The Mineral Composition of Wild-Type and Cultivated Varieties of Pasture Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Analysis
2.2. Statistical Analysis
3. Results
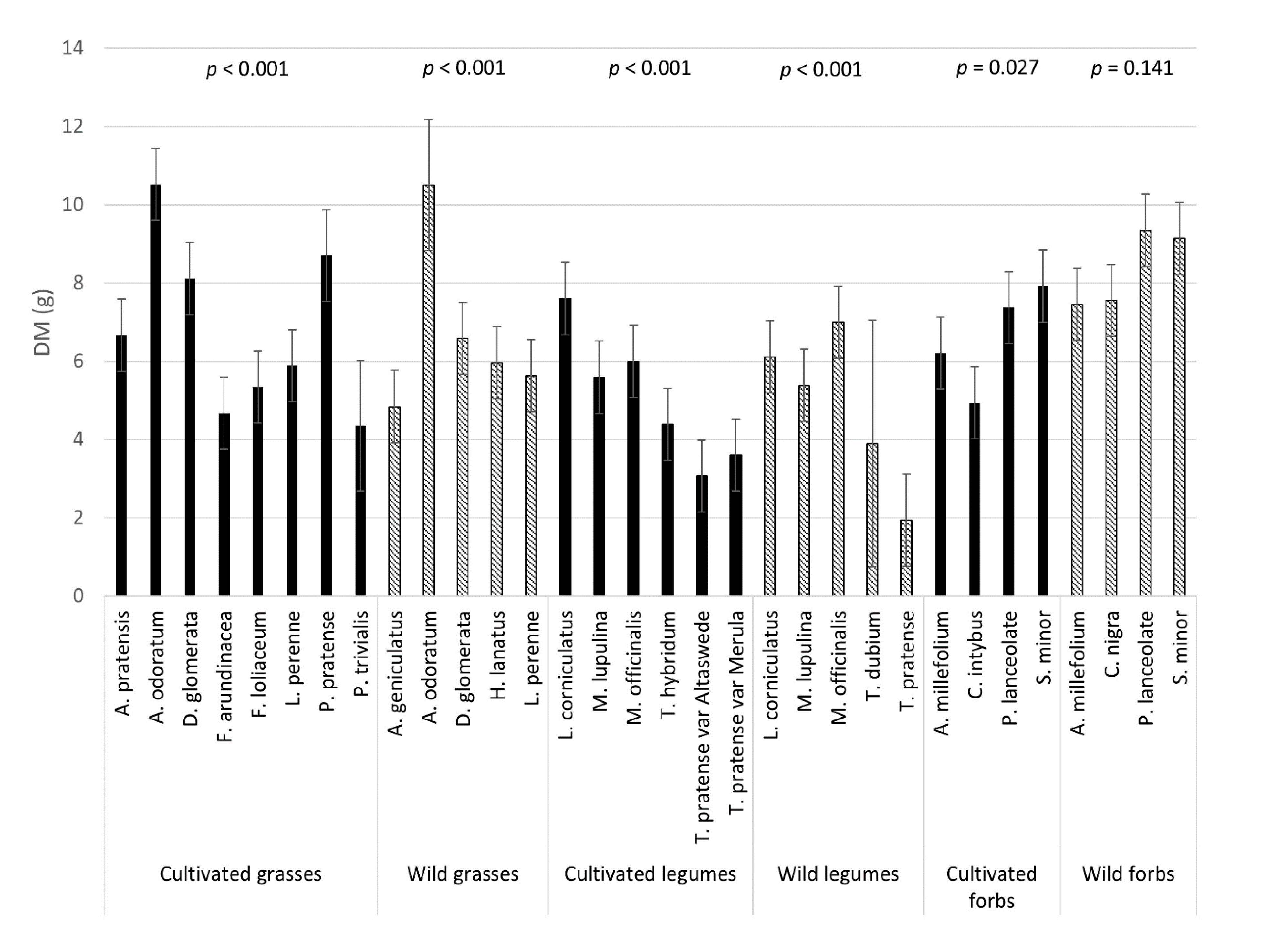
3.1. Plant Yield
3.2. Mineral Composition: Comparison of Botanical Groups
3.3. Mineral Composition: Comparison of Plant Type
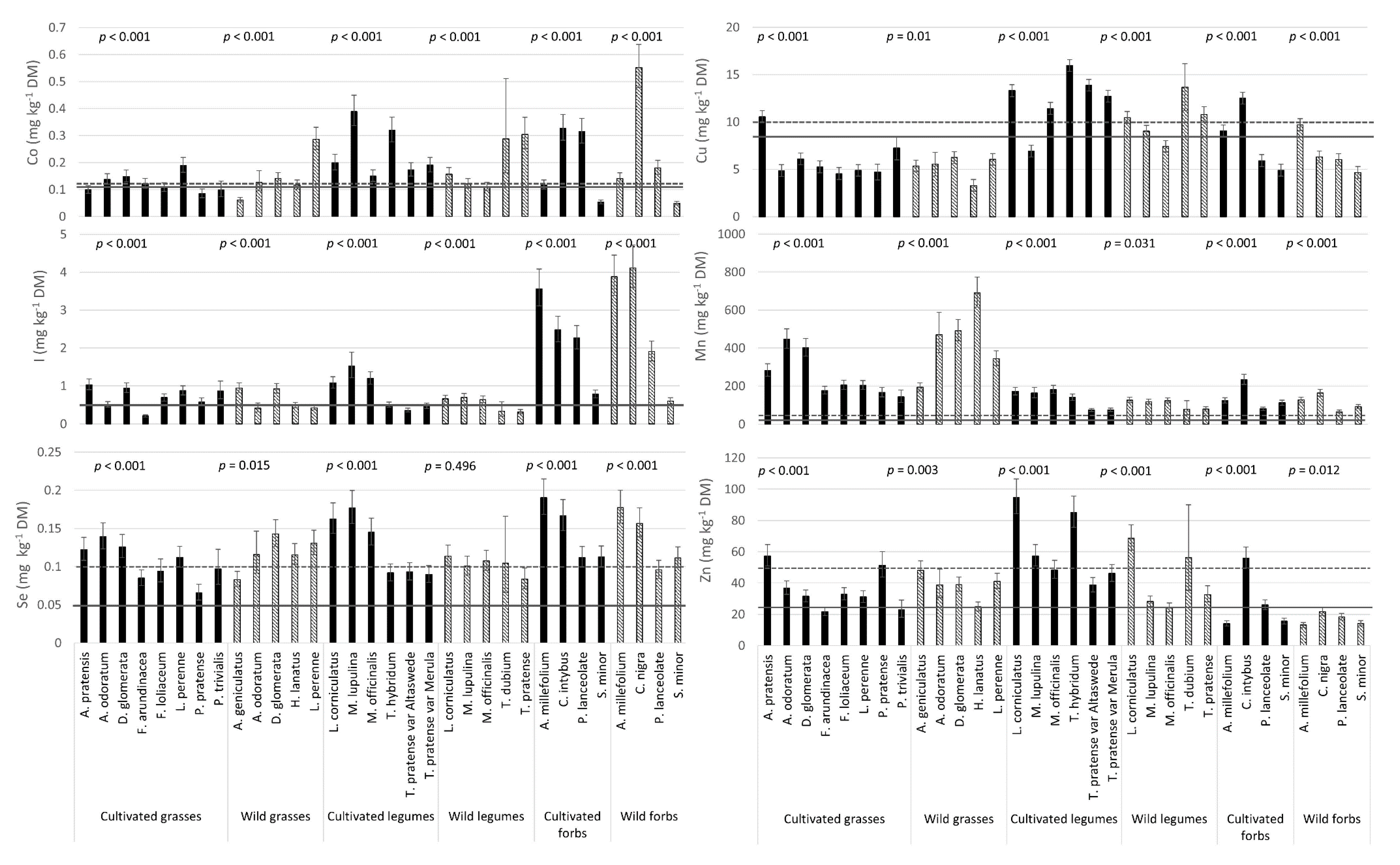
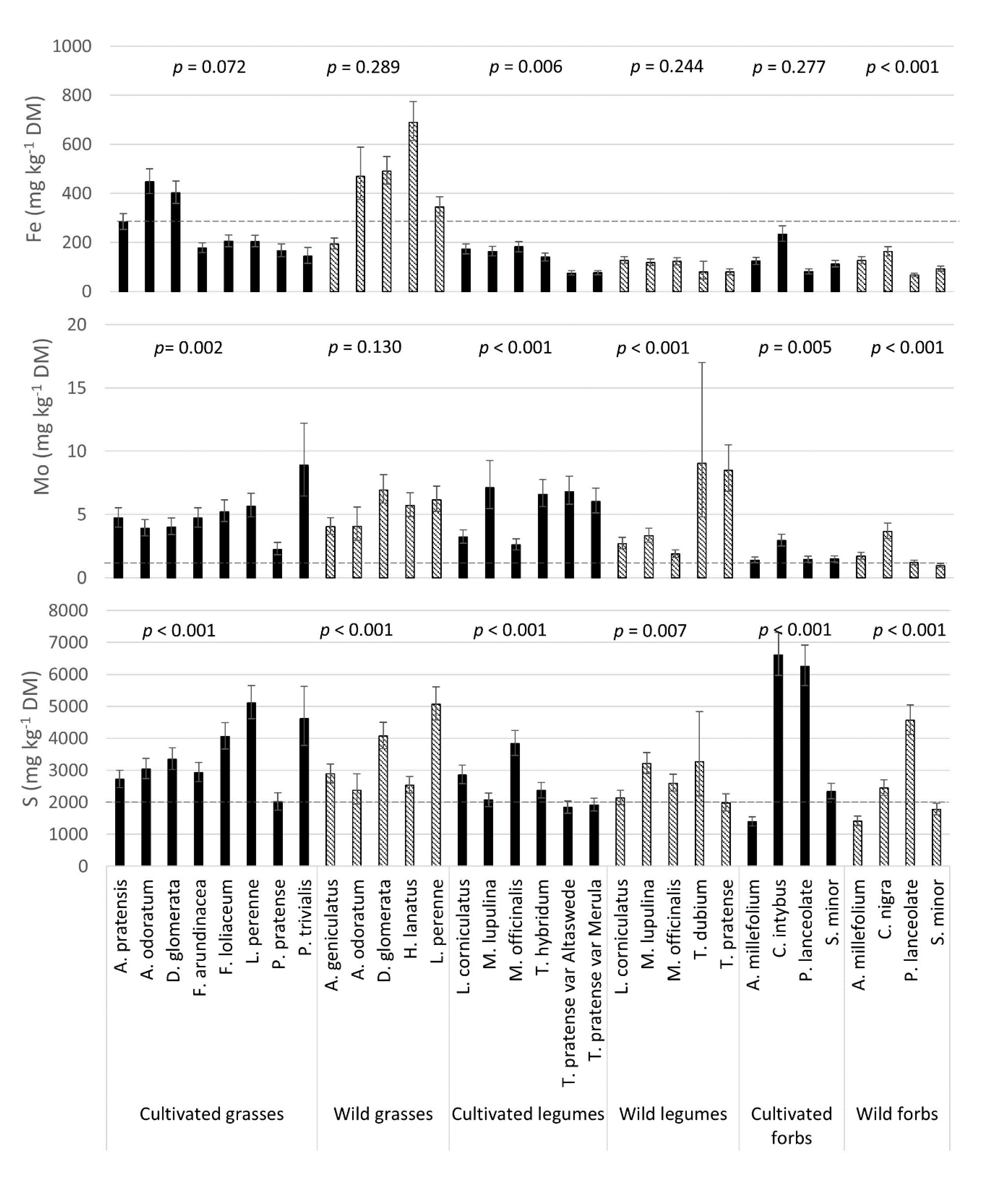
3.4. Mineral Composition: Variation between Species
3.5. Plant Concentrations Relative to Recommended Levels for Livestock Intake
4. Discussion
4.1. Impact of Plant Yield
4.2. Differences between Wild and Cultivated Species
4.3. Inter- and Intra-Botanical Group Variability
4.4. Selecting Promising Species for Further Research
4.5. Wider Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, U.C.; Wu, K.; Liang, S. Micronutrients in Soils, Crops, and Livestock. Earth Sci. Front. 2008, 15, 110–125. [Google Scholar] [CrossRef]
