Water and Soil Nutrient Dynamics of Huanglongbing-Affected Citrus Trees as Impacted by Ground-Applied Nutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Conditions, Experimental Design, and Irrigation
2.2. Leaf Area Index
2.3. Water Relations
2.3.1. Stomatal Conductance (gs)
2.3.2. Stem Water Potential (Ψw)
2.3.3. Sap Flow
2.4. Soil Nutrient Concentrations
2.5. Statistics and Data Analysis
3. Results and Discussion
3.1. Leaf Area Index
3.2. Meteorological Conditions
3.3. Water Relations
3.3.1. Stomatal Conductance
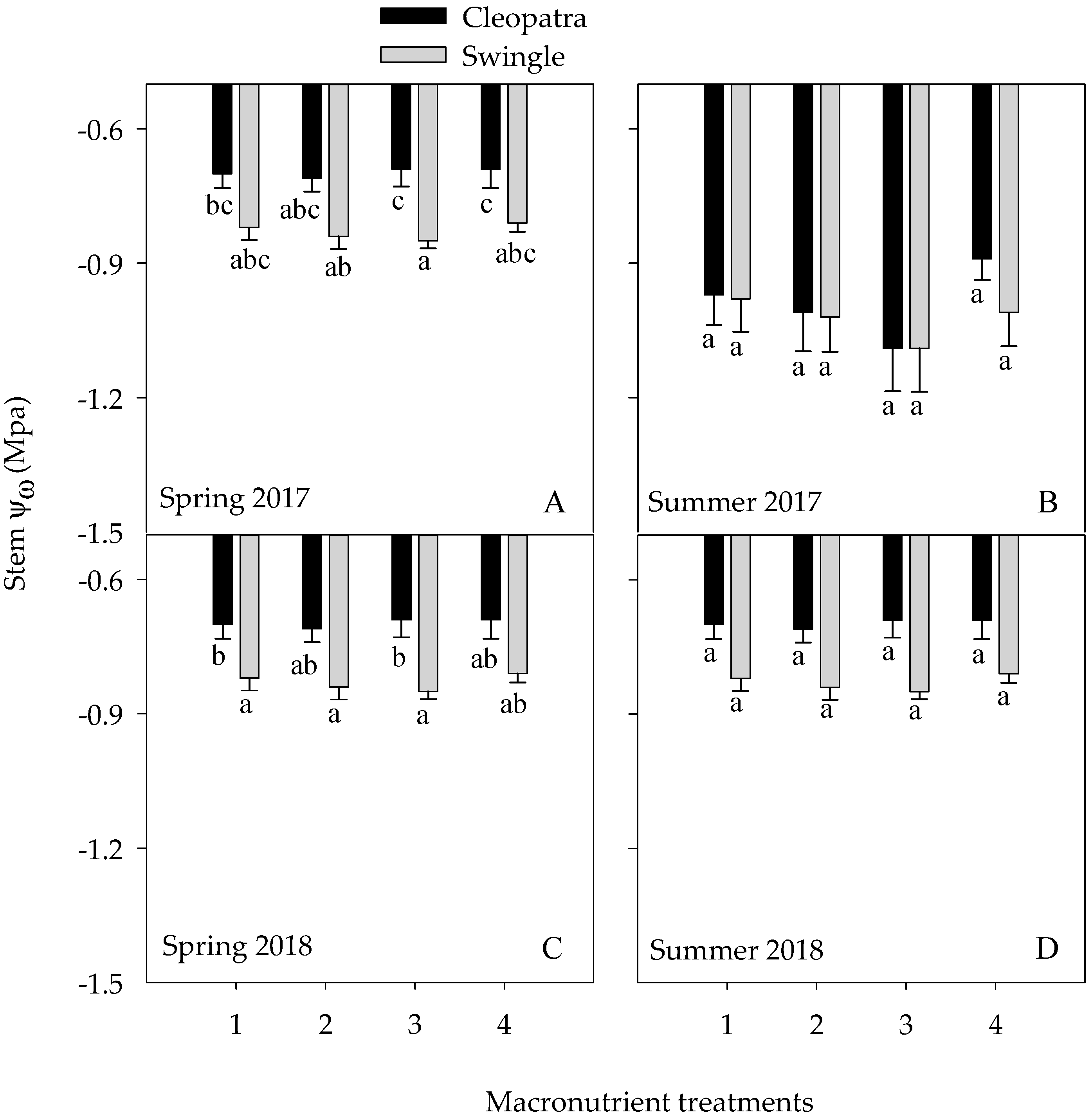
3.3.2. Stem Water Potential (Ψw)
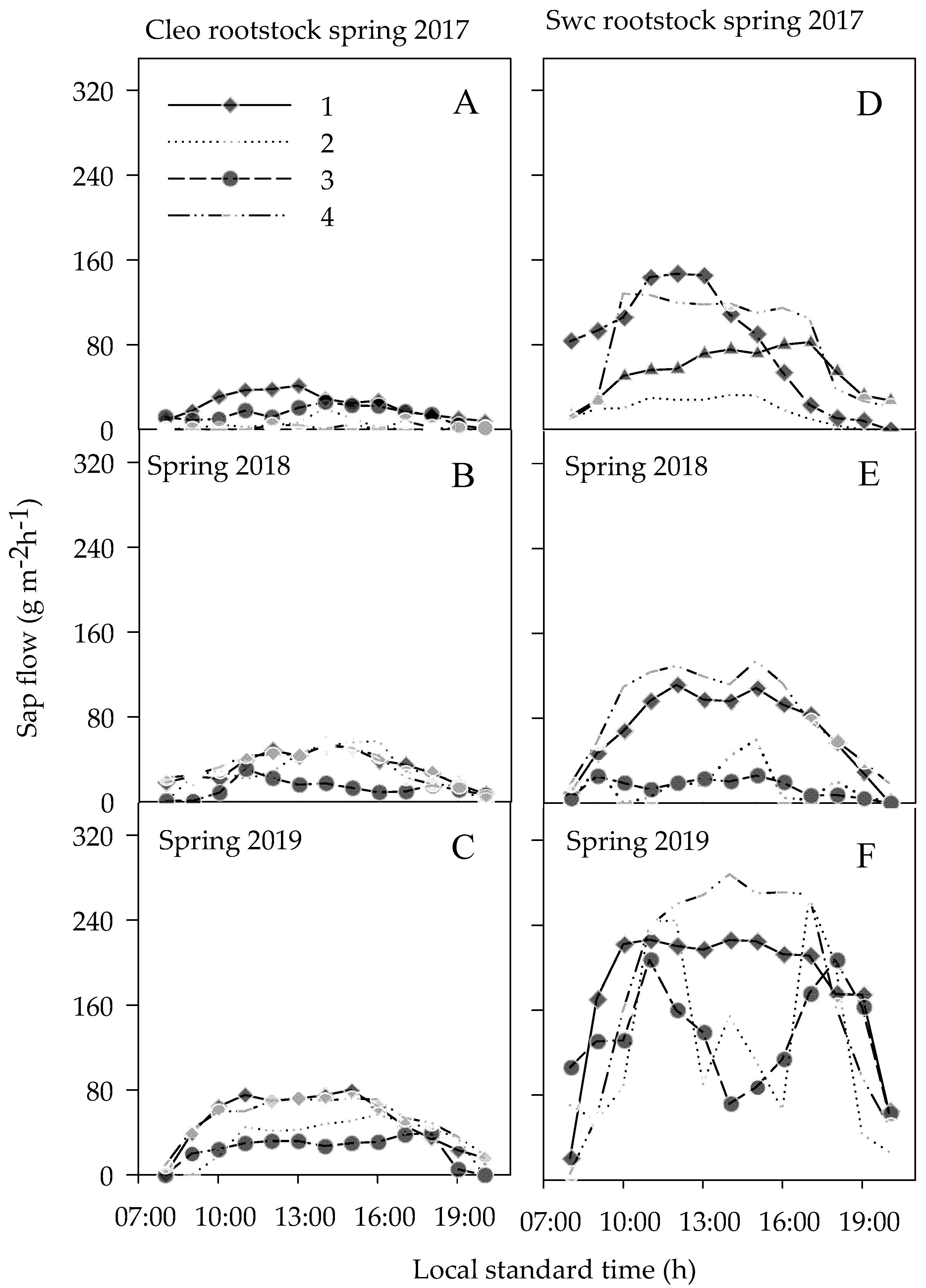
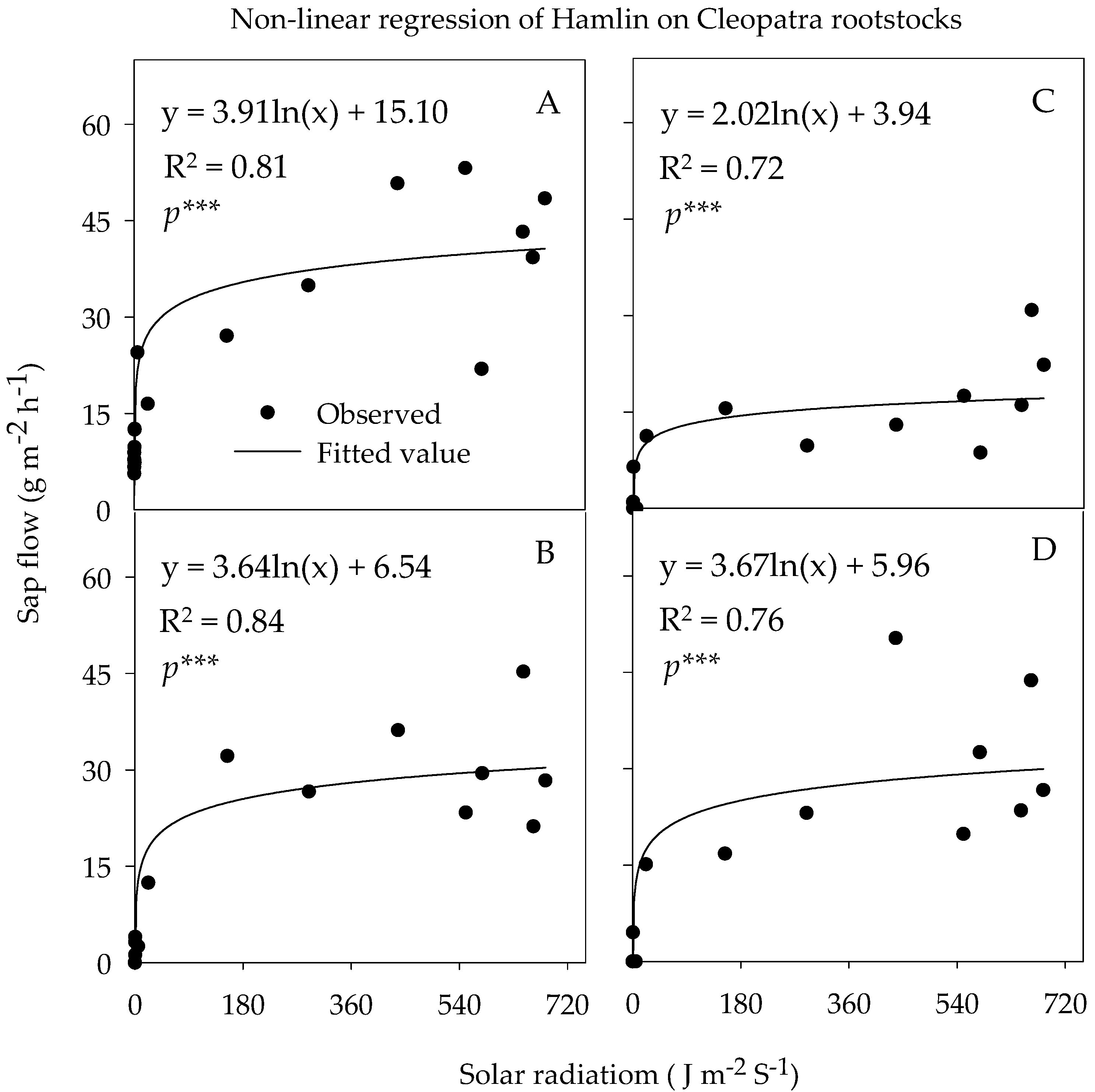
