Abstract
Basal stem rot of oil palms (OPs) is caused by Ganoderma boninense, a white-rot fungus. Root tissues are the primary route for G. boninense penetration and subsequent pathogenesis on OPs. Little is known on the host lignin biochemistry and selectivity for G. boninense degradation. Oil palm genotypes with different defense responses to G. boninense (highly tolerant, intermediately tolerant, and susceptible) were assessed for root lignin biochemistry (lignin content and composition), plant functional traits (height, fresh weight, girth), chlorophyll content, and root elemental nutrient content. One-year-old seedlings and five-year-old trees were screened for root thioglycolic acid lignin (TGA) content, lignin composition, and elemental nutrient depositions, while plant functional traits were evaluated in the one-year-old seedlings only. The TGA lignin in all the oil palm seedlings and trees ranged from 6.37 to 23.72 pM µg−1, whereas the nitrobenzene oxidation products showed a syringyl (S)-to-guaiacyl (G) ratios of 0.18–0.48. Tolerant genotypes showed significantly lower lignin content compared to the intermediately tolerant and susceptible genotypes. Likewise, the S/G ratio was higher in genotypes with lower lignin content. The depositions of root Fe, Si, Ti, S, and Cu were significantly different among the oil palm genotypes with the susceptible genotypes showing greater content than the tolerant genotypes.
1. Introduction
Oil palm (Elaeis guineensis Jacq.), also the world’s most efficient oil-bearing tree [], is highly valued for its vegetable oil []. Palm oil input (3−4 tonnes of oil per hectare per year) to output ratio exceeds 10 times greater than other major oil-seed crops such as soybean, rapeseed, and sunflower. In 2019/2020, palm oil yield accounted for 30% of global vegetable oil production. After soybean and cottonseed oils, palm oil scored the highest production with about 72.27 million metric tonnes of oil generated from about 5.5% of the total agricultural land []. World demand for vegetable oil is estimated to reach 240 million tonnes by 2050 [] and for this reason, oilseed crops, particularly oil palm, are constantly subjected to yield improvements [,].
Basal stem rot (BSR) of oil palm, caused by the white-rot fungi, Ganoderma boninense Pat. May causes devastating palm yield losses in the South-East Asian region. The disease can reduce up to 80% of the total plantation’s yield potential [,]. The saprophytic soil-inhabiting pathogen penetrates host tissue using needle-like micro-hyphae and rapidly colonizes (biotrophic phase) the cortex and lower stem [,,,]. During the G. boninense pathogenesis, lignin, a potential barrier to the starch reservoir in plant cells is breached and broken down for nutrient absorption [,]. Although Ganoderma resistant planting materials are yet to be described in the oil palm industry, several oil palm genotypes with different tolerance levels to BSR have been reported: DuraXDura (highly susceptible), DeliXDeli (moderately susceptible), DuraXPsifera (moderately tolerant), and ZaireXCameroon (highly tolerant) [,].
Lignins, together with other hydrophobic polymers, are synthesized by the phenylpropanoid pathway []. The synthesis begins with the deamination of phenylalanine amino acids into cinnamic acids, which then undergo a series of hydroxylations, O-methylations, and reductions to form three different types of alcohol moieties (monolignols): p-coumaryl (4-hyroxycinnamyl) alcohol, coniferyl (3-methoxy 4-hydroxycinnamyl) alcohol), and sinapyl (3,5-dimethoxy 4-hydroxycinnamyl) alcohol. Upon incorporation into a lignin polymer, these monolignols are referred as p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) subunits [,,]. The polymerization of different lignin subunits is mediated by the wall-bound laccase and/or peroxidase enzymes via the bimolecular radical coupling process []. Constitutive lignin in plant cells differ by content and composition (syringyl to guaiacyl subunits) and the extent of lignifications is associated with plant species, environmental cues, growth stages, and cell types [,]. The fraction of the subunits in a lignin polymer is affected by tissue identity within the same plant, subcellular locations and a wide array of responses to biotic and abiotic factors [].
The level of plant tissue lignin deposition, often measured as lignin content and the monomeric compositions, are poorly understood, particularly in terms of host defense response against pathogens []. This complex heteropolymer deposited mainly in the plant cell wall provides mechanical support to plant organs, imparts strength and rigidity to cells, aids water transport of the plant vascular system, and confers protection and resilience against mechanical stress and pathogens [,,,,,,]. Over the years, accumulating evidence has shown that types of monomers (S/G) and the degree of lignification may serve as a tool for host tolerance or susceptibility detection. For instance, the induced syringyl-rich lignin has been found accumulated during the wheat cells defense response against Puccinia graminis []. In Camelina sativa, it was shown that the resistant lines conferred a higher S/G ratio compared to the susceptible lines []. In studies conducted on poplar wood meals, a correlation between the lignin content and S/G ratio was observed []. In contrast, no correlation between these two variables was reported in the Eucalyptus species [].
Mineral nutrition, an important factor in plant growth and development affects plant physiological processes and plant-disease interactions [,,]. A balanced nutrient supply ensures optimal plant growth and tolerance to pests and diseases. Nutritional deficiency is associated with impaired plant metabolism, which would eventually result in a weakened plant with reduced disease resistance []. Categorized into essential and non-essential metal ions, elemental nutrients display adverse roles in a plant–disease complex. When a pathogen infects a plant, the host physiological processes such as the mineral nutrient uptake, assimilation, and translocation are disturbed significantly. In others, mineral nutrition measured in terms of the constitutive elemental depositions has been associated to plant defense response to pathogens [].
