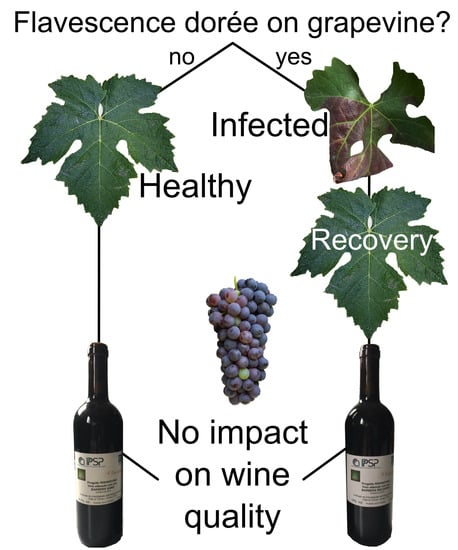
Recovery from Grapevine Flavescence Dorée in Areas of High Infection Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Vineyards, Plot Selection, and Assessment of Flavescence Dorée-Infected Vines
2.2. Nucleic Acid Extraction and Phytoplasma Molecular Detection
2.3. Maturation Curves
2.4. Microvinification, Enological, and Sensory Analyses
2.5. Virus Detection
3. Results
3.1. Disease Incidence and Recovery Rate
3.2. Maturation Curves
3.3. Microvinifications and Enological and Sensory Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schvester, D.; Carle, P.; Montous, G. Transmission de la Flavescence dorée de la vigne par S. littoralis Ball. Ann. Epiphyt. 1963, 14, 175–198. [Google Scholar]
- The IRPCM Phytoplasma/Spiroplasma Working Team–Phytoplasma Taxonomy Group. ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. Int. J. Syst. Evol. Microbiol. 2004, 54, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Morone, C.; Boveri, M.; Giosuè, S.; Gotta, P.; Rossi, V.; Scapin, I.; Marzachi, C. Epidemiology of Flavescence dorée in vineyards in Northwestern Italy. Phytopathology 2007, 97, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, F.; Zhao, Y.; Casati, P.; Bulgari, D.; Bianco, P.A.; Wei, W.; Davis, R.E. ‘Candidatus Phytoplasma solani’, a novel taxon associated with stolbur- and bois noir-related diseases of plants. Int. J. Syst. Evol. Microbiol. 2013, 63, 2879–2894. [Google Scholar] [CrossRef]
- EFSA Panel on Plant Health PLH; Jeger, M.; Bragard, C.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Jaques Miret, J.A.; MacLeod, A.; et al. Risk to plant health of Flavescence dorée for the EU territory. EFSA J. 2016, 14, 4603. [Google Scholar] [CrossRef]
- Marzachì, C.; Alma, A.; d’Aquilio, M.; Minuto, G.; Boccardo, G. Detection and identification of phytoplasmas infecting cultivated and wild plants in Liguria (Italian riviera). J. Plant Pathol. 1999, 81, 127–136. [Google Scholar]
- Gotta, P. Nuovi Strumenti Normativi Per La Limitazione Dei Serbatoi Di Infezione Di Flavescenza Dorata Nei Terreni Abbandonati. In Proceedings of the COSTIGLIOLE D’ASTI: Gerbidi, Rifiuto o Risorsa? Costigliole d’Asti (AT), Italy, 22 March 2019. [Google Scholar]
- Roggia, C.; Caciagli, P.; Galetto, L.; Pacifico, D.; Veratti, F.; Bosco, D.; Marzachì, C. Flavescence dorée phytoplasma titre in field-infected Barbera and Nebbiolo grapevines. Plant Pathol. 2014, 63, 31–41. [Google Scholar] [CrossRef]
- Kuzmanovic, S.; Martini, M.; Ermacora, P.; Ferrini, F.; Starovic, M.; Tosic, M.; Carraro, L.; Osler, R. Incidence and molecular characterization of Flavescence dorée and stolbur phytoplasmas in grapevine cultivars from different viticultural areas of Serbia. Vitis 2008, 45, 105–111. [Google Scholar]
- Eveillard, S.; Jollard, C.; Labroussaa, F.; Khalil, D.; Perrin, M.; Desqué, D.; Salar, P.; Razan, F.; Hévin, C.; Bordenave, L.; et al. Contrasting susceptibilities to Flavescence dorée in Vitis vinifera, rootstocks and wild Vitis species. Front. Plant Sci. 2016, 7, 1762. [Google Scholar] [CrossRef]
- Galetto, L.; Miliordos, D.; Roggia, C.; Rashidi, M.; Sacco, D.; Marzachì, C.; Bosco, D. Acquisition capability of the grapevine Flavescence dorée by the leafhopper vector Scaphoideus titanus Ball correlates with phytoplasma titre in the source plant. J. Pest Sci. 2014, 87, 671–679. [Google Scholar] [CrossRef]
- Galetto, L.; Miliordos, D.; Pegoraro, M.; Sacco, D.; Veratti, F.; Marzachì, C.; Bosco, D. Acquisition of Flavescence dorée phytoplasma by Scaphoideus titanus Ball from different grapevine varieties. Int. J. Mol. Sci. 2016, 17, 1563. [Google Scholar] [CrossRef] [PubMed]
- Caudwell, A. Les phénomènes de rétablissement chez la Flavescence dorée de la vigne. Ann. Epiphyt. 1961, 12, 347–354. [Google Scholar]
- Musetti, R.; di Toppi, L.S.; Ermacora, P.; Favali, M.A. Recovery in apple trees infected with the apple proliferation phytoplasma: An ultrastructural and biochemical study. Phytopathology 2004, 94, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Musetti, R.; di Toppi, L.S.; Martini, M.; Ferrini, F.; Loschi, A.; Favali, M.A.; Osler, R. Hydrogen peroxide localization and antioxidant status in the recovery of apricot plants from European Stone Fruit Yellows. Eur. J. Plant Pathol. 2005, 112, 53–61. [Google Scholar] [CrossRef]
- Osler, R.; Borselli, S.; Ermacora, P.; Loschi, A.; Martini, M.; Musetti, R.; Loi, N. Acquired tolerance in apricot plants that stably recovered from European Stone Fruit Yellows. Plant Dis. 2014, 98, 492–496. [Google Scholar] [CrossRef]
- Osler, R.; Borselli, S.; Ermacora, P.; Ferrini, F.; Loschi, A.; Martini, M.; Moruzzi, S.; Musetti, R.; Giannini, M.; Serra, S.; et al. Transmissible tolerance to European stone fruit yellows (ESFY) in apricot: Cross-protection or a plant mediated process? Phytoparasitica 2016, 44, 203–211. [Google Scholar] [CrossRef]
- Osler, R.; Loi, N.; Carraro, L.; Musetti, R.; Loschi, A.; Ermacora, P. Spontaneous recovery in grapevines affected by Flavescence dorée phytoplasma. Acta Physiol. Plant. 2004, 26, 134. [Google Scholar]
- Rossi, M.; Mori, N.; Beal, D.; Veratti, F.; Marzachì, C.; Ripamonti, M. Are grapevine plants recovered from Flavescence dorée susceptible to new infections of this phytoplasma? In Proceedings of the IOBC-WPRS Meeting of the Working Group, Vila Real, Portugal, 5–8 November 2019. [Google Scholar]
- Romanazzi, G.; Murolo, S.; Feliziani, E. Effects of an innovative strategy to contain grapevine Bois Noir: Field treatment with resistance inducers. Phytopathology 2013, 103, 785–791. [Google Scholar] [CrossRef]
- Bulgari, D.; Casati, P.; Quaglino, F.; Bianco, P.A. Endophytic bacterial community of grapevine leaves influenced by sampling date and phytoplasma infection process. BMC Microbiol. 2014, 14, 198. [Google Scholar] [CrossRef]
- Albertazzi, G.; Milc, J.; Caffagni, A.; Francia, E.; Roncaglia, E.; Ferrari, F.; Tagliafico, E.; Stefani, E.; Pecchioni, N. Gene expression in grapevine cultivars in response to Bois Noir phytoplasma infection. Plant Sci. 2009, 176, 792–804. [Google Scholar] [CrossRef]
- Hren, M.; Ravnikar, M.; Brzin, J.; Ermacora, P.; Carraro, L.; Bianco, P.A.; Casati, P.; Borgo, M.; Angelini, E.; Rotter, A.; et al. Induced expression of sucrose synthase and alcohol dehydrogenase I genes in phytoplasma-infected grapevine plants grown in the field. Plant Pathol. 2009, 58, 170–180. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Catarcione, G.; Ederli, L.; Zadra, C.; Pasqualini, S.; Badiani, M.; Musetti, R.; Santi, S.; Ciaffi, M. Correction to: Jasmonate-mediated defence responses, unlike salicylate-mediated responses, are involved in the recovery of grapevine from bois noir disease. BMC Plant Biol. 2018, 18, 39. [Google Scholar] [CrossRef]
- Santi, S.; De Marco, F.; Polizzotto, R.; Grisan, S.; Musetti, R. Recovery from stolbur disease in grapevine involves changes in sugar transport and metabolism. Front. Plant Sci. 2013, 4, 171. [Google Scholar] [CrossRef] [PubMed]
- Vitali, M.; Chitarra, W.; Galetto, L.; Bosco, D.; Marzachì, C.; Gullino, M.L.; Spanna, F.; Lovisolo, C. Flavescence dorée phytoplasma deregulates stomatal control of photosynthesis in Vitis vinifera: Grapevine ecophysiology upon phytoplasma infection. Ann. Appl. Biol. 2013, 162, 335–346. [Google Scholar] [CrossRef]
- Musetti, R.; Marabottini, R.; Badiani, M.; Martini, M.; Sanità di Toppi, L.; Borselli, S.; Borgo, M.; Osler, R. On the role of H2O2 in the recovery of grapevine (Vitis vinifera cv. Prosecco) from Flavescence dorée disease. Funct. Plant Biol. 2007, 34, 750. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Boccacci, P.; Margaria, P.; Palmano, S.; Gribaudo, I. Hydrogen peroxide accumulation and transcriptional changes in grapevines recovered from Flavescence dorée disease. Phytopathology 2013, 103, 776–784. [Google Scholar] [CrossRef]
- Margaria, P.; Abbà, S.; Palmano, S. Novel aspects of grapevine response to phytoplasma infection investigated by a proteomic and phospho-proteomic approach with data integration into functional networks. BMC Genom. 2013, 14, 38. [Google Scholar] [CrossRef]
- Margaria, P.; Ferrandino, A.; Caciagli, P.; Kedrina, O.; Schubert, A.; Palmano, S. Metabolic and transcript analysis of the flavonoid pathway in diseased and recovered Nebbiolo and Barbera grapevines (Vitis vinifera L.) following infection by Flavescence dorée phytoplasma: Flavonoid changes in phytoplasma infected vines. Plant Cell Environ. 2014, 37, 2183–2200. [Google Scholar] [CrossRef]
- Pacifico, D.; Margaria, P.; Galetto, L.; Legovich, M.; Abbà, S.; Veratti, F.; Marzachì, C.; Palmano, S. Differential gene expression in two grapevine cultivars recovered from Flavescence dorée. Microbiol. Res. 2019, 220, 72–82. [Google Scholar] [CrossRef]
- Oliveira, M.J.R.A.; Roriz, M.; Vasconcelos, M.W.; Bertaccini, A.; Carvalho, S.M.P. Conventional and novel approaches for managing “flavescence dorée” in grapevine: Knowledge gaps and future prospects. Plant Pathol. 2019, 68, 3–17. [Google Scholar] [CrossRef]
- Pavan, F.; Mori, N.; Bigot, G.; Zandigiacomo, P. Border effect in spatial distribution of Flavescence dorée affected grapevines and outside source of Scaphoideus titanus vectors. Bull. Insectol. 2012, 65, 281–290. [Google Scholar]
- Maggi, F.; Bosco, D.; Galetto, L.; Palmano, S.; Marzachì, C. Space-time point pattern analysis of Flavescence dorée epidemic in a grapevine field: Disease progression and recovery. Front. Plant Sci. 2017, 7, 1987. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Hammond, R.W.; Davis, R.E.; Gundersen, D.E. Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology 1993, 83, 834–842. [Google Scholar] [CrossRef]
- Martini, M.; Murari, E.; Mori, N.; Bertaccini, A. Identification and epidemic distribution of two Flavescence dorée—Related phytoplasmas in Veneto (Italy). Plant Dis. 1999, 83, 925–930. [Google Scholar] [CrossRef]
- Martini, M.; Botti, S.; Marcone, C.; Marzachi, C.; Casati, P.; Bianco, P.A.; Benedetti, R.; Bertaccini, A. Genetic variability among flavescence dorée phytoplasmas from different origins in Italy and France. Mol. Cell. Probes 2002, 16, 197–208. [Google Scholar] [CrossRef]
- Deng, S.; Hiruki, C. Amplification of 16S rRNA genes from culturable and nonculturable Mollicutes. J. Microbiol. Meth. 1991, 14, 53–61. [Google Scholar] [CrossRef]
- Schneider, B.; Cousins, M.T.; Klinkong, S.; Seemüller, E. Taxonomic relatedness and phylogenetic positions of phytoplasmas associated with diseases of faba bean, sunnhemp, sesame, soybean, and eggplant. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 1995, 102, 225–232. [Google Scholar]
- Angelini, E.; Clair, D.; Borgo, M.; Bertaccini, A.; Boudon-Padieu, E. Flavescence dorée in France and Italy-Occurrence of closely related phytoplasma isolates and their near relationships to Palatinate grapevine yellows and an alder yellows phytoplasma. Vitis 2001, 40, 79–86. [Google Scholar]
- Marzachì, C.; Boarino, A.; Vischi, A.; Palermo, S.; Morone, C.; Loria, A.; Boccardo, G. Flavescenza dorata, legno nero e giallume dell’astro in vitigni del Piemonte sud orientale. Inf. Fitopatol. 2001, 9, 58–63. [Google Scholar]
- Pavan, F.; Mori, N.; Bressan, S.; Mutton, P. Control strategies for grapevine phytoplasma diseases: Factors influencing the profitability of replacing symptomatic plants. Phytopath. Medit. 2012, 51, 11–22. [Google Scholar]
- Maixner, M.; Krohner, D.; Kappel, Y. Symptom remission and recovery in “bois noir” infected grapevines. Bull. Insectol. 2011, 64, S175–S176. [Google Scholar]
- Garau, R.; Sechi, A.; Prota, V.A.; Moro, G. Productive parameters in Chardonnay and Vermentino grapevines infected with Bois noir and recovered in Sardinia. Bull. Insectol. 2007, 60, 233–234. [Google Scholar]
- Bellomo, C.; Carraro, L.; Ermacora, P.; Pavan, F.; Osler, R.; Frausin, C.; Governatori, G. Recovery phenomena in grapevines affected by grapevine yellows in Friuli Venezia Giulia. Bull. Insectol. 2007, 60, 235–236. [Google Scholar]
- Rossi, M.; Pegoraro, M.; Ripamonti, M.; Abbà, S.; Beal, D.; Giraudo, A.; Veratti, F.; Malembic-Maher, S.; Salar, P.; Bosco, D.; et al. Genetic diversity of Flavescence dorée phytoplasmas at the vineyard scale. Appl. Environ. Microbiol. 2019, 85, e03123-18. [Google Scholar] [CrossRef]
- Mannini, F.; Digiaro, M. The effects of viruses and viral diseases on grapes and wine. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G., Golino, D., Fuchs, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 453–482. [Google Scholar]
- Lessio, F.; Tota, F.; Alma, A. Tracking the dispersion of Scaphoideus titanus Ball (Hemiptera: Cicadellidae) from wild to cultivated grapevine: Use of a novel mark–capture technique. Bull. Entomol. Res. 2014, 104, 432–443. [Google Scholar] [CrossRef]
- Ripamonti, M.; Pegoraro, M.; Rossi, M.; Bodino, N.; Beal, D.; Panero, L.; Marzachì, C.; Bosco, D. Prevalence of Flavescence dorée phytoplasma-infected Scaphoideus titanus in different vineyard agroecosystems of Northwestern Italy. Insects 2020, 11, 301. [Google Scholar] [CrossRef]
- Murolo, S.; Garbarino, M.; Mancini, V.; Romanazzi, G. Spatial pattern of Bois noir: Case study of a delicate balance between disease progression and recovery. Sci. Rep. 2020, 10, 9801. [Google Scholar] [CrossRef]
- Roggia, C. (Enocontrol S.C.A.R.L, Alba CN, Italy). Personal communication, 2019.
- Oliveira, M.J.R.A.; Castro, S.; Paltrinieri, S.; Bertaccini, A.; Sottomayor, M.; Santos, C.S.; Vasconcelos, M.W.; Carvalho, S.M.P. “Flavescence dorée” impacts growth, productivity and ultrastructure of Vitis vinifera plants in Portuguese “Vinhos Verdes” region. Sci. Hortic. 2020, 261, 108742. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Abbà, S.; Boccacci, P.; Birello, G.; Rossi, M.; Palmano, S.; Marzachì, C.; Perrone, I.; Gambino, G. miRVIT: A novel miRNA database and its application to uncover Vitis responses to Flavescence dorée infection. Front. Plant Sci. 2018, 9, 1034. [Google Scholar] [CrossRef]
- Kanwar, P.; Jha, G. Alterations in plant sugar metabolism: Signatory of pathogen attack. Planta 2019, 249, 305–318. [Google Scholar] [CrossRef]
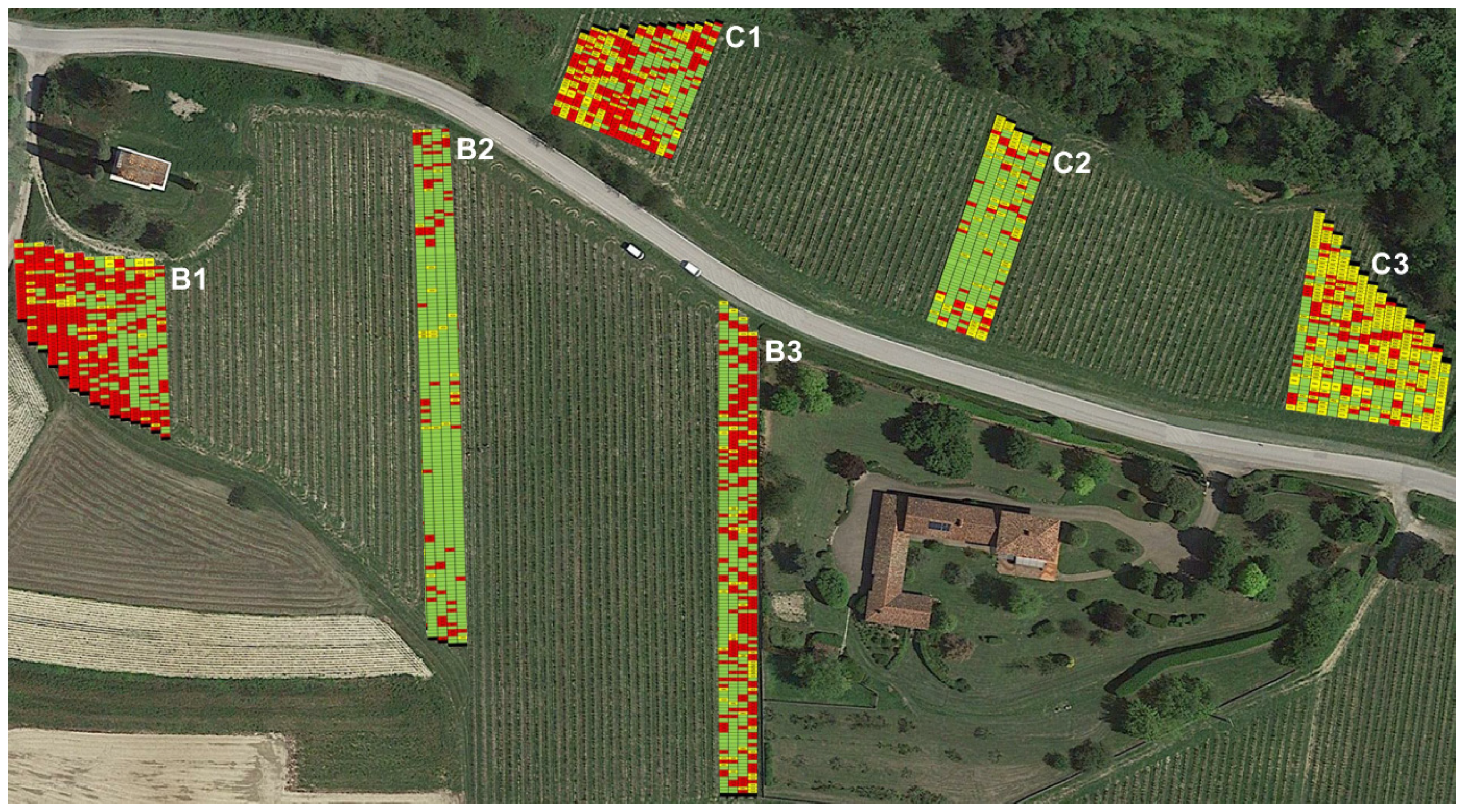
| Cultivar | Plot | N° of Plants | 2015 | 2014 | 2013 | 2012 | 2011 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % FD | % Rec | % FD | % Rec | % FD | % Rec | % FD | % Rec | % FD | |||
| Barbera | B1 | 537 | 52% | 14% | 56% | 19% | 34% | 46% | 16% | 61% | 8% |
| B2 | 569 | 13% | 18% | 11% | 0% | 0% | 100% | 1% | 100% | 1% | |
| B3 | 546 | 39% | 13% | 39% | 15% | 20% | 27% | 5% | 58% | 4% | |
| Chardonnay | C1 | 442 | 49% | 11% | 55% | 10% | 41% | / | / | / | / |
| C2 | 334 | 14% | 12% | 14% | 16% | 15% | / | / | / | / | |
| C3 | 339 | 40% | 3% | 41% | 6% | 39% | / | / | / | / | |
| Sample | Sampling Date | pH | °Brix | Sugar (g L−1) | Total Acidity (Tartaric Acid g L−1) | Alcoholic Potential Degree (% vol) |
|---|---|---|---|---|---|---|
| Bh | 17 August 2015 | 2.77 | 21.3 | 194.9 | 15.53 | 11.46 |
| 24 August 2015 | 2.96 | 24.3 | 226.6 | 11.62 | 13.32 | |
| 28 August 2015 | 3.05 | 26.2 | 249.2 | 10.18 | 14.65 | |
| 07 September 2015 | 3.13 | 29.4 | 285.1 | 8.91 | 17.11 | |
| 15 September 2015 | 3.24 | 28.9 | 277.4 | 8.86 | 16.65 | |
| Br | 17 August 2015 | 2.76 | 22.2 | 204.4 | 14.23 | 12.02 |
| 24 August 2015 | 3.03 | 22.4 | 207.7 | 11.63 | 12.21 | |
| 28 August 2015 | 3.05 | 25.8 | 241.3 | 12.38 | 14.19 | |
| 07 September 2015 | 3.06 | 27.5 | 267.8 | 9.52 | 16.07 | |
| 15 September 2015 | 3.29 | 28.3 | 273.5 | 8.43 | 16.41 |
| Sample | Sampling Date | pH | °Brix | Sugar (g L−1) | Total Acidity (Tartaric Acid g L−1) | Alcoholic Potential Degree (% vol) |
|---|---|---|---|---|---|---|
| Ch | 03 August 2015 | 2.87 | 17.3 | - | 14.30 | 9.50 |
| 07 August 2015 | 3.05 | 19.6 | - | 11.10 | 11.05 | |
| 17 August 2015 | 3.11 | 22.6 | 217.38 | 8.15 | 12.78 | |
| 24 August 2015 | 3.21 | 22.7 | 216.19 | 7.06 | 12.71 | |
| Cr | 03 August 2015 | 2.90 | 15.8 | - | 12.92 | 8.50 |
| 07 August 2015 | 2.93 | 16.2 | - | 12.81 | 8.80 | |
| 17 August 2015 | 3.08 | 19.2 | 177.11 | 9.92 | 10.41 | |
| 24 August 2015 | 3.22 | 22.5 | 214.17 | 6.91 | 12.59 |
| Sample | Must | End of Fermentation | Refinement Phase | ||
|---|---|---|---|---|---|
| °Brix | Alcoholic Strength (% vol) | pH | Total Acidity (Tartaric Acid g L−1) | Total Acidity (Tartaric Acid g L−1) | |
| Bh | 29.1 | 15.34 | 3.22 | 9.17 | 6.50 |
| Br | 28.2 | 14.56 | 3.44 | 8.00 | 5.41 |
| Ch | 22.9 | 13.60 | 3.33 | 6.98 | 5.53 |
| Cr | 20.4 | 12.39 | 3.31 | 6.80 | 5.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ripamonti, M.; Pacifico, D.; Roggia, C.; Palmano, S.; Rossi, M.; Bodino, N.; Marzachì, C.; Bosco, D.; Galetto, L. Recovery from Grapevine Flavescence Dorée in Areas of High Infection Pressure. Agronomy 2020, 10, 1479. https://doi.org/10.3390/agronomy10101479
Ripamonti M, Pacifico D, Roggia C, Palmano S, Rossi M, Bodino N, Marzachì C, Bosco D, Galetto L. Recovery from Grapevine Flavescence Dorée in Areas of High Infection Pressure. Agronomy. 2020; 10(10):1479. https://doi.org/10.3390/agronomy10101479
Chicago/Turabian StyleRipamonti, Matteo, Davide Pacifico, Chiara Roggia, Sabrina Palmano, Marika Rossi, Nicola Bodino, Cristina Marzachì, Domenico Bosco, and Luciana Galetto. 2020. "Recovery from Grapevine Flavescence Dorée in Areas of High Infection Pressure" Agronomy 10, no. 10: 1479. https://doi.org/10.3390/agronomy10101479
APA StyleRipamonti, M., Pacifico, D., Roggia, C., Palmano, S., Rossi, M., Bodino, N., Marzachì, C., Bosco, D., & Galetto, L. (2020). Recovery from Grapevine Flavescence Dorée in Areas of High Infection Pressure. Agronomy, 10(10), 1479. https://doi.org/10.3390/agronomy10101479