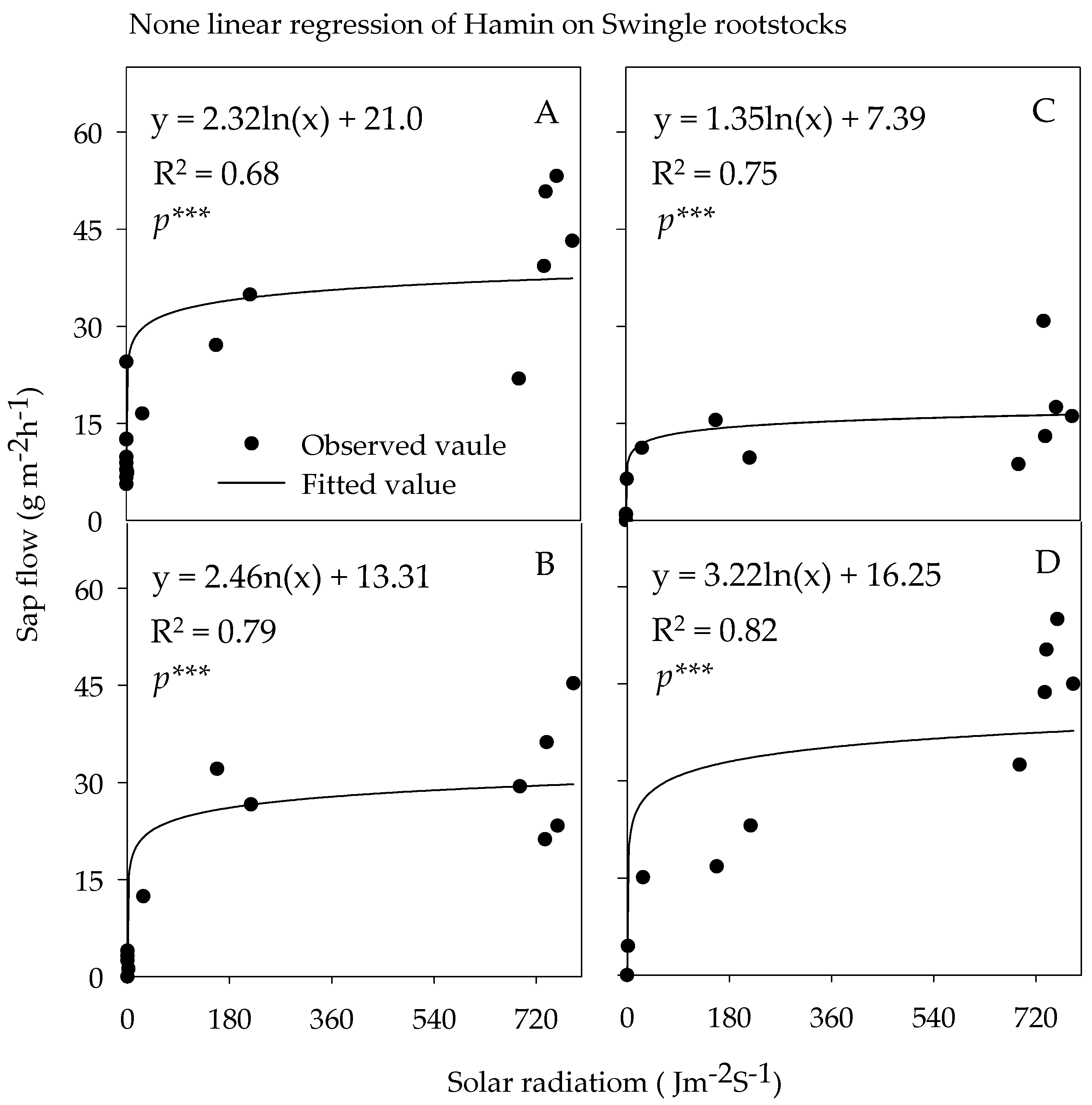
3.3.3. Sap Flow
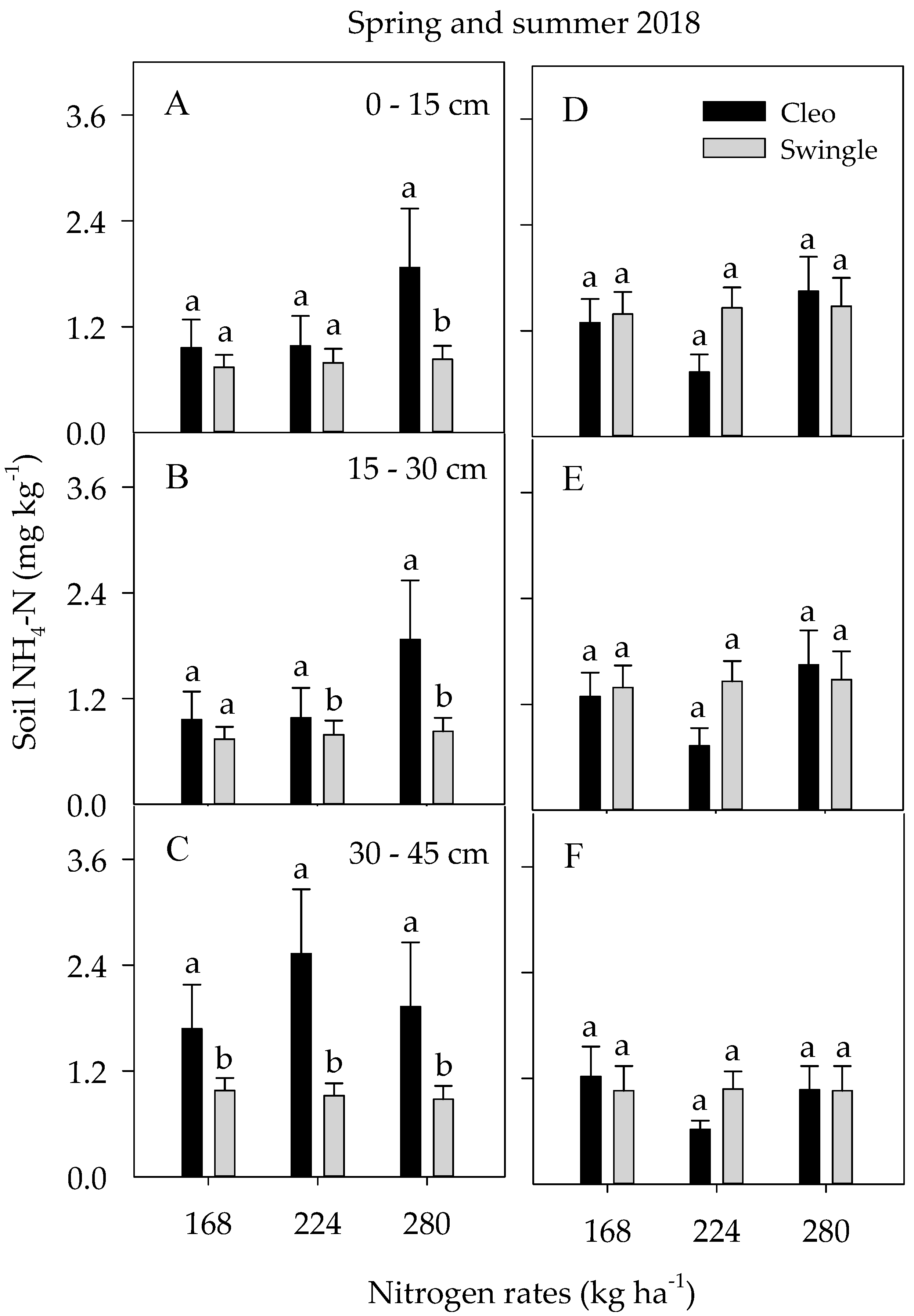
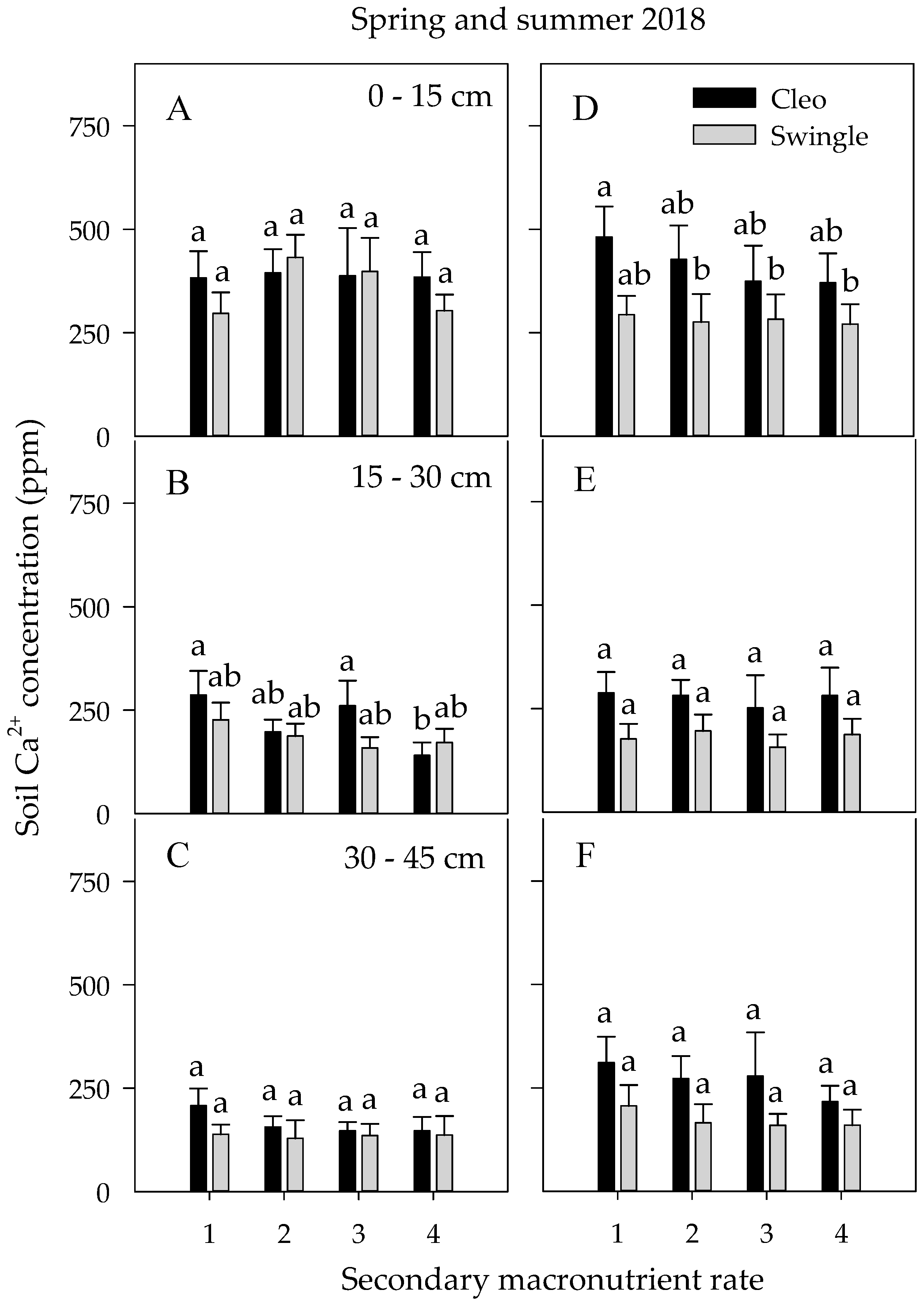
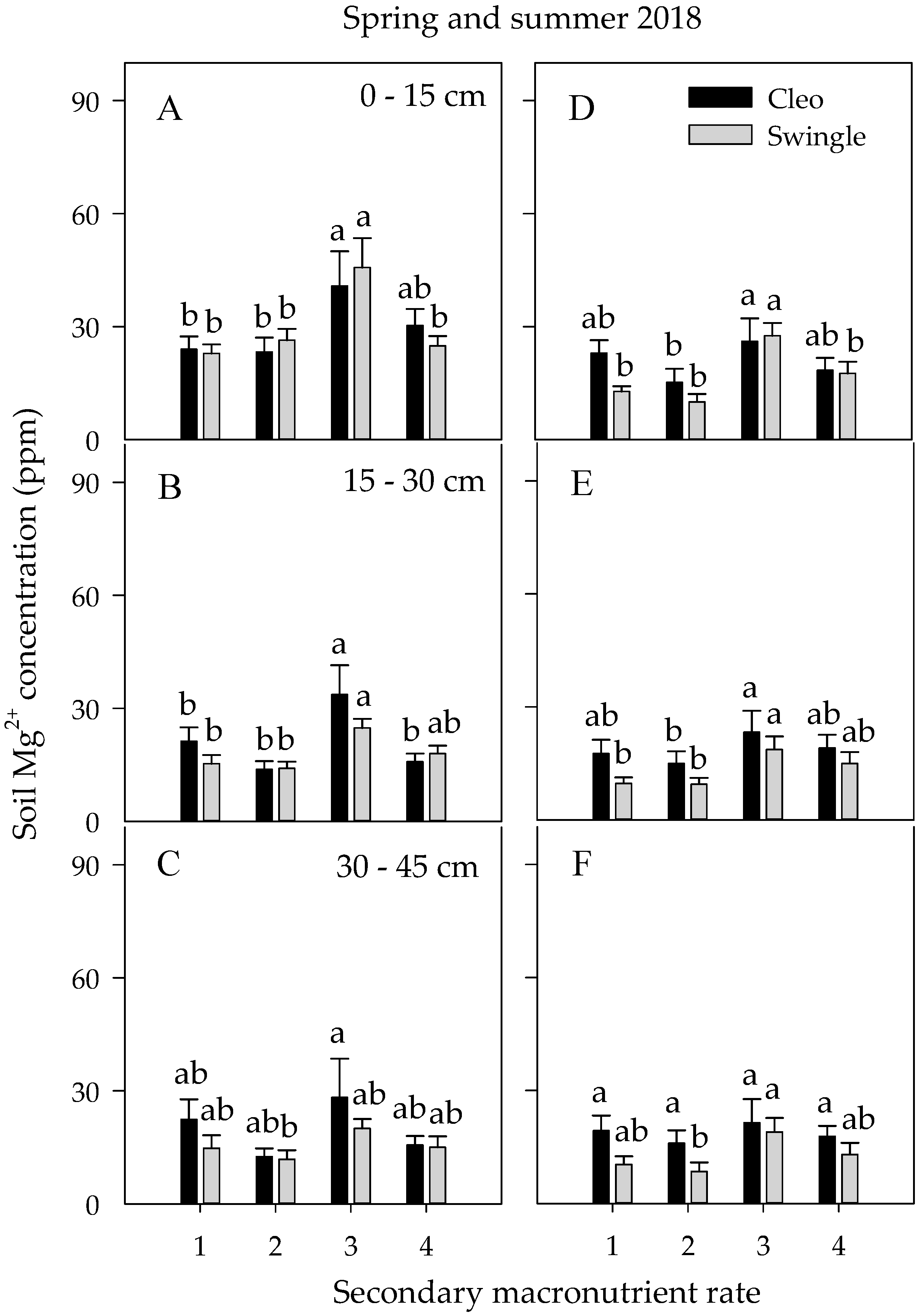
3.4. Soil Nutrient Concentrations and Dynamics
3.4.1. Soil Macronutrients
3.4.2. Secondary Macronutrients
3.4.3. Micronutrients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Citrus Fruits 2019 Summary; United States Department of Agriculture: Washington, DC, USA, 2019.
- Graham, J.H.; Johnson, E.G.; Gottwald, T.R.; Irey, M.S. Presymptomatic fibrous root decline in citrus trees caused by huanglongbing and potential interaction with Phytophthora spp. Plant. Dis. 2013, 97, 1195–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadyampa, K.; Davie, M.; Morgan, K.T.; SchuMann, A.W. Root density distribution and water uptake of citrus trees infected with huanglongbing. Proc. Fla. State Hort. Soc. 2014, 127, 37–41. [Google Scholar]
- Coleman, M. Spatial and temporal patterns of root distribution in developing stands of four woody crop species grown with drip irrigation and fertilization. Plant. Soil 2007, 299, 195–213. [Google Scholar] [CrossRef] [Green Version]
- Aubert, B. Huanglongbing (HLB) a Diagnosis and strategies for control in Reunion Island—A case study. IRCHLB Proc. 2008, 2008, 1–34. [Google Scholar]
- Nwugo, C.C.; Duan, Y.; Lin, H. Study on citrus response to huanglongbing highlights a down-regulation of defense-related proteins in lemon plants upon ‘ca. Liberibacter asiaticus’ infection. PLoS ONE 2013, 8, e67442. [Google Scholar] [CrossRef]
- Morgan, K.T.; Kadyampakemi, D. Open field hydroponics: Concept and application. Adv. Citrus Nutr. 2012, 271–280. [Google Scholar] [CrossRef]
- Paramasivam, S.; Alva, A.K.; Fares, A.; Sajwan, K.S. Estimation of nitrate leaching in an entisol under optimum citrus production. Soil Sci. Soc. Am. J. 2010, 65, 914. [Google Scholar] [CrossRef]
- Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M. Effect of essential nutrients on roots growth and lifespan of huanglongbing affected citrus trees. Plants 2020, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Hamido, S.A.; Ebel, R.C.; Morgan, K.T. Interaction of huanglongbing and foliar applications of copper on water relations of citrus sinensis cv. Valencia. Plants 2019, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P. Effect of irrigation pattern and timing on root density of young citrus trees infected with Huanglongbing disease. Horttechnology 2014, 24, 209–221. [Google Scholar] [CrossRef]
- Ebel, R.C.; Hamido, S.; Morgan, K.T. Interaction of huanglongbing and foliar applications of copper on growth and nutrient acquisition of citrus sinensis cv. Valencia. Plants 2019, 54, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Obreza, T.A.; Schumann, A. Keeping water and nutrients in the Florida citrus tree root zone. Horttechnology 2010, 20, 67–73. [Google Scholar] [CrossRef]
- Kiggundu, N.; Migliaccio, K.W.; Schaffer, B.; Li, Y.; Crane, J.H. Water savings, nutrient leaching, and fruit yield in a young avocado orchard as affected by irrigation and nutrient management. Irrig. Sci. 2012, 30, 275–286. [Google Scholar] [CrossRef]
- Quiñones, A.; Martínez-Alcántara, B.; Legaz, F. Influence of irrigation system and fertilization management on seasonal distribution of N in the soil profile and on N-uptake by citrus trees. Agric. Ecosyst. Environ. 2007, 122, 399–409. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Sax, S.M. Fertigation frequency, wetting patterns and nitrate leaching from lysimeter-grown citrus trees. Proc. Fla. State Hortic. Soc. 1999, 112, 9–13. [Google Scholar]
- Alva, A.K.; Paramasivam, S. Nitrogen management for high yield and quality of citrus in sandy soils. Soil Sci. Soc. Am. J. 1998, 62, 1335–1342. [Google Scholar] [CrossRef]
- Spann, T.M.; Schumann, A.W. The role of plant nutrients in disease development with emphasis on citrus and huanglongbing. Proc. Fla. State Hortic. Sci. 2009, 122, 169–171. [Google Scholar]
- Spann, T.M.; Atwood, R.A.; Dewdney, M.M.; Ebel, R.C.; Ehsani, R.; England, G.; Rogers, M.E. IFAS guidance for huanglongbing (greening) management. Citrus Ind. 2010, 1–8. [Google Scholar] [CrossRef]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant. Soil 2013, 368, 101–128. [Google Scholar] [CrossRef] [Green Version]
- Kleiber, T.; Golcz, A.; Krzesiński, W. Effect of magnesium nutrition of onion (Allium cepa L.). Part, I. Yielding and nutrient status. Ecol. Chem. Eng. S 2012, 19, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Mbabazi, D.; Migliaccio, K.W.; Crane, J.H.; Fraisse, C.; Zotarelli, L.; Morgan, K.T.; Kiggundu, N. An irrigation schedule testing model for optimization of the Smartirrigation avocado app. Agric. Water Manag. 2017, 179, 390–400. [Google Scholar] [CrossRef]
- UN WWAP (United Nations World Water Assessment Programme). The United Nations World Water Development Report 2015: Water for a Sustainable World; UNESCO: Paris, France, 2015.
- Han, S.H.; Yun, S.; Lee, J.; Kim, S.; Chang, H.; Son, Y. Estimating the production and mortality of fine roots using minirhizotrons in a Pinus densiflora forest in Gwangneung, Korea. J. For. Res. 2016, 27, 1029–1035. [Google Scholar] [CrossRef]
- Morgan, K.T. Improved irrigation management of sweet orange with huanglongbing. HortScience 2017, 52, 916–921. [Google Scholar] [CrossRef]
- Barry, G.H.; Castle, W.S.; Davies, F.S. Rootstocks and plant water relations affect sugar accumulation of citrus fruit via osmotic adjustment. J. Am. Soc. Hortic. Sci. 2004, 129, 881–889. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Achor, D.S. Anatomical rootstocks that of characteristics vary in specific roots root of citrus length. New Phytol. 2010, 141, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Hamido, S.A.; Morgan, K.T.; Kadyampakeni, D.M. The effect of huanglongbing on young citrus tree water use. HortTechnology 2017, 27, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Syvertsen, J.P.; Smith, M.L. Nitrogen uptake efficiency and leaching losses from lysimeter-grown citrus trees fertilized at three nitrogen rates. J. Am. Soc. Hortic. Sci. 1996, 121, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Christos, D. Review article Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar]
- Bréda, N.J.J. Ground-based measurements of leaf area index: A review of methods, instruments and current controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef]
- Cimo, G.; Lo Bianco, R.; Gonzalez, P.; Bandaranayake, W.; Etxeberria, E.; Syvertsen, J.P. Leaf starch and nutrient responses to stem girdling and drought stress with respect to understanding HLB (greening) symptoms in citrus. Acta Hortic. 2016, 1130, 293–299. [Google Scholar] [CrossRef]
- Keel, S.G.; Pepin, S.; Leuzinger, S.; Körner, C. Stomatal conductance in mature deciduous forest trees exposed to elevated CO2. Trees Struct. Funct. 2007, 21, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Barkataky, S.; Ebel, R.C.; Morgan, K.T.; Dansereau, K. Water relations of well-watered citrus exposed to cold-acclimating temperatures. HortScience 2013, 48, 1309–1312. [Google Scholar] [CrossRef] [Green Version]
- Naor, A. Midday stem water potential as a plant water stress indicator for irrigation scheduling in fruit trees. Acta Hortic. 2000, 537, 447–454. [Google Scholar] [CrossRef]
- Cimò, G.; Bianco, R.L.; Gonzalez, P.; Bandaranayake, W.; Etxeberria, E.; Syvertsen, J.P. Carbohydrate and nutritional responses to stem girdling and drought stress with respect to understanding symptoms of Huanglongbing in citrus. HortScience 2013, 48, 920–928. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Mccutchan, H.; Shackel, K.A. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L.). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Barry, G.H.; Castle, W.S.; Davies, F.S. Soluble solids accumulation in ‘Valencia’ sweet orange as related to rootstock selection and fruit size. J. Am. Soc. Hortic. Sci. 2019, 129, 594–598. [Google Scholar] [CrossRef]
- Braun, P.; Schmid, J. Sap flow measurements in grapevines (Vitis vinifera L.) 1. Stem morphology and use of the heat balance method. Plant. Soil 1999, 215, 39–45. [Google Scholar] [CrossRef]
- Ham, J.M.; Heilman, J.L.; Lascano, R.J. Determination of soil water evaporation and transpiration from energy balance and stem flow measurements. Agric. For. Meteorol. 1990, 52, 287–301. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.W.; Nkedi-Kizza, P.; Obreza, T.A. Water use in drip- and microsprinkler-irrigated citrus trees. Soil Sci. Soc. Am. J. 2014, 78, 1351. [Google Scholar] [CrossRef]
- Ringuet, S.; Sassano, L.; Johnson, Z.I. A suite of microplate reader-based colorimetric methods to quantify ammonium, nitrate, orthophosphate and silicate concentrations for aquatic nutrient monitoring. J. Environ. Monit. 2011, 13, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Scrimgeour, C. Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; p. 1224. ISBN 978-0-8593-3586-0. [Google Scholar]
- Mehlich, A.; Carolina, N. Communications in soil science and plant analysis mehlich 3 soil test extractant: A modification of mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 2008, 3624, 1409–1416. [Google Scholar]
- Mylavarapu, R.S.; Sanchez, J.F.; Nguyen, J.H.; Bartos, J.M. Evaluation of mehlich-1 and mehlich-3 extraction procedures for plant nutrients in acid mineral soils of Florida. Commun. Soil Sci. Plant Anal. 2002, 33, 807–820. [Google Scholar] [CrossRef]
- Brunetto, G.; Melo, G.W.B.; de Toselli, M.; Quartieri, M.; Tagliavini, M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef] [Green Version]
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen nutrition of fruit trees to reconcile productivity and environmental concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.B.; Li, H.X.; Zhao, M.X.; Mei, X.L.; Kang, Y.L.; Dong, C.X.; Yangchun, X.U. Strategies for timing nitrogen fertilization of pear trees based on the distribution, storage, and remobilization of 15N from seasonal application of (15NH4)2SO4. J. Integr. Agric. 2020, 19, 1340–1353. [Google Scholar] [CrossRef]
- Quartieri, M.; Millard, P.; Tagliavini, M. Storage and remobilisation of nitrogen by pear (Pyrus communis L.) trees as affected by timing of N supply. Eur. J. Agron. 2002, 17, 105–110. [Google Scholar] [CrossRef]
- Hall, D.C. Estimating the relative abundance of flush shoots in citrus with implications on monitoring insects associated with flush. HortScience 2007, 42, 364–368. [Google Scholar] [CrossRef] [Green Version]
- Morgan, K.T.; Zotarelli, L.; Dukes, M.D. Use of irrigation technologies for citrus trees in Florida. Hort Technol. 2010, 20, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.M.; Xia, R.X.; Xiao, Z.Y.; Huang, R.H.; Tan, M.L.; Wang, M.Y.; Wu, Q.S. Reduced leaf photosynthesis at midday in citrus leaves growing under field or screenhouse conditions. J. Hortic. Sci. Biotechnol. 2007, 82, 387–392. [Google Scholar] [CrossRef]
- Gasque, M.; Martí, P.; Granero, B.; González-Altozano, P. Effects of long-term summer deficit irrigation on ‘Navelina’ citrus trees. Agric. Water Manag. 2016, 169, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Vahrmeijer, J.T.; Taylor, N.J. We Are Intech Open, the World’s Leading Publisher of Open Access Books Built by Scientists, for Scientists TOP 1%; Intech Open: London, UK, 2018; pp. 1–15. [Google Scholar]
- Sato, S.; Morgan, K.T.; Ozores-Hampton, M.; Simonne, E.H. Spatial and temporal distributions in sandy soils with seepage irrigation: I. Ammon. Nitrate 2009, 73, 1044–1052. [Google Scholar]
- Kadyampakeni, D.M.; Morgan, K.T.; Schumann, A.; Alfred, L.; Nkedi-Kizza, P. Phosphorus and Potassium distribution and adsorption on two Florida sandy soils. Soil Sci. Soc. Am. J. 2014, 78, 325–334. [Google Scholar] [CrossRef]
- Busman, B.L.; Lamb, J.; Randall, G.; Rehm, G.; Schmitt, M. Nutrient management: The nature of phosphorus in soils. Univ. Minn. Ext. 2017, 1–4. Available online: https://extension.umn.edu/crop-production#nutrient-management (accessed on 28 July 2001).