The role of oil palm root tissue lignin (content and composition) on host defense response against G. boninense is poorly understood. This, in turn, imparts significant importance to investigate the effect of constitutive lignin at natural settings on the extent of host defense response against G. boninense. The present work aims to investigate lignin content and composition differences (root tissues) in oil palm genotypes with different defense response to G. boninense (highly tolerant, intermediately tolerant, and susceptible to BSR). The thioglycolic acid lignin content was determined spectrophotometrically and lignin composition was quantified as a product of nitrobenzene oxidation procedure [,,]. Further, plant functional traits and root elemental nutrient content were evaluated simultaneously.
2. Materials and Methods
2.1. Oil Palm Genotypes
One-year-old oil palm seedlings (nursery condition) were collected at Applied Agricultural Resources (AAR) Sdn. Bhd., Sungai Buloh, Selangor. Three genotypes previously described as tolerant/susceptible to basal stem rot (BSR) disease were selected as following: ZaireXCameroon (highly tolerant), DuraXPsifera (intermediately tolerant) and DuraXDura (susceptible). Five-year-old oil palm trees were selected at Malaysian Palm Oil Board (MPOB) Research Station, Teluk Intan, Perak; DuraXCameroon (highly tolerant), DeliXPsifera (intermediately tolerant) and DuraXDura (susceptible) []. The one-year-old oil palm seedlings were subjected to destructive sampling whereas, only root tissues were collected from the 5-year-old oil palm trees (lignin content and lignin composition assessments). To characterize each oil palm genotype utilized in this study, an artificial infection by G. boninense inocula was performed on all the selected genotypes and the BSR disease incidence (%) was scored.
2.2. Basal Stem Rot Disease Trial
2.2.1. Large-Scale Fungal Inoculum Preparation
The Ganoderma boninense-T10 super-virulent isolate was gifted by Applied Agricultural Resources, Sungai Buloh. The seven-day-old fungal culture on maltose extract agar (MEA) plate was used for large-scale inoculum preparation. The G. boninense-rubberwood block (RWB) inoculum was prepared according to our previous study []. Briefly, air-dried RWB (6 × 6 × 6 cm) was soaked into distilled water (an overnight), washed and sterilized twice at 121 °C. About 20 mL of molten MEA was applied onto each RWB placed in a polyethylene bag. Each bag was sterilized again and left to cool at room temperature. Three pieces of G. boninense plugs (1 × 1 cm) were inoculated onto each RWB. The G. boninense inoculated-RWB was incubated in a dark chamber for 6–8 weeks. Only RWB fully covered with mycelium were utilized for subsequent artificial infection.
2.2.2. Artificial Infection of Oil Palm Genotypes
The defense responses of the oil palm genotypes during G. boninense pathogenesis were measured as basal stem rot (BSR) disease incidence. A BSR disease trial was performed for six months. Three-month-old oil palm seedlings were challenged with pathogenic Ganoderma boninense (T10) inoculum. The experiment was designed in a completely randomized design (CRD) with five treatments (oil palm genotypes) and four biological replicates per treatment. The 3-month-old oil palm seedlings raised in sterilized potting media composed of 3:2:1 v/v/v topsoil: peat: sand, were grown for a month before the artificial infection. Artificial infection of the oil palm seedlings was conducted under a glasshouse condition and disease incidence (DS%) was computed at six-months-post-inoculation according to our previous study []. Briefly, the oil palm seedlings were uprooted and washed thoroughly with tap water. The oil palm seedling was placed on a sitting position onto the RWB-G. boninense inoculum. Roots were distributed evenly around the RWB-inoculum and loosely secured with a gentle press. Both the positioned oil palm seedling and the RWB-inoculum were planted into the potting medium. The artificially infected plants were watered regularly and the absence/presence of BSR disease symptoms were recorded on a monthly basis.
2.3. Plant Functional Traits
Height, fresh weight, bole diameter, and chlorophyll content of the one-year-old oil palm seedlings were determined. Height was measured from the soil surface to highest point of the seedling (leaf), using a tape, Vertex III Hypsometer (Haglof, Långsele, Sweden). The fresh weight and BD were measured using a CAS−ED Digital Weighing Scale (Hoegtogler, Columbia, MD, USA) and a metal caliper, respectively. The relative chlorophyll content was determined at three random positions on a fully developed leaf using a SPAD−502 (Minolta, Tokyo, Japan) portable chlorophyll meter.
2.4. Lignin Content and Composition
2.4.1. Preparation of Extractive-Free Root Samples
Root tissues were excavated at 12–15 cm from the soil surface. Only roots with a diameter in between 1.5 and 2.0 cm (primary roots) and 0.5 and 0.8 mm (secondary roots) were selected for the chemical analysis. The primary and secondary root tissues were manually separated. Each material was washed thoroughly and dried at 80 °C for 2 days. The dried root tissues were ground into powders (50–60 mesh) using a 40-mesh screen, Wiley mill.
Extractive-free root powders were prepared according to Brinkmann [] with slight modifications. Root powder (2 g) was extracted in 20 mL of washing buffer (100 mM K2HPO4/KH2PO4, pH 7.4, 0.5% Triton X-100. The extraction was repeated six times using 100% methanol. Samples were subjected to centrifugation at 5 500× g for 20 min before drying at 25 °C. The extractive-free root powder was directly used for lignin content, lignin composition and nutrient element assessment.