- Fisher, G.E.J. Micronutrients and animal nutrition and the link between the application of Micronutrients to crops and animal health. Turk. J. Agric. For. 2008, 32, 221–233. [Google Scholar]
- Kao, P.T.; Darch, T.; McGrath, S.P.; Kendall, N.R.; Buss, H.L.; Warren, H.; Lee, M.R.F. Factors influencing elemental micronutrient supply from pasture systems for grazing ruminants. Adv. Agron. 2020, 164, 161–229. [Google Scholar]
- Lee, M.R.F.; Fleming, H.R.; Whittington, F.; Hodgson, C.; Suraj, P.T.; Davies, D.R. The potential of silage lactic acid bacteria-derived nano-selenium as a dietary supplement in sheep. Anim. Prod. Sci. 2019, 59, 1999–2009. [Google Scholar] [CrossRef] [Green Version]
- Goff, J.P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid–base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef]
- Kendall, N.R.; Hession, D.; Keady, T. Mineral nutrition of grazing sheep—Problems and solutions. In Proceedings of the Teagasc National Sheep Conference, Letterkenny, Ireland, 31 January 2019; pp. 10–16. [Google Scholar]
- Lee, M.R.F.; Rivero-Viera, J.; Cone, J.W. The role of pasture in the diet of ruminant livestock. In Improving Grassland and Pasture Management in Temperate Agriculture; Marshall, A., Collins, R., Eds.; Burleigh Dodds: Cambridge, UK, 2018; pp. 1–24. [Google Scholar]
- O’Donovan, M.; Lewis, E.; O’Kiely, P. Requirements of future grass-based ruminant production systems in Ireland. Ir. J. Agric. Food Res. 2011, 50, 1–21. [Google Scholar]
- Wilkinson, J.M.; Lee, M.R.F.; Rivero, M.J.; Chamberlain, A.T. Some challenges and opportunities for grazing dairy cows on temperate pastures. Grass Forage Sci. 2020, 75, 1–17. [Google Scholar] [CrossRef]
- Connolly, J.; Finn, J.A.; Black, A.D.; Kirwan, L.; Brophy, C.; Lüscher, A. Effects of multi-species swards on dry matter production and the incidence of unsown species at three Irish sites. Ir. J. Agric. Food Res. 2009, 48, 243–260. [Google Scholar]
- Storkey, J.; Döring, T.; Baddeley, J.; Collins, R.; Roderick, S.; Jones, H.; Watson, C. Engineering a plant community to deliver multiple ecosystem services. Ecol. Appl. 2015, 25, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Kumssa, D.B.; Lovatt, J.A.; Graham, N.S.; Palmer, S.; Hayden, R.; Wilson, L.; Young, S.D.; Lark, R.M.; Penrose, B.; Ander, E.L.; et al. Magnesium biofortification of Italian ryegrass (Lolium multiflorum L.) via agronomy and breeding as a potential way to reduce grass tetany in grazing ruminants. Plant Soil 2019. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. Review: Biofortification of Durum Wheat with Zinc and Iron. Cereal Chem. J. 2010, 87, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.S.; Zhao, F.J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2008, 22, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.R.; Epp, M.D.; Riordan, H.D. Changes in USDA food composition data for 43 garden crops, 1950 to 1999. J. