2004).
- Limagrain Ibérica. La densidad óptima. In Maíz y Girasol, Catálogo 2018; Limagrain Ibérica: Navarra, Spain, 2018; pp. 34–35. [Google Scholar]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, K.D.; Cai, L.; Cannon, P.F.; Crouch, J.A.; Crous, P.W.; Damm, U.; Goodwin, P.H.; Chen, H.; Johnston, P.R.; Jones, E.; et al. Colletotrichum—Names in current use. Fungal Divers. 2009, 39, 147–182. [Google Scholar]
- Lu, G.; Cannon, P.F.; Reid, A.; Simmons, C.M. Diversity and molecular relationships of endophytic Colletotrichum isolates from the Iwokrama Forest Reserve, Guyana. Mycol. Res. 2004. [Google Scholar] [CrossRef]
- Götz, M.; Nirenberg, H.; Krause, S.; Wolters, H.; Draeger, S.; Buchner, A.; Lottmann, J.; Berg, G.; Smalla, K. Fungal endophytes in potato roots studied by traditional isolation and cultivation-independent DNA-based methods. FEMS Microbiol. Ecol. 2006. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, 163–173. [Google Scholar]
- Rakotoniriana, E.F.; Munaut, F.; Decock, C.; Randriamampionona, D.; Andriambololoniaina, M.; Rakotomalala, T.; Rakotonirina, E.J.; Rabemanantsoa, C.; Cheuk, K.; Ratsimamanga, S.U.; et al. Endophytic fungi from leaves of Centella asiatica: Occurrence and potential interactions within leaves. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2008. [Google Scholar] [CrossRef]
- Rojas, E.I.; Rehner, S.A.; Samuels, G.J.; Van Bael, S.A.; Herre, E.A.; Cannon, P.; Chen, R.; Pang, J.; Wang, R.; Zhang, Y.; et al. Colletotrichum gloeosporioides s.l. associated with Theobroma cacao and other plants in Panamá: Multilocus phylogenies distinguish host-associated pathogens from asymptomatic endophytes. Mycologia 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzaga, L.L.; Costa, L.E.O.; Santos, T.T.; Araújo, E.F.; Queiroz, M.V. Endophytic fungi from the genus Colletotrichum are abundant in the Phaseolus vulgaris and have high genetic diversity. J. Appl. Microbiol. 2015, 118, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miransari, M.; Bahrami, H.A.; Rejali, F.; Malakouti, M.J.; Torabi, H. Using arbuscular mycorrhiza to reduce the stressful effects of soil compaction on corn (Zea mays L.) growth. Soil Biol. Biochem. 2007, 39, 2014–2026. [Google Scholar] [CrossRef]
- Mejía, L.C.; Herre, E.A.; Sparks, J.P.; Winter, K.; García, M.N.; Van Bael, S.A.; Stitt, J.; Shi, Z.; Zhang, Y.; Guiltinan, M.J.; et al. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front. Microbiol. 2014, 5, 479. [Google Scholar] [CrossRef] [PubMed]
- Redman, R.S.; Dunigan, D.D.; Rodriguez, R.J. Fungal symbiosis from mutualism to parasitism: Who controls the outcome, host or invader? New Phytol. 2001, 151, 705–716. [Google Scholar] [CrossRef] [Green Version]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef] [Green Version]
- Kogel, K.-H.; Franken, P.; Hückelhoven, R. Endophyte or parasite—What decides? Curr. Opin. Plant Biol. 2006, 9, 358–363. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.S.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.M.; Chandra Nayak, S. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef] [Green Version]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.-J.; Sessitsch, A. Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Annu. Rev. Phytopathol. 2017. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Murata, H.; Nakano, S.; Yamanaka, T.; Shimokawa, T.; Abe, T.; Ichida, H.; Hayashi, Y.; Tahara, K.; Ohta, A. Conversion from mutualism to parasitism: A mutant of the ectomycorrhizal agaricomycete Tricholoma matsutake that induces stunting, wilting, and root degeneration in seedlings of its symbiotic partner, Pinus densiflora, in vitro. Botany 2019. [Google Scholar] [CrossRef] [Green Version]
- Kohler, A.; Kuo, A.; Nagy, L.G.; Morin, E.; Barry, K.W.; Buscot, F.; Canbäck, B.; Choi, C.; Cichocki, N.; Clum, A.; et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat. Genet. 2015. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-H.; Su, Z.-Z.; Wang, C.; Kubicek, C.P.; Feng, X.-X.; Mao, L.-J.; Wang, J.-Y.; Chen, C.; Lin, F.-C.; Zhang, C.-L. The rice endophyte Harpophora oryzae genome reveals evolution from a pathogen to a mutualistic endophyte. Sci. Rep. 2015, 4, 5783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashmi, M.; Kushveer, J.S.; Sarma, V.V. A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 2019, 10, 798–1079. [Google Scholar] [CrossRef]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium verticillioides: Managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Šišić, A.; Baćanović-Šišić, J.; Al-Hatmi, A.M.S.; Karlovsky, P.; Ahmed, S.A.; Maier, W.; De Hoog, G.S.; Finckh, M.R. The “forma specialis” issue in Fusarium: A case study in Fusarium solani f. sp. pisi. Sci. Rep. 2018. [Google Scholar] [CrossRef]
- Skiada, V.; Faccio, A.; Kavroulakis, N.; Genre, A.; Bonfante, P.; Papadopoulou, K.K. Colonization of legumes by an endophytic Fusarium solani strain FsK reveals common features to symbionts or pathogens. Fungal Genet. Biol. 2019. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. Dissecting endophytic lifestyle along the parasitism/mutualism continuum in Arabidopsis. Curr. Opin. Microbiol. 2016, 32, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Nzanza, B.; Marais, D.; Soundy, P. Yield and nutrient content of tomato (Solanum lycopersicum L.) as influenced by Trichoderma harzianum and Glomus mosseae inoculation. Sci. Hortic. 2012. [Google Scholar] [CrossRef] [Green Version]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crop. Res. 2012. [Google Scholar] [CrossRef]
- Berta, G.; Copetta, A.; Gamalero, E.; Bona, E.; Cesaro, P.; Scarafoni, A.; D’Agostino, G. Maize development and grain quality are differentially affected by mycorrhizal fungi and a growth-promoting pseudomonad in the field. Mycorrhiza 2014, 24, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; Pascale, S.; De Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Polcyn, W.; Paluch-Lubawa, E.; Lehmann, T.; Mikuła, R. Arbuscular mycorrhiza in highly fertilized maize cultures alleviates short-term drought effects but does not improve fodder yield and quality. Front. Plant Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Weiss, M.; Kogel, K.H.; Schäfer, P. Piriformospora indica-a mutualistic basidiomycete with an exceptionally large plant host range. Mol. Plant Pathol. 2012. [Google Scholar] [CrossRef]
- Varma, A.; Savita, V.; Sahay, N.; Butehorn, B.; Franken, P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 1999, 65, 2741–2744. [Google Scholar] [CrossRef] [Green Version]
- Fakhro, A.; Andrade-Linares, D.R.; von Bargen, S.; Bandte, M.; Büttner, C.; Grosch, R.; Schwarz, D.; Franken, P. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 2010, 20, 191–200. [Google Scholar] [CrossRef]
- Sarma, M.V.R.K.; Kumar, V.; Saharan, K.; Srivastava, R.; Sharma, A.K.; Prakash, A.; Sahai, V.; Bisaria, V.S. Application of inorganic carrier-based formulations of fluorescent pseudomonads and Piriformospora indica on tomato plants and evaluation of their efficacy. J. Appl. Microbiol. 2011, 111, 456–466. [Google Scholar] [CrossRef]
- Andrade-Linares, D.R.; Müller, A.; Fakhro, A.; Schwarz, D.; Franken, P. Impact of Piriformospora indica on Tomato. In Piriformospora indica; Varma, A., Kost, G., Oelmüller, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 107–117. [Google Scholar]
- Andrade-Linares, D.R.; Grosch, R.; Franken, P.; Rexer, K.-H.; Kost, G.; Restrepo, S.; de Garcia, M.C.C.; Maximova, E. Colonization of roots of cultivated Solanum lycopersicum by dark septate and other ascomycetous endophytes. Mycologia 2011, 103, 710–721. [Google Scholar] [CrossRef]
- Xia, Y.; Sahib, M.R.; Amna, A.; Opiyo, S.O.; Zhao, Z.; Gao, Y.G. Culturable endophytic fungal communities associated with plants in organic and conventional farming systems and their effects on plant growth. Sci. Rep. 2019. [Google Scholar] [CrossRef]
- Kabir, Z.; O’Halloran, I.P.; Fyles, J.W.; Hamel, C. Dynamics of the mycorrhizal symbiosis of corn (Zea mays L.): Effects of host physiology, tillage practice and fertilization on spatial distribution of extra-radical mycorrhizal hyphae in the field. Agric. Ecosyst. Environ. 1998. [Google Scholar] [CrossRef]
- Liu, A.; Hamel, C.; Hamilton, R.I.; Smith, D.L. Mycorrhizae formation and nutrient uptake of new corn (Zea mays L.) hybrids with extreme canopy and leaf architecture as influenced by soil N and P levels. Plant Soil 2000. [Google Scholar] [CrossRef]
- Pan, J.J.; Baumgarten, A.M.; May, G. Effects of host plant environment and Ustilago maydis infection on the fungal endophyte community of maize (Zea mays). New Phytol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.J.; de Armas, R.D.; Soares, C.R.F.S.; Ogliari, J.B. Communities of endophytic microorganisms in different developmental stages from a local variety as well as transgenic and conventional isogenic hybrids of maize. World J. Microbiol. Biotechnol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, C.; Baldauf, J.A.; Tai, H.; Gutjahr, C.; Hochholdinger, F.; Li, C. Root type and soil phosphate determine the taxonomic landscape of colonizing fungi and the transcriptome of field-grown maize roots. New Phytol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Brisson, V.L.; Schmidt, J.E.; Northen, T.R.; Vogel, J.P.; Gaudin, A.C.M. Impacts of maize domestication and breeding on rhizosphere microbial community recruitment from a nutrient depleted agricultural soil. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, J.G.; May, G. Endophytes: The other maize genome. In The Maize Genome; Bennetzen, J.L., Flint-Garcia, S., Hirsch, C.N., Tuberosa, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 213–246. [Google Scholar]