2.4.2. Thioglycolic Acid (TGA) Lignin Determination
Quantification of lignin content in the root tissues was performed with modifications according to the thioglycolic acid (TGA) method described by Bruce and West (1989) []. Briefly, 2 mg of the extractive-free root powder was treated with 2N HCl: TGA at a 5:1 ratio. Samples were incubated at 95 °C for 4 h under regular shaking at 150 rpm. Following incubation, samples were rapidly cooled on ice and centrifuged at 15 000× g for 10 min. After removing the supernatant, the pellet was washed thrice with distilled water. Each air-dried sample received 1 mL of 0.5 N NaOH before incubation (regular shaking at 150 rpm) at room temperature for 18 h. Next, centrifugation was repeated, and the resultant supernatant received 0.3 mL of concentrated HCl. Samples were incubated at 4 °C for an overnight before centrifugation (as described earlier). The pellet was dissolved in 1 mL of 0.5 N NaOH and absorbance at 280 nm was measured using a MultiScanitGo spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Lignin content was expressed using an extinction coefficient = 0.513 × 10−12 (cm−1) (M−1) derived from lignin alkali (Sigma-Aldrich, St. Louis, MO, USA) at 30–300 μg/mL, concentrations.
2.4.3. Lignin Composition
Nitrobenzene oxidation was carried out according to Lin and Dence [] with minor modifications on the extraction process (heated at 160 °C for 3 h) and HPLC conditions. All HPLC analyses were performed on HPLC Agilent 1100 System using a reversed-phase (Luna C5, 5 µm particle size, 150 × 4.6 mm dimension) column. The injector and the column temperatures were set at 25 °C, while the injection volume was fixed at 10 µL. The wavelength of the detector was set at 280 nm. The mobile phase, acetonitrile/water solution (1:6, v/v), adjusted to pH 2.6 using trifluoroacetic acid (TFA) buffer was set at 1 mL min−1, flow rate. All standards (Sigma-Aldrich, St. Louis, MO, USA), prepared at 0.05–2 mg mL−1 were used for lignin content quantification based on the retention times and characteristic spectra. The alkaline nitrobenzene products were classified as syringyl (S)-lignin and guaiacyl (G)-lignin based on a standard peak for syringaldehdye and vanillin, respectively. The S/G ratio was calculated using the sum of areas from S units over sum of areas of the G subunits.
2.5. Mineral Element Analysis
The extractive-free root powder was dried at 65 °C for 72 h. Each sample (2.5 g) was compacted to form a thin layer to fit the sample cups. A total of ten biological replicates per treatment were analyzed. The elemental composition of root powder expressed in percentages was determined using an Energy Dispersive X-ray Fluorescence Spectrometer (EDX 720, Shimadzu, Kyoto, Japan).
2.6. Statistical Analysis
Data on lignin content and plant functional traits were subjected to one-way analysis of variance (ANOVA) and significant differences between the oil palm genotypes of different defense responses to G. boninense were detected by Tukey Test at p < 0.05. Statistical analysis was carried out using SAS, version 9.3. For the grouping of the oil palm genotypes, a Jaccard-based distance dendrogram was plotted using the vegan package, an R library (version 3.4.0) [].
3. Results
3.1. Oil Palm Genotypes and Basal Stem Rot Disease Incidence (%)
The defense responses of all genotypes used in this study were based on a multi-location progeny trial conducted by Applied Agricultural Resources Sdn Bhd. and Malaysian Palm Oil Board. To further confirm the description of each cross, a basal stem rot disease trial was conducted in a glasshouse condition. At six month post-inoculation, the disease incidence (DS%) of the infected oil palm genotypes were as following; (i) tolerant (ZaireXCameroon and DuraXCameroon); DS = 23.6−20.4%, (ii) intermediately tolerant (DuraXPsifera and DeliXPsifera); DS = 54.2%−57.8%, and (iii) susceptible (DuraXDura); DS = 84.6% (Table 1). Generally, all seedlings evident progressive BSR disease symptoms throughout the six-month disease trial: formation of fruiting bodies, yellowing and browning of leaves or plant death (Figure 1).

Table 1.
Disease incidence (DI%) of three-month-old oil palm genotypes/crosses challenged with Ganoderma boninense.
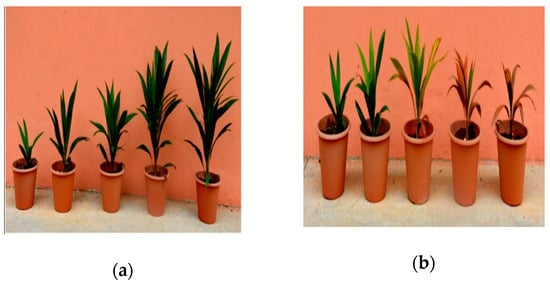
Figure 1.
Oil palm seedlings and basal stem rot disease progression: Three-month-old oil palm seedlings (DuraXPsifera) challenged by pathogenic Ganoderma boninense. (a) Uninfected seedlings (control); (b) infected seedlings at one-to-five-month post-inoculation (mpi). Pots arranged from the most left represent one mpi and increases thereafter.
3.2. Variation in Plant Functional Traits Among the Oil Palm Genotypes
Height, bole diameter (BD), and chlorophyll content showed significant differences among the one-year-old genotypes, but not fresh weight. The average height of DuraXDura (BSR-susceptible) was significantly highest at 187 cm followed by DuraXPsifera (BSR-intermediately tolerant) at 177 cm and ZaireXCameroon (BSR-tolerant) at 93 cm. Bole diameter displayed a similar trend as the height. DuraXDura, DuraXPsifera, and ZaireXCameroon showed an average BD of 100.4, 94.63, and 69.3 cm, respectively. Conversely, the average chlorophyll content of ZaireXCameroon was significantly highest (55.92 units) among the genotypes. DuraXDura and DuraXPsifera showed no significant differences in chlorophyll content at 48 and 49.92 units, respectively. The average fresh weight of the oil palm genotypes ranged from 20 to 25 kg, with no significant difference (Figure 2a).
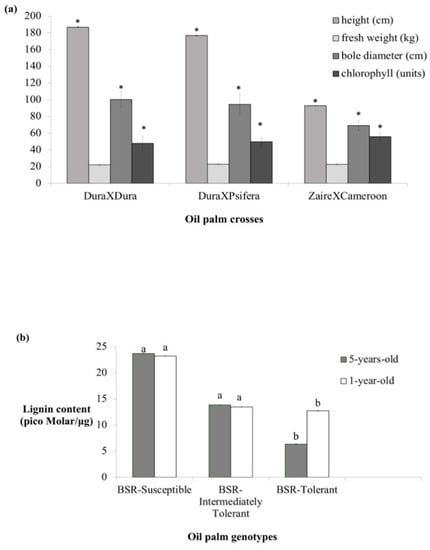
Figure 2.
Characterization of oil palm seedlings/trees. (a) Plant functional traits in one-year-old seedlings. (b) Root lignin content by oil palm crosses/genotyes in one and five-year old seedlings/trees. The oil palm genotypes are described as following: BSR-susceptible; DuraXDura; BSR-intermediately; DuraXPsifera and DeliXPsifera and BSR-tolerant; ZaireXCameroon and DuraXCameroon. Values are expressed as average mean ± standard deviation of thirty biological replicates. Asterisks, a and b represent the presence of significant differences among the oil palm genotypes at p < 0.05.
3.3. Lignin Content Distinguishes the Oil Palm Genotypes
The lignin content of the one-year-old oil palm seedlings displayed a similar trend with the five-year-old trees. The BSR-tolerant genotypes (ZaireXCameroon and DuraXCameroon) showed a lower lignin content in comparison to the BSR-susceptible genotypes (DuraXDura, DuraXPsifera and DeliXPsifera). Among the one-year-old genotypes, DuraXDura showed the significantly highest lignin content (23.24 pM/µg), followed by DuraXPsifera (13.87 pM/µg) and ZaireXCameroon (6.37 pM/µg). Likewise, the five-year-old DuraXDura showed the highest lignin content (23.72 pM/µg) in comparison to DeliXPsifera (13.47 pM/µg) and DuraXCameroon (12.75 pM/µg) (Figure 2b). The results suggest that the BSR tolerant genotypes are more likely to confer a lower root lignin content in comparison to the BSR susceptible genotypes. The classification of the one-year-old seedlings (different genotypes) by lignin content differentiated the seedlings into two different groups on a Jaccard-based distance dendrogram. At height = 0.4, the BSR-susceptible DuraXDura and BSR-intermediately tolerant DuraXPsifera were grouped under a cluster, whereas the BSR-tolerant (ZaireXCameroon) was distint (Figure 3).
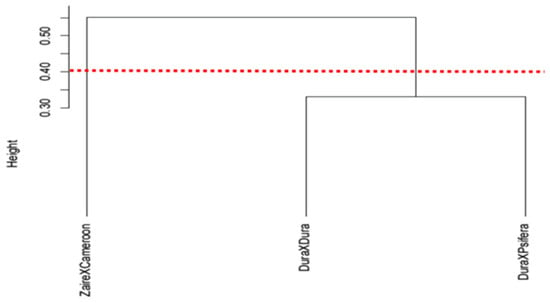
Figure 3.
Jaccard-based distance dendrogram of one-year-old oil palm crosses/genotypes plotted by root lignin content shows grouping of DuraXDura and DuraXPsifera under a clade at height = 0.35. Red dotted line indicates height cut-off at 0.40. The oil palm seedlings are characterized as following: ZaireXCameroon; tolerant to BSR, DuraXPsifera; intermediately tolerant to BSR, and DuraXDura; susceptible to BSR.
3.4. Lignin Composition in Oil Palm Genotypes
The nitrobenzene oxidation products obtained from the oil palm root tissues were vanilin and syringaldehyde. Each resulted from the degradation of non-condensed guaiacyl (G) and syringyl (S) units, respectively. All genotypes showed both S and G subunits in common with apparent differences in their compositions. The S/G (relative ratio) of the one and five-year-old oil palm seedlings/trees ranged from 0.18 to 0.48. The S/G ratio of the one-year-old ZaireXCameroon, DuraXPsifera and DuraXDura genotypes were 0.34, 0.19, and 0.24, respectively, whereas the five-year-old DuraXCameroon, DeliXPsifera and DuraXDura showed a ratio of 0.48, 0.19, and 0.18, respectively (Table 2).

Table 2.
The nitrobenzene oxidation products in oil palm root tissues expressed as.syringyl (S) and guaiacyl (G) subunit.
3.5. Elemental Nutrient Content of Oil Palm Genotypes with Differing Defense Response to G. boninense
In the present study, a total of eight elemental nutrients were found present in the oil palm root tissues from different genotypes; potassium (K), calcium (Ca), iron (Fe), silicon (Si), titanium (Ti), sulphur (S), phosphorus (P), and copper (Cu). Generally, macronutrients comprised of both K and Ca (60–70%) dominated the total content of root elemental nutrients. Ranging at 59% to 63%, K was found present as the most abundant nutrient element. The deposition of K was significantly higher in the commercial variety, DuraXPsifera (BSR-intermediately tolerant) at 62.22%. Both Ca and Fe minerals were found present at 9%–14%. Silicon deposition ranged at 4-7.04% whilst Ti, S, and P ranged at 1.5–2.8% in the oil palm root tissues. Copper was found present at the least (0.1–0.2%). Root Ca and P content showed no significant differences among the oil palm genotypes. The susceptible genotype (DuraXDura) showed a significantly higher percentage of Fe, Si, Ti, S, and Cu in nutrient element deposition in comparison to the tolerant (ZaireXCameroon) and intermediately tolerant (DuraXPsifera) genotypes (Table 3).

Table 3.
Elemental nutrient deposition (%) in root tissues of one-year-old oil palm seedlings.
4. Discussion
In plants, constitutive lignin, also the first line barrier during a pathogen attack is expressed throughout growth and development. Lignin and lignification have been associated with resistance against pathogens in oil palm [,], banana [], flax [], and many other economically important crops. The accumulation of syringyl (S)-enriched lignin following artificial infection with G. boninense inoculum had been observed in oil palm. This study conducted by our research group, evidences immediate lignification as a key host defense response against basal stem rot (BSR) of oil palm []. Many other crop species have shown such similar findings. During the early stage infection of Populus tremuloides, stem tissue lignin content increased significantly with an elevated S/G ratio []. Significant differences in lignin content and S/G composition were reported among the Eucalyptus genotypes [,]. In others, resistant plant varieties with reduced lignin content had shown acceptable agronomic properties [,] for large-scale cultivation purpose. As such, switchgrass with low lignin content showed resistance to insects [] and enhanced biomass []. In biofuel crops, the forage digestibility [] and fermentable sugar yield [] were improved in plants engineered with low lignin levels. Furthermore, low lignin feed crops were shown to have a higher nutritional content. However, a profound reduction of lignin impaired plant growth [] and altered plant immunity [].
In this study, the BSR highly tolerant oil palm genotypes (ZaireXCameroon and DuraXCameroon) showed a significantly reduced constitutive lignin content coupled with a high S/G ratio in the root tissues of one- and five-year-old seedlings and trees. On the other hand, the BSR intermediately tolerant (DuraXPsifera and DeliXPsifera) and susceptible (DuraXDura) genotypes displayed a significantly higher lignin content with a low S/G ratio. Concurrently, the occurrence of low lignin content with a high S/G ratio in the tolerant cross may suggest the ability of this genotype to synthesize a greater amount of lignin with the S subunit during the oil palm-G. boninense interaction, which subsequently results in lower disease incidence. Plants with constitutively higher lignin content may have a weaker ability to accumulate lignin during defense response compared to a plant with constitutively lower lignin content.
The S and G subunits in lignin differ in the number of methoxyl groups. Lignin variability among the genotypes may infer a difference in the occurrence of cross-specific methoxylation rate of monolignols during lignin formation. The S subunit carries two methoxyl groups, each at the 3’ and 5’ position of the aromatic ring, whereas, the G-subunit holds only one methoxyl group at the 3’ position []. The vacant 5’ in the G subunit readily participates in branching reactions, and this, in turn, explains the occurrence of the branching pattern among the G subunits. Degradation of lignin has been closely linked to the types of monomers present in the wood tissues [,,]. Linear structured lignin (S subunits) is more recalcitrant to oxidizing agents compared to a branched (G subunits) pattern []. The same may apply for the degradation of lignin in oil palm by G. boninense. The BSR tolerant genotypes with high S/G ratio could potentially delay or inhibit degradation of lignin, the primary barrier for G. boninense pathogenesis. The average fresh weight showed no significant differences among the oil palm genotypes. Although other functional traits such as the height, chlorophyll content, and bole girth showed significant differences among the oil palm genotypes, the apparent observation could not be associated with host BSR tolerance/susceptibility. Our findings were comparable to previous studies conducted on woody trees; no clear relationship between biomass and total lignin content was obtained in field-grown poplar [,].
Nutritional status of a plant affects the plant tolerance or susceptibility to pathogens; however, the effect of nutrients on plant disease complex is poorly understood. There are about 16 mineral nutrients expressed at variable relative abundance to support plant growth, yield, and disease resistance. In plants, the micronutrients, zinc (Zn), iron (Fe), copper (Cu), manganese (Mn), boron (B), chloride (Cl), and molybdenum (Mo) are required in very small concentrations, below 100 mg/kg DW, whereas the macronutrients, calcium (Ca), magnesium (Mg), nitrogen (N), potassium (K), phosphorus (P), and sulfur (S) are needed in relatively large amounts, at 1000 mg/kg DW. Despite having an undefined specific role, the elimination of elemental minerals could potentially cease plant growth and in extreme cases, the plant may die [,]. Mineral nutrition affects the primary resistance mechanism through the formation of mechanical barriers and/or synthesis of natural defense compounds. In this study, potassium was significantly highest in DuraxPsifera (BSR-intermediately tolerant), also a commercial cross with the highest yield. The results suggest that potassium may have been used by the plant for the synthesis of cellulose, protein, and starch (yield components). Calcium and phosphorus showed no significant differences among the three different genotypes with differing defense response to G. boninense.
The following elements were found significantly highest in the intermediate and susceptible genotypes: Fe, Si, Ti, S, and Cu. Found incorporated in plant cell walls in the form of hydrated amorphous silica, the accumulation of Si helps to form a protective physical barrier which resists fungal penetration []. Reinforcement of the cell wall by solid silica increases plant resistance against pests and diseases, reduces the transpiration rate, improves tolerance to water stress, and alleviates the effects of heavy metals and salt stress [,]. Cell wall fortification by silica bodies has been related to the phenylpropanoid metabolism, whereby marked predisposition of phenolic polymers was followed with induced silicification. The Cu-containing laccase and/or peroxidase families are responsible for monolignol polymerization, a key event to lignin complex formation. In plants, an increase in the reactive oxygen species (ROS) enhanced the catalase, laccase, and peroxidase activities and lignin content [,,]. In Silene paradoxa, Cu was reported to influence root lignification, vessel differentiation, and mucilage production [,]. In another similar study conducted on oil palm seedlings, single and combined application of copper, boron, and manganese showed no significant difference in host lignification, BSR incidence, and severity [,]. Although the relationship between elemental nutrients and lignin content have been reported to vary tremendously between and within plant species, our findings showed consistent agreement between Fe, Si, Ti, S, and Cu depositions and root lignin content accumulation. These elemental nutrients may have collectively enhanced lignin content in the root tissues investigated in this study.
5. Conclusions
The present findings suggest that natural variation in lignin content and lignin composition among the oil palm genotypes showed a non-discernible effect on agricultural fitness measured in terms of functional traits. However, host constitutive lignin could positively impact oil palm defense response to G. boninense (different genotypes). A higher root lignin content was observed in basal stem rot susceptible oil palm genotypes in comparison to the tolerant genotypes. The oil palm genetic make-up affected both the mineral element deposition and lignification (lignin content and composition). The oil palm genotypes with a high root lignin content parallelly showed a high Fe, Si, Ti, S, and Cu depositions. The findings suggest that these elements may have been involved the phenylpropanoid pathway governing lignin biosynthesis and the subsequent accumulation of lignin content.
Author Contributions
Conceptualization, N.G. and W.M.-Y.; methodology, formal analysis, investigation, data curation, writing—original draft preparation, visualization, N.G.; supervision, funding acquisition, I.A.-S. and W.M.-Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education Malaysia (MOHE), grant number FRGS552425.
Acknowledgments
We thank Malaysian Palm Oil Board (MPOB) And Applied Agricultural Resources (AAR) Sdn. Bhd. for providing the planting materials required in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wahid, B.; Siti, N.A.A.; Henson, I.E. Oil Palm-Achievements and Potentials. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Barcelos, E.; Rios, S.A.; Cunha, R.N.V.; Lopes, R.; Motoike, S.Y.; Babiychuk, E.; Skirycz, A.; Kushnir, S. Oil palm natural diversity and the potential for yield improvement. Front. Plant Sci. 2015, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Palm Oil Industry Worldwide-Statistics & Facts. Available online: www.statista.com/topics/6079/global-palm-oil-industry (accessed on 6 July 2020).
- Corley, R.H.V. How much palm oil do we need? Environ. Sci. Policy 2009, 12, 134–139. [Google Scholar] [CrossRef]
- Singh, G. Ganoderma—The scourge of oil palm in the coastal areas. Planter 1991, 786, 421–444. [Google Scholar]
- Singh, R.; Ong-Abdullah, M.; Low, E.L.; Manaf, M.A.A.; Rosli, R.; Nookiah, R.; Ooi, L.C.; Ooi, S.; Chan, K.; Halim, M.A.; et al. Oil palm genome sequence reveals divergence of infertile species in old and new worlds. Nature 2013, 500, 335–339. [Google Scholar] [CrossRef]
- Pilotti, C.A. Stem rots of oil palm caused by Ganoderma boninense: Pathogen biology and epidemiology. Mycopathologia 2005, 159, 129–137. [Google Scholar] [CrossRef]
- Flood, J.; Hasan, Y. Basal stem rot—Taxonomy, biology, epidemiology, economic status and control in South East Asia and Pacific Islands. In Proceedings of the Malaysian Palm Oil Board Conference, Kuala Lumpur, Malasia, 18–19 May 2004; MPOB: Kuala Lumpur, Malaysia, 2004; pp. 117–133. [Google Scholar]
- Rees, R.W.; Flood, J.; Hassan, Y.; Potterd, U.; Cooper, R.M. Basal stem rot of oil palm (Elaies guineensis): Mode of infection and lower stem invasion by Ganoderma boninense. Plant Pathol. 2009, 58, 982–989. [Google Scholar] [CrossRef]
- Govender, N.T.; Seman, I.A.; Mahmood, M.; Wong, M.Y. The phenylpropanoid pathway and lignin in defence against Ganoderma boninense colonized root tissues in oil palm (Elaeis guineensis Jacq.). Front. Plant Sci. 2017, 8, 1395. [Google Scholar] [CrossRef]
- Govender, N.T.; Wong, M.-Y. Detection of oil palm root penetration by Agrobacterium-mediated transformed Ganoderma boninense, expressing green fluorescent protein. Phytopathology 2017, 107, 483–490. [Google Scholar] [CrossRef]
- Idris, A.S.; Ariffin, D. Basal Stem Rot-Biology, Detection and Control. In Proceedings of the International Conference on Pest and Disease of Importance to the Palm Industry, Kuala Lumpur, Malaysia, 18–19 May 2004. [Google Scholar]
- Idris, A.S.; Khusairi, D.; Ismail, S.; Ariffin, D. Selection for partial resistance in oil palm to Ganoderma basal stem rot. In Proceedings of the Seminar Recent Progress in the Management of Peat and Ganoderma, Bangi, Malaysia, 6–7 May 2002. [Google Scholar]
- Umezawa, T. The cinnamate/monolignol pathway. Phytochem. Rev. 2009, 9, 1–17. [Google Scholar] [CrossRef]
- Bose, S.K.; Francis, R.C.; Govender, M.; Bush, T.; Saprk, A. Lignin content versus syringyl to guaiacyl ratio amongst poplars. Bioresour. Technol. 2009, 100, 1628–1633. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Cheng, X.; Sun, J.; Marita, J.M.; Ralph, J.; Chiang, V.L. Combinatorial modification of multiple lignin traits in trees through multigene co-transformation. Proc. Natl. Acad. Sci. USA 2003, 100, 4939–4944. [Google Scholar] [CrossRef] [PubMed]
- Skyba, O.; Douglas, C.J.; Mansfielda, S.D. Syringyl-rich lignin renders Poplars more resistant to degradation by wood decay fungi. Appl. Environ. Microbiol. 2013, 79, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
- Wallis, A.F.A.; Wearne, R.H.; Wright, P.J. Analytical characteristics of plantation eucalypt woods relating to kraft pulp yields. Appita J. 1996, 49, 427–432. [Google Scholar]
- Lapierre, C.; Pollet, B.; Petit-Conil, M.; Toval, G.; Romero, J.; Pilate, G.; Leple, J.C.; Boerjan, W.; Ferret, V.V.; De Nadai, V.; et al. Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid o-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol. 1999, 119, 153–163. [Google Scholar] [CrossRef]
- Gallego-Giraldo, L.; Posé, S.; Pattathil, S.; Peralta, A.; Hahn, M.G.; Ayre, B.G.; Sunuwar, J.; Hernandez, J.; Patel, M.A.; Shah, J.; et al. Elicitors and defence gene induction in plants with altered lignin compositions. New Phytol. 2018, 219, 1235–1251. [Google Scholar] [CrossRef]
- Agrios, N.G. Plant Pathology; Elsevier-Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure: Updates on lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Altering the cell wall and its impact on plant disease: From forage to bioenergy. Annu. Rev. Phytopathol. 2014, 52, 69–91. [Google Scholar] [CrossRef]
- Eynck, C.; Seguin-swartz, G.; Clarke, W.E.; Parkin, I.A.P. Monolignol biosynthesis is associated with resistance to Sclerotinia sclerotiorum in Camelia sativa. Mol. Plant Pathol. 2012, 13, 887–899. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Rodrigues, F.A.; Seebold, K.W. Silicon and plant disease. In Silicon in Agriculture; Datnoff, L.E., Elmer, W.H., Huber, D.M., Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Huber, D.M.; Graham, R.D. The role of nutrition in crop resistance and tolerance to disease. In Mineral Nutrition of Crops Fundamental Mechanisms and Implications; Rengel, Z., Ed.; Food Product Press: New York, NY, USA, 1999; pp. 205–226. [Google Scholar]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. Agro. Sustain. Develop. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Bruce, R.J.; West, C.A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989, 91, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, K.; Blaschke, L.; Polle, A. Comparisons of different methods for lignin determination as a basis for calibration of near-infrared reflectance spectroscopy and implications of lignoproteins. J. Chem. Ecol. 2002, 28, 2483–2501. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1992; pp. 518–523. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 25 July 2020).
- Paterson, R.R.M. Ganoderma disease of oil palm-A white-rot perspective necessary for integrated control. Crop Prot. 2007, 26, 1369–1376. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Meon, S.; Lima, N. The feasibility of producing oil palm with altered lignin content to control Ganoderma disease. J. Phytopathol. 2009, 157, 649–656. [Google Scholar] [CrossRef]
- Ana, R.D.; Ian, A.D. Dubery Panama Disease: Cell wall reinforcement in banana roots in response to elicitors from Fusarium oxysporum f. s. cubense Race Four. Biochem. Cell Biol. 2000, 9, 1173–1180. [Google Scholar]
- Kostyn, K.; Czemplik, M.; Kulma, A.; Bortniczuk, M.; Skala, J.; Szopa, J. Genes of phenylpropanoid pathway are activated in early response to Fusarium attack in flax plants. Plant Sci. 2012, 190, 103–115. [Google Scholar] [CrossRef]
- Hancock, J.E.; Loya, W.M.; Giardina, C.P.; Chiang, V.L.; Pregitzer, K.S. Plant growth, biomass partitioning and soil carbon formation in response to latered lignin biosynthesis in Populus tremuloides. New Phytol. 2007, 173, 732–742. [Google Scholar] [CrossRef]
- Elissetche, J.P.; Valenzzuela, S.; Garcia, R.; Norambuena, M.; Iturra, C.; Rodriguez, J.; Mendonea, R.T.; Balocchi, C. Transcript abundance of enzymes involved in lignin biosynthesis of Eucalyptus globules genotypes with contrasting levels of pulp yield and wood density. Tree Genet. Genomes 2011, 7, 697–705. [Google Scholar] [CrossRef]
- Klash, A.; Ncube, E.; Toit, B.; Meincken, M. Determination of the cellulose and lignin content on wood fibre surfaces of Eucalypts as a function of genotype and site. Eur. J. For. Res. 2010, 129, 741–748. [Google Scholar] [CrossRef]
- Dowd, P.F.; Johnson, E.T. Differential resistance of switchgrass Panicum virgatum L. lines to fall armyworms Spodoptera frugiperda (J.E. Smith). Genet. Resour. Crop Evol. 2009, 56, 1077–1089. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Vogel, K.P.; Funnell, D. Impact of reduced lignin on plant fitness. Crop Sci. 2005, 45, 812–819. [Google Scholar] [CrossRef]
- Dowd, P.F.; Sarath, G.; Mitchell, R.B.; Saathoff, A.J.; Vogel, K.P. Insect resistance of a full sib family of tetraploid switchgrass Panicum virgatum L. with varying lignin levels. Genet. Resour. Crop Evol. 2013, 60, 975–984. [Google Scholar] [CrossRef]
- Ray, P.; Ishiga, T.; Decker, S.R.; Turner, G.B.; Craven, K.D. A novel delivery system for the root symbiotic fungus, Sebacina vermifera and consequent biomass enhancement of low lignin COMT Switchgras lines. Bioenergy Res. 2015, 8, 922–933. [Google Scholar] [CrossRef]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Escamilla-Trevino, L.L.; Sathitsuksanoh, N.; Shen, Z.; Shen, H.; Zhang, Y.H.; Dixon, R.A.; Zhao, B. Silencing of 4-coumarate: Coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytol. 2011, 192, 611–625. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef]
- Sarkanen, K.V. Lignins: Occurrence, Formation, Structure and Reactions; Sarkanen, K.V., Ludwig, C.H., Eds.; Wiley-Science: New York, NY, USA, 1971. [Google Scholar]
- Blanchette, R.A. Delignification by wood-decay fungi. Annu. Rev. Phytopathol. 1991, 29, 381–403. [Google Scholar] [CrossRef]
- Highley, T.L. Influence of type and amount of lignin on decay by Coriolus versicolor. Can. J. For. Res. 1982, 12, 435–438. [Google Scholar] [CrossRef]
- Syafii, W.; Yoshimoto, T. Effect of lignin structure on decay resistance of some tropical woods. Indones. J. Trop. Agric. 1991, 3, 32–37. [Google Scholar]
- Stewart, J.J.; Akiyama, T.; Chapple, C.; Ralph, J.; Mansfield, S.D. The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiol. 2009, 150, 621–635. [Google Scholar] [CrossRef]
- Stout, A.T.; Davis, A.A.; Domec, J.C.; Yang, C.; Shi, R.; King, J.S. Growth under field conditions affects lignin content and productivity in transgeneuc Populus trichocarpa with altered lignin biosynthesis. Biomass Bioenergy 2014, 68, 228–239. [Google Scholar] [CrossRef]
- Voelker, S.; Lachenbruch, B.; Meinzer, F.; Jourdes, M.; Ki, C.; Patten, A. Antisense down-regulation of 4CL expression alters lignifications, tree growth, and saccharification potential of field-grown poplar. Plant Physiol. 2010, 154, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.; Palacios-Rojas, N.; Feil, R.; Stit, M. Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: Nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J. 2006, 46, 533–548. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological function of mineral micronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Marafon, A.C.; Endres, L. Silicon: Fertilization and nutrition in higher plants. Amazon. J. Agric. Environ. Sci. 2013, 56, 380–388. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Elmer, W.; Huber, D.M. Mineral Nutrition and Plant Disease; APS Press: St Paul, MN, USA, 2007. [Google Scholar]
- Liang, Y.C.; Sun, W.; Zhu, Y.G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, L.; He, F.; Zhao, F.J.; Shen, Z.; Zheng, L. Transcriptional and physiological analyses identify a regulatory role for hydrogen peroxide in the lignin biosynthesis of copper-stressed rice roots. Plant Soil 2014, 387, 323–336. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhang, H.; Zhang, Q.; Jin, G.M.; Jiang, S.J.; Jiang, D.; He, Q.Y.; Li, Z.P. Copper-induced oxidative stress and responses of the antioxidant system in roots of Medicago sativa. J. Agron. Crop Sci. 2011, 197, 418–429. [Google Scholar] [CrossRef]
- Colzi, I.; Arnetoli, M.; Gallo, A.; Doumett, S.; Del Bubba, M.; Pignattelli, S.; Gabbrielli, R.; Gonnelli, C. Copper tolerance strategies involving the root cell wall pectins in Silene paradoxa. Environ. Exp. Bot. 2015, 78, 91–98. [Google Scholar] [CrossRef]
- Colzi, I.; Doumett, S.; Del Bubba, M.; Fornaini, J.; Arnetoli, M.; Gabbrielli, R.; Gonnelli, C. On the role of the cell wall in the phenomenon of copper tolerance in Silene paradoxa L. Environ. Exp. Bot. 2012, 72, 77–83. [Google Scholar] [CrossRef]
- Tengoua, F.F.; Hanafi, M.M.; Idris, A.S.; Syed-Omar, S.R. Comparative study of lignin in roots of different oil palm progenies in relation to Ganoderma basal stem rot disease. J. Oil Palm Res. 2015, 27, 128–134. [Google Scholar]
- Tengoua, F.F.; Hanafi, M.M.; Idris, A.S.; Kadir, J.; Jamaludin, N.M.A.; Mohidin, H.; Syed-Rastan, S.O. Basal stem rot disease incidence and severity on oil palm (Elaeis guineensis jacq.) seedlings. Am. J. Appl. Sci. 2014, 11, 1841–1859. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).