Balancing Forage Production, Seed Yield, and Pest Management in the Perennial Sunflower Silphium integrifolium (Asteraceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Locations
2.2. Plant Description and Experimental Design
- Control: untrimmed.
- Mid-cut: stems trimmed above the first visible node, about 5 cm above ground level.
- Low-cut: stems trimmed at ground level.
- Low-cuttwice: trimmed at ground level on 15 November 2017 for the first time and on 9 February 2018 for the second time.
2.3. Forage and Seed Yield Determination
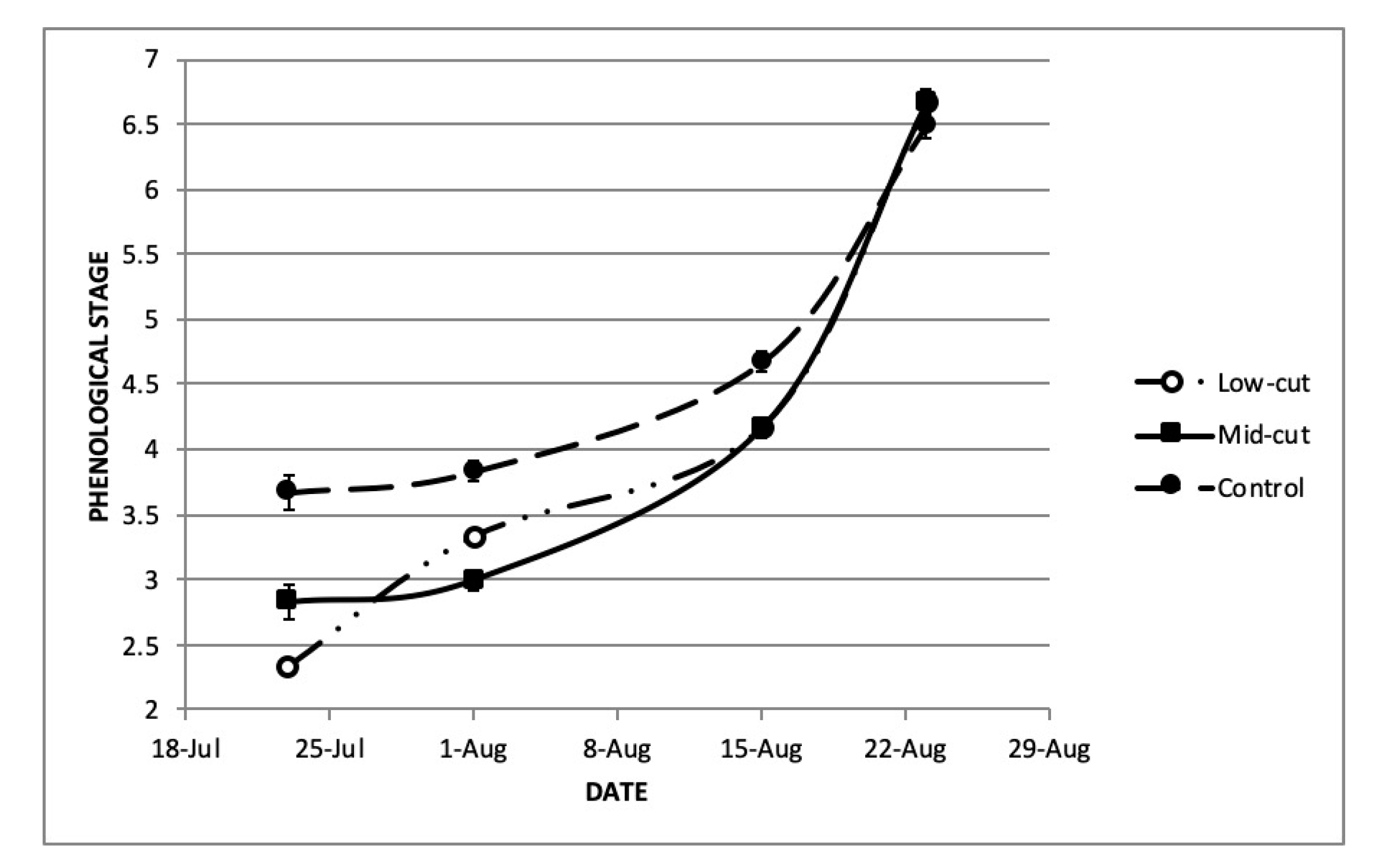
- Stage 1: Vegetative rosette.
- Stage 2: Bolting (stems growing in height from the rosette, without visible reproductive structures).
- Stage 3: Pre-flowering (visible buds, without visible ray florets).
- Stage 4: Anthesis (first capitulum with ray florets fully enlarged).
- Stage 5: Full bloom (multiple capitula in anthesis).
- Stage 6: Capitula mostly green with loss or withering of corollas and visible seeds.
- Stage 7: Mature capitula with brown seeds.
2.4. Statistics
3. Results
3.1. Evaluation of Silflower as a Dual-Purpose Crop
3.1.1. Biomass Production and Forage Quality
3.1.2. Yield Components and Seed-Yield
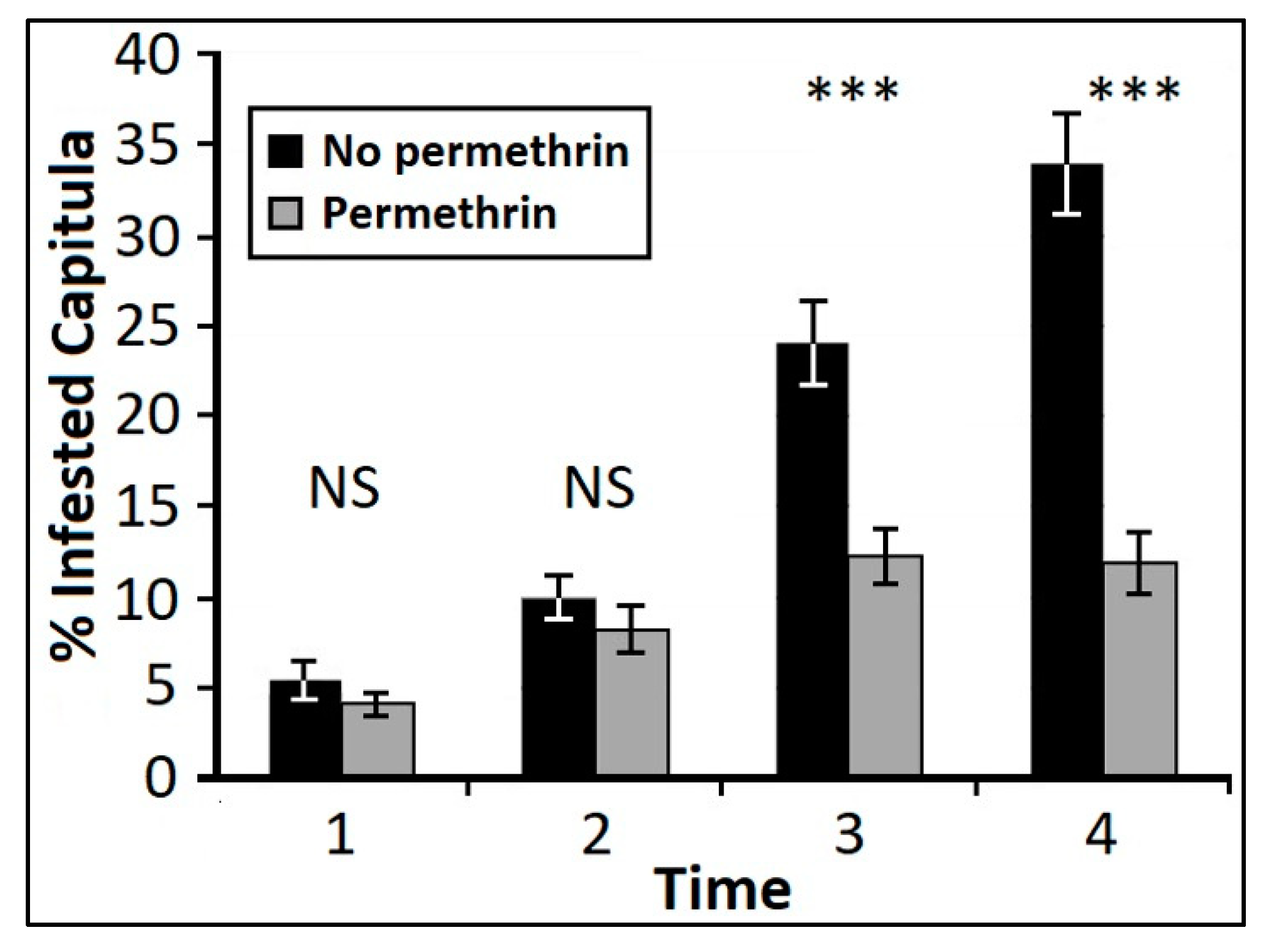
3.1.3. Harvest Effects on Phenology, Plant Architecture and Insect Damage
Plant Phenology
Plant Architecture
Response to Insecticide Application
4. Discussion
4.1. Can Silflower Be Used as a Fodder?
4.2. Can Silflower Be Used as a Dual-Purpose Crop?
4.3. Do Delays in Crop Phenology Reduce Insect Damage?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stetter, M.G.; Gates, D.J.; Mei, W.; Ross-Ibarra, J. How to make a domesticate. Curr. Biol. 2017, 27, R896–R900. [Google Scholar] [CrossRef]
- Gepts, P. Crop Domestication as a Long-term Selection Experiment. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; Volume 24, pp. 1–44. ISBN 0470650281. [Google Scholar]
- Sati, V.P. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Power, A.G. Ecosystem services and agriculture: Tradeoffs and synergies. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2959–2971. [Google Scholar] [CrossRef]
- Crews, T.E.; Blesh, J.; Culman, S.W.; Hayes, R.C.; Jensen, E.S.; Mack, M.C.; Peoples, M.B.; Schipanski, M.E. Going where no grains have gone before: From early to mid-succession. Agric. Ecosyst. Environ. 2016, 223, 223–238. [Google Scholar] [CrossRef]
- Adebiyi, J.; Schmitt Olabisi, L.; Snapp, S. Understanding perennial wheat adoption as a transformative technology: Evidence from the literature and farmers. Renew. Agric. Food Syst. 2016, 31, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Van Tassel, D.L.; Dehaan, L.R.; Cox, T.S. Missing domesticated plant forms: Can artificial selection fill the gap? Evol. Appl. 2010, 3, 434–452. [Google Scholar] [CrossRef]
- Pimentel, D.; Cerasale, D.; Stanley, R.C.; Perlman, R.; Newman, E.M.; Brent, L.C.; Mullan, A.; Chang, D.T.I. Annual vs. perennial grain production. Agric. Ecosyst. Environ. 2012, 161, 1–9. [Google Scholar] [CrossRef]
- Cox, T.S.; Glover, J.D.; Van Tassel, D.L.; Cox, C.M.; Dehaan, L.R. Prospects for Developing Perennial Grain Crops. Bioscience 2006, 56, 649. [Google Scholar] [CrossRef]
- DeHaan, L.R.; Van Tassel, D.L.; Cox, T.S. Perennial grain crops: A synthesis of ecology and plant breeding. Renew. Agric. Food Syst. 2005, 20, 5–14. [Google Scholar] [CrossRef]
- Ryan, M.R.; Crews, T.E.; Culman, S.W.; Dehaan, L.R.; Hayes, R.C.; Jungers, J.M.; Bakker, M.G. Managing for Multifunctionality in Perennial Grain Crops. Bioscience 2018, 68, 294–304. [Google Scholar] [CrossRef]
- van der Ploeg, J.D.; Barjolle, D.; Bruil, J.; Brunori, G.; Costa Madureira, L.M.; Dessein, J.; Drąg, Z.; Fink-Kessler, A.; Gasselin, P.; Gonzalez de Molina, M.; et al. The economic potential of agroecology: Empirical evidence from Europe. J. Rural Stud. 2019, 71, 46–61. [Google Scholar] [CrossRef] [Green Version]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff-Hönninger, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crops Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Gansberger, M.; Montgomery, L.F.R.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crops Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- Sokolov, V.S.; Gritsak, Z.I. Silphium—A valuable fodder and nectariferous crop. World Crop. 1972, 24, 299–301. [Google Scholar]
- Stanford, G. Silphium perfoliatum (cup-plant) as a new forage. In Proceedings of the 12th North American Prairie Conference, Cedar Falls, IA, USA, 5–9 August 1990; pp. 33–38. [Google Scholar]
- Bell, L.W.; Moore, A.D.; Kirkegaard, J.A. Evolution in crop-livestock integration systems that improve farm productivity and environmental performance in Australia. Eur. J. Agron. 2014, 57, 10–20. [Google Scholar] [CrossRef]
- Arzadun, M.J.; Arroquy, J.I.; Laborde, H.E.; Brevedan, R.E. Grazing Pressure on Beef and Grain Production of Dual-Purpose Wheat in Argentina. Agron. J. 2003, 95, 1157–1162. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Calzolari, D.; Musio, S.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Magagnini, G.; Amaducci, S. A comprehensive study of planting density and nitrogen fertilization effect on dual-purpose hemp (Cannabis sativa L.) cultivation. Ind. Crops Prod. 2017, 107, 427–438. [Google Scholar] [CrossRef]
- Sprague, S.J.; Kirkegaard, J.A.; Graham, J.M.; Dove, H.; Kelman, W.M. Crop and livestock production for dual-purpose winter canola (Brassica napus) in the high-rainfall zone of south-eastern Australia. Field Crop. Res. 2014, 156, 30–39. [Google Scholar] [CrossRef]