- Sartain, J.B. Soil Testing and Interpretation for Florida Turfgrasses; Elec. Data Info. Source, SL181; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2018; pp. 1–6. [Google Scholar]
- Quaggio, J.A.; Cantarella, H.; van Raij, B. Phosphorus and potassium soil test and nitrogen leaf analysis as a base for citrus fertilization. Nutr. Cycl. Agroecosyst. 1998, 52, 67–74. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Morton, A.R.; Trolove, S.N.; Kerckhoffs, L.H.J. Magnesium deficiency in citrus grown in the Gisborne district of New Zealand. N. Z. J. Crop Hortic. Sci. 2008, 36, 199–213. [Google Scholar] [CrossRef]
- Elrashidi, M.A.; O’Connor, G.A. Boron sorption and desorption in soils1. Soil Sci. Soc. Am. J. 1982, 46, 27. [Google Scholar] [CrossRef]
- Mesquita, G.L.; Zambrosi, F.C.; Tanaka, F.A.; Boaretto, R.M.; Quaggio, J.A.; Ribeiro, R.V.; Mattos, D. Anatomical and physiological responses of citrus trees to varying boron availability are dependent on rootstock. Front. Plant Sci. 2016, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Eller, F.; Brix, H. Influence of low calcium availability on cadmium uptake and translocation in a fast-growing shrub and a metal accumulating herb. AoB Plants 2016, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]

| 2018 | 2019 | |||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Spring | Summer | |||||
| Rootstocks 2 | ||||||||
| Treatments 1 | Cleo | Swc | Cleo | Swc | Cleo | Swc | Cleo | Swc |
| 1 | 0.67 b 3 | −0.34 a | 0.62 b | 0.50 b | −0.46 a | −0.01 b | 0.28 a | 0.09 b |
| 2 | 0.75 a | −0.29 a | 0.73 a | 0.66 a | −0.39 b | 0.18 a | 0.86 b | 0.40 a |
| 3 | 0.87 a | −0.13 a | 0.91 a | 0.65 a | −0.45 b | 0.35 a | 0.89 b | 0.23 a |
| 4 | 0.87 a | −0.05 b | 0.94 a | 0.95 a | −0.63 a | 0.08 b | 0.98 b | 0.24 a |
| Significance 4 | NS | * | *** | *** | * | ** | *** | *** |
| Effect 5 | Significance level | |||||||
| R | *** | * | NS | * | ||||
| S | ** | *** | *** | *** | ||||
| N | * | ** | NS | NS | ||||
| 2017 | 2018 | |||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Spring | Summer | |||||
| Rootstocks 1 | ||||||||
| Macro 2 | Cleo | Swc | Cleo | Swc | Cleo | Swc | Cleo | Swc |
| Stomatal conductance (mmol m−2 s−1) | ||||||||
| 1 | 2.66 a 3 | 2.56 a | 4.84 a | 4.43 a | 4.43 a | 5.31 a | 4.14 a | 4.27 b |
| 2 | 2.70 a | 2.58 a | 5.12 a | 4.43 a | 4.63 a | 5.24 a | 4.41 a | 4.72 ab |
| 3 | 2.66 a | 2.54 a | 5.01 a | 4.12 a | 4.35 a | 5.23 a | 4.53 a | 4.87 a |
| 4 | 2.61 a | 2.55 a | 4.79 a | 4.61 a | 4.59 a | 5.12 a | 4.61 a | 5.07 a |
| Significance 4 | NS | NS | NS | NS | NS | NS | NS | * |
| Effect 5 | ANOVA | |||||||
| R | *** | *** | *** | *** | ||||
| S | NS | NS | NS | *** | ||||
| N | NS | NS | NS | NS | ||||
| Spring 2017 | Summer 2017 | |||||||
|---|---|---|---|---|---|---|---|---|
| NO3-N | NH4-N | Ca | Mg | NO3-N | NH4-N | Ca | Mg | |
| Effect 1 | Significance level 2 | |||||||
| D | *** | *** | NS | *** | * | *** | *** | *** |
| R | *** | *** | NS | * | ** | *** | *** | *** |
| N | NS | NS | NS | NS | *** | NS | NS | NS |
| S | ** | NS | * | NS | * | NS | NS | NS |
| D × R | NS | NS | NS | NS | NS | * | *** | * |
| R × S | NS | NS | ** | NS | NS | ** | ** | NS |
| R × N | NS | ** | NS | NS | NS | NS | NS | NS |
| Spring 2018 | Summer 2018 | |||||||
| D | NS | *** | *** | *** | *** | *** | *** | ** |
| R | *** | *** | NS | NS | NS | *** | *** | NS |
| N | * | NS | NS | * | NS | NS | NS | NS |
| S | NS | ** | NS | *** | NS | NS | NS | *** |
| R × S | NS | NS | NS | NS | NS | NS | NS | * |
| R × N | NS | NS | * | NS | ** | * | ** | NS |
| N × S | NS | NS | NS | ** | NS | NS | ** | *** |
| N × R × S | NS | NS | * | ** | NS | NS | ** | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, A.A.; Morgan, K.T.; Hamido, S.A.; Kadyampakeni, D.M.; Mahmoud, K.A. Water and Soil Nutrient Dynamics of Huanglongbing-Affected Citrus Trees as Impacted by Ground-Applied Nutrients. Agronomy 2020, 10, 1485. https://doi.org/10.3390/agronomy10101485
Atta AA, Morgan KT, Hamido SA, Kadyampakeni DM, Mahmoud KA. Water and Soil Nutrient Dynamics of Huanglongbing-Affected Citrus Trees as Impacted by Ground-Applied Nutrients. Agronomy. 2020; 10(10):1485. https://doi.org/10.3390/agronomy10101485
Chicago/Turabian StyleAtta, Alisheikh A., Kelly T. Morgan, Said A. Hamido, Davie M. Kadyampakeni, and Kamal A. Mahmoud. 2020. "Water and Soil Nutrient Dynamics of Huanglongbing-Affected Citrus Trees as Impacted by Ground-Applied Nutrients" Agronomy 10, no. 10: 1485. https://doi.org/10.3390/agronomy10101485
APA StyleAtta, A. A., Morgan, K. T., Hamido, S. A., Kadyampakeni, D. M., & Mahmoud, K. A. (2020). Water and Soil Nutrient Dynamics of Huanglongbing-Affected Citrus Trees as Impacted by Ground-Applied Nutrients. Agronomy, 10(10), 1485. https://doi.org/10.3390/agronomy10101485