- Rozpądek, P.; Nosek, M.; Domka, A.; Ważny, R.; Jędrzejczyk, R.; Tokarz, K.; Pilarska, M.; Niewiadomska, E.; Turnau, K. Acclimation of the photosynthetic apparatus and alterations in sugar metabolism in response to inoculation with endophytic fungi. Plant. Cell Environ. 2019, 42, 1408–1423. [Google Scholar] [CrossRef]
- Vargas, W.A.; Mandawe, J.C.; Kenerley, C.M. Plant-derived sucrose is a key element in the symbiotic association between Trichoderma virens and maize plants. Plant Physiol. 2009. [Google Scholar] [CrossRef] [Green Version]
- Sirrenberg, A.; Göbel, C.; Grond, S.; Czempinski, N.; Ratzinger, A.; Karlovsky, P.; Santos, P.; Feussner, I.; Pawlowski, K. Piriformospora indica affects plant growth by auxin production. Physiol. Plant. 2007. [Google Scholar] [CrossRef]
- Barazani, O.; Von Dahl, C.C.; Baldwin, I.T. Sebacina vermifera promotes the growth and fitness of Nicotiana attenuata by inhibiting ethylene signaling. Plant Physiol. 2007. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, P.; Pfiffi, S.; Voll, L.M.; Zajic, D.; Chandler, P.M.; Waller, F.; Scholz, U.; Pons-Kühnemann, J.; Sonnewald, S.; Sonnewald, U.; et al. Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant J. 2009. [Google Scholar] [CrossRef]
- Lee, Y.C.; Johnson, J.M.; Chien, C.T.; Sun, C.; Cai, D.; Lou, B.; Oelmüller, R.; Yeh, K.W. Growth promotion of Chinese cabbage and Arabidopsis by Piriformospora indica is not stimulated by mycelium-synthesized auxin. Mol. Plant-Microbe Interact. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franken, P. The plant strengthening root endophyte Piriformospora indica: Potential application and the biology behind. Appl. Microbiol. Biotechnol. 2012, 96, 1455–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadassery, J.; Ranf, S.; Drzewiecki, C.; Mithöfer, A.; Mazars, C.; Scheel, D.; Lee, J.; Oelmüller, R. A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J. 2009. [Google Scholar] [CrossRef] [PubMed]
- Camehl, I.; Drzewiecki, C.; Vadassery, J.; Shahollari, B.; Sherameti, I.; Forzani, C.; Munnik, T.; Hirt, H.; Oelmüller, R. The OXI1 kinase pathway mediates piriformospora indica-induced growth promotion in arabidopsis. PLoS Pathog. 2011, 7, e1002051. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Torres, M.S. Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiol. Plant. 2010, 138, 440–446. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schäfer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, V.; Tuteja, N.; Johri, A.K. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology 2009, 155, 780–790. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Johnson, J.M.; Cai, D.; Sherameti, I.; Oelmüller, R.; Lou, B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J. Plant Physiol. 2010. [Google Scholar] [CrossRef]
- Xu, L.; Wang, A.; Wang, J.; Wei, Q.; Zhang, W. Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J. 2017, 5, 251–258. [Google Scholar] [CrossRef] [Green Version]

| Treatment Name 1 | Dose (Conidia·Seed−1) 2 | Dose (Conidia·ha−1) |
|---|---|---|
| M-Zm | - | - |
| CtST1-Zm | 2 × 102 | 2 × 107 |
| CtST2-Zm | 2 × 103 | 2 × 108 |
| CtST3-Zm | 2 × 104 | 2 × 109 |
| Treatment Name 1 | Dose (Conidia·Seed−1 or Plant−1) | Dose (Conidia·ha−1) | Application 2 |
|---|---|---|---|
| M-Sl | - | - | |
| CtST-Sl | 105 | 109 | Seed treatment |
| CtIR-Sl | 2 × 106 | 2 × 1010 | Irrigation |
| Treatments | Root Length (cm) | Root Weight (mg) | Shoot Length (cm) | Shoot Weight (mg) |
|---|---|---|---|---|
| Maize | ||||
| Control | 9.5 ± 5.3 a | 190.3 ± 121.5 a | 10.3 ± 5.3 a | 175.7 ± 122.0 a |
| Ct0861 | 10.5 ± 4.0 a | 198.1 ± 118.2 a | 16.1 ± 6.2 b | 246.5 ± 141.1 b |
| Tomato | ||||
| Control | 5.7 ± 2.2 a | 8.0 ± 2.1 a | 3.3 ± 1.3 a | 28.5 ± 14.5 a |
| Ct0861 | 7.3 ± 2.1 b | 8.2 ± 2.4 a | 4.2 ± 1.1 b | 40.7 ± 14.5 b |
| Treatments 1 | Stem Height (m) | Number of Cobs Per Plant | Mean Cob Weight Per Plant (g) | 1000 Kernels Weight (g) | Mean Yield·Plant−1 (g) | Yield (t·ha−1) |
|---|---|---|---|---|---|---|
| M-Zm | 2.47 ± 0.24 b | 1.25 ± 0.45 a | 198.2 ± 30.0 a | 381 ± 18 a | 169.0 ± 28.8 b | 12.5 ± 2.1 b |
| CtST1-Zm | 2.62 ± 0.26 a | - | 211.2 ± 18.4 a | 386 ± 11 a | 181.6 ± 15.0 ab | 13.4 ± 1.1 ab |
| CtST2-Zm | 2.70 ± 0.21 a | - | 224.4 ± 26.9 a | 388 ± 20 a | 191.7 ± 24.2 ab | 14.1 ± 1.8 ab |
| CtST3-Zm | 2.63 ± 0.28 a | 1.31 ± 0.50 a | 237.3 ± 30.4 a | 395 ± 11 a | 206.8 ± 23.3 a | 15.2 ± 1.7 a |
| Treatments 1 | Root Weight (g) | N. Buds·Plant−1 2 | N. Open Flowers·Plant−1 2 | Total Fruits Plant−1 | Mean Fruit Weight (g) | Yield· Plant−1 (kg) | Yield (t·ha−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M1 | M2 | ||||||
| M-Sl | 363.5 ± 11.2 a | 5.1 ± 1.6 a | 6.6 ± 1.5 a | 2.9 ± 1.1 a | 3.5 ± 1.3 a | 24.8 ± 1.6 a | 128.5 ± 2.1 a | 3.2 ± 0.2 a | 63.5 ± 2.1 a |
| CtST-Sl | 369.8 ± 8.1 b | 7.6 ± 2.4 b | 10.8 ± 3.4 b | 3.7 ± 1.3 b | 4.8 ± 1.5 b | 25.8 ± 1.6 b | 130.4 ± 5.1 b | 3.4 ± 0.3 b | 67 ± 2.1 b |
| CtIR-Sl | 372.0 ± 9.8 b | 8.0 ± 2.4 b | 10.7 ± 2.6 b | 3.5 ± 1.7 ab | 5.4 ± 1.7 b | 27.1 ± 2.1 c | 130.4 ± 2.1 b | 3.5 ± 0.3 c | 71.1 ± 1.8 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-González, S.; Marín, P.; Sánchez, R.; Arribas, C.; Kruse, J.; González-Melendi, P.; Brunner, F.; Sacristán, S. Mutualistic Fungal Endophyte Colletotrichum tofieldiae Ct0861 Colonizes and Increases Growth and Yield of Maize and Tomato Plants. Agronomy 2020, 10, 1493. https://doi.org/10.3390/agronomy10101493
Díaz-González S, Marín P, Sánchez R, Arribas C, Kruse J, González-Melendi P, Brunner F, Sacristán S. Mutualistic Fungal Endophyte Colletotrichum tofieldiae Ct0861 Colonizes and Increases Growth and Yield of Maize and Tomato Plants. Agronomy. 2020; 10(10):1493. https://doi.org/10.3390/agronomy10101493
Chicago/Turabian StyleDíaz-González, Sandra, Patricia Marín, Roberto Sánchez, Cristina Arribas, John Kruse, Pablo González-Melendi, Frédéric Brunner, and Soledad Sacristán. 2020. "Mutualistic Fungal Endophyte Colletotrichum tofieldiae Ct0861 Colonizes and Increases Growth and Yield of Maize and Tomato Plants" Agronomy 10, no. 10: 1493. https://doi.org/10.3390/agronomy10101493
APA StyleDíaz-González, S., Marín, P., Sánchez, R., Arribas, C., Kruse, J., González-Melendi, P., Brunner, F., & Sacristán, S. (2020). Mutualistic Fungal Endophyte Colletotrichum tofieldiae Ct0861 Colonizes and Increases Growth and Yield of Maize and Tomato Plants. Agronomy, 10(10), 1493. https://doi.org/10.3390/agronomy10101493