- Smart, A.J.; Redfearn, D.; Mitchell, R.; Wang, T.; Zilverberg, C.; Bauman, P.J.; Derner, J.D.; Walker, J.; Wrigh, C. Forum: Integration of Crop-Livestock Systems: An Opportunity to Protect Grasslands from Conversion to Cropland in the US Great Plains. Rangel. Ecol. Manag. 2019, in press. [Google Scholar] [CrossRef]
- USAD: National Agriculture Statistics Service. News Release: Kansas Rank in US Agriculture; USAD: Lincoln, NE, USA, 2016; Volume 3.
- Wahid, R.; Nielsen, S.F.; Hernandez, V.M.; Ward, A.J.; Gislum, R.; Jørgensen, U.; Møller, H.B. Methane production potential from Miscanthus sp.: Effect of harvesting time, genotypes and plant fractions. Biosyst. Eng. 2015, 133, 71–80. [Google Scholar] [CrossRef]
- Giunta, F.; Cabigliera, A.; Virdis, A.; Motzo, R. Dual-purpose use affects phenology of triticale. Field Crop. Res. 2015, 183, 111–116. [Google Scholar] [CrossRef]
- Summers, C.G. Integrated pest management in forage alfalfa. Integr. Pest. Manag. Rev. 1998, 3, 127–154. [Google Scholar] [CrossRef]
- Rogers, C.E. Insect pests and strategies for their management in cultivated sunflower. Field Crop. Res. 1992, 30, 301–332. [Google Scholar] [CrossRef]
- Pilson, D. Herbivory and natural selection on flowering phenology in wild sunflower. Helianthus Annuus. Oecologia 2000, 122, 72–82. [Google Scholar] [CrossRef]
- Prasifka, J.R.; Mallinger, R.E.; Hulke, B.S.; Larson, S.R.; Van Tassel, D. Plant-Herbivore and Plant-Pollinator Interactions of the Developing Perennial Oilseed Crop, Silphium Integrifolium. Environ. Entomol. 2017, 46, 1339–1345. [Google Scholar] [CrossRef]
- Johnson, P.J.; Boe, A. Three interesting insects and the cause of reduced vigor of cup plant (Silphium perfoliatum) in agronomic plantings. Proc. S. Dak. Acad. Sci. 2011, 90, 209. [Google Scholar]
- Brévault, T.; Clouvel, P. Pest management: Reconciling farming practices and natural regulations. Crop. Prot. 2019, 115, 1–6. [Google Scholar] [CrossRef]
- Cabrera, A.L. Regiones Fitogeográficas Argentinas; Acme: Buenos Aires, Argentina, 1994. [Google Scholar]
- Neumerkel, W.; Märtin, B.; Linke, G. Silphium perfoliatum L.—Eine Nutzpflanze? Wiss. Z. Der Martin-Luther-Univ. Halle-Wittenb. Math.-Nat. R. 1978, 27, 31–38. [Google Scholar]
- AOAC Official Methods of Analysis, 15th ed.; Association of Official Analytical Collaboration International: Arlington, VA, USA, 2002.
- National Research Council. Nutrient Requirement of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Linn, J.G.; Martin, N.P. Forage Quality Tests and Interpretation; University of Minnesota Extension Service Publication: St. Paul, MN, USA, 1989. [Google Scholar]
- Evans, L.T. Crop Evolution. In Adaptation and Yield; Cambridge University Press: Cambridge, UK, 1993; p. 498. [Google Scholar]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Bynum, E.D.; Rogers, C.E.; Archer, T.L. Evaluation of New Insecticide Application Strategies for Controlling the Sunflower Moth (Lepidoptera: Pyralidae) on Sunflower. J. Econ. Entomol. 1985, 78, 933–936. [Google Scholar] [CrossRef]
- Charlet, L.D.; Busacca, J.D. Insecticidal Control of Banded Sunflower Moth, Cochylis hospes (Lepidoptera: Cochylidae), Larvae at Different Sunflower Growth Stages and Dates of Planting in North Dakota1. J. Econ. Entomol. 1986, 79, 648–650. [Google Scholar] [CrossRef]
- Davis, R.S.; Peterson, R.K.D.; MacEdo, P.A. An Ecological Risk Assessment for Insecticides Used in Adult Mosquito Management. Hum. Ecol. Risk Assess. 2007, 3, 373–382. [Google Scholar] [CrossRef]
- Piccolomini, A.M.; Whiten, S.R.; Flenniken, M.L.; O’Neill, K.M.; Peterson, R.K.D. Acute toxicity of permethrin, deltamethrin, and etofenprox to the Alfalfa leafcutting bee. J. Econ. Entomol. 2018, 111, 1001–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theiling, K.M.; Croft, B.A. Pest Side-Effects on Arthropod Natural Enemies: A Database Summary. Agric. Ecosyst. Environ. 1988, 21, 191–218. [Google Scholar] [CrossRef]
- Holson, R.R.; Freshwater, L.; Maurissen, J.P.J.; Moser, V.C.; Phang, W. Statistical issues and techniques appropriate for developmental neurotoxicity testing. A report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints. Neurotoxicol. Teratol. 2008, 30, 326–348. [Google Scholar] [CrossRef] [PubMed]
- Bretagnolle, V.; Berthet, E.; Gross, N.; Gauffre, B.; Plumejeaud, C.; Houte, S.; Badenhausser, I.; Monceau, K.; Allier, F.; Monestiez, P.; et al. Towards sustainable and multifunctional agriculture in farmland landscapes: Lessons from the integrative approach of a French LTSER platform. Sci. Total Environ. 2018, 627, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Moon, N.J. A short review of the role of lactobacilli in silage fermentation. Food Microbiol. 1984, 1, 333–338. [Google Scholar] [CrossRef]
- Ates, S.; Cicek, H.; Gultekin, I.; Yigezu, Y.A.; Keser, M.; Filley, S.J. Bio-economic analysis of dual-purpose management of winter cereals in high and low input production systems. Field Crop. Res. 2018, 227, 56–66. [Google Scholar] [CrossRef]
- Epplin, F.M.; Hossain, I.; Krenzer, E.G. Winter wheat fall-winter forage yield and grain yield response to planting date in a dual-purpose system. Agric. Syst. 2000, 63, 161–173. [Google Scholar] [CrossRef]
- Harrison, M.T.; Evans, J.R.; Dove, H.; Moore, A.D. Dual-purpose cereals: Can the relative influences of management and environment on crop recovery and grain yield be dissected? Crop. Pasture Sci. 2011, 62, 930–946. [Google Scholar] [CrossRef]
- Castillo, F.M.; Vásquez, S.C.; Calderini, D.F. Does the pre-flowering period determine the potential grain weight of sunflower? Field Crop. Res. 2017, 212, 23–33. [Google Scholar] [CrossRef]
- Cantagallo, J.E.; Medan, D.; Hall, A.J. Grain number in sunflower as affected by shading during floret growth, anthesis and grain setting. Field Crop. Res. 2004, 85, 191–202. [Google Scholar] [CrossRef]
- Masnatta, W.J. Compromisos en la Asignación de Recursos, Perennidad y la Estabilidad del Rendimiento, Durante el Proceso de Domesticación de Hierbas Xerofíticas; Universidad de Buenos Aires: Buenos Aires, Argentina, 2018. [Google Scholar]
- González-Paleo, L.; Vilela, A.E.; Ravetta, D.A. Back to perennials: Does selection enhance tradeoffs between yield and longevity? Ind. Crops Prod. 2016, 91, 272–278. [Google Scholar] [CrossRef]
| Control | Mid-Cut | Low-Cut | Low-Cuttwice | F | |
|---|---|---|---|---|---|
| Biomass Yield (kg dw plant−1) | ------ | 0.57 ± 0.06 | 0.51 ± 0.03 | 0.51 ± 0.04 | 0.29 NS |
| ------ | 0.17 ± 0.02 a | 0.18 ± 0.02 a | 0.17 ± 0.02 a | 0.32 NS | |
| Heads Per Plant | 114.28 ± 9.15 a | 17.85 ± 6.14 b | 13.04 ± 4.57 b | 0 c | 45.75 *** |
| 316.64 ± 19.08 a | 207.45 ± 20.01 b | 174.65 ± 21.71 b | 0 c | 13.92 *** | |
| Seeds Per Head | 30.77 ± 2.28 | 26.00 ± 2.28 | 21.61 ± 2.94 | ---- | 2.51 NS |
| 48.23 ± 2.92 | 46.24 ± 2.35 | 46.63 ± 2.23 | ---- | 0.17 NS | |
| Seed Weight (100 seeds; g) | 2.01 ± 0.16 | 2.14 ± 0.16 | 2.40 ± 0.21 | ---- | 1.14 NS |
| 1.86 ± 0.09 | 2.07 ± 0.11 | 2.18 ± 0.11 | ---- | 2.73 NS | |
| Potential Seed Yield (g plant−1) | 76.30 ± 4.32 a | 12.42 ± 5.26 b | 9.18 ± 4.82 b | ---- | 62.77 *** |
| 279.36 ± 24.74 a | 188.98 ± 24.17 b | 198.44 ± 31.60 b | ----- | 4.11 * |
| Forage | Silage | ||
|---|---|---|---|
| Kansas | Patagonia | Kansas | |
| Crude Protein, %DM | 21.08 ± 2.7 | 27.7 ± 0.71 | 9.63 ± 0.63 |
| ADF, %DM | 18.97 ± 1.6 | 14.6 ± 0.79 | 42.28 ± 1.37 |
| aNDF, %DM | 27.03 ± 2.87 | 17.9 ± 0.71 | 54.53 ± 1.69 |
| ND-ICP, %DM | 1.44 ± 0.16 | 4.4 ± 0.12 | 1.44 ± 0.09 |
| NFC, %DM | 41.34 ± 3.09 | 45.4 ± 0.65 | 23.58 ± 1.15 |
| RFV | 257.98 ± 31.56 | 406 ± 20.5 | 95.69 ± 4.79 |
| TDN-1x, %DM | 74.13 ± 1.27 | 77.5 ± 0.62 | 55.93 ± 1.07 |
| Nel-3x, Mcal/cwt | 77.17 ± 1.41 | 81.0 ± 0.68 | 56.94 ± 4.79 |
| Neg, Mcal/cwt | 41.80 ± 1.99 | 49.6 ± 0.71 | 25.23 ± 0.95 |
| Nem, Mcal/cwt | 68.72 ± 2.23 | 77.5 ± 0.82 | 50.52 ± 1.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, A.E.; González-Paleo, L.; Ravetta, D.A.; Murrell, E.G.; Van Tassel, D.L. Balancing Forage Production, Seed Yield, and Pest Management in the Perennial Sunflower Silphium integrifolium (Asteraceae). Agronomy 2020, 10, 1471. https://doi.org/10.3390/agronomy10101471
Vilela AE, González-Paleo L, Ravetta DA, Murrell EG, Van Tassel DL. Balancing Forage Production, Seed Yield, and Pest Management in the Perennial Sunflower Silphium integrifolium (Asteraceae). Agronomy. 2020; 10(10):1471. https://doi.org/10.3390/agronomy10101471
Chicago/Turabian StyleVilela, Alejandra E., Luciana González-Paleo, Damián A. Ravetta, Ebony G. Murrell, and David L. Van Tassel. 2020. "Balancing Forage Production, Seed Yield, and Pest Management in the Perennial Sunflower Silphium integrifolium (Asteraceae)" Agronomy 10, no. 10: 1471. https://doi.org/10.3390/agronomy10101471
APA StyleVilela, A. E., González-Paleo, L., Ravetta, D. A., Murrell, E. G., & Van Tassel, D. L. (2020). Balancing Forage Production, Seed Yield, and Pest Management in the Perennial Sunflower Silphium integrifolium (Asteraceae). Agronomy, 10(10), 1471. https://doi.org/10.3390/agronomy10101471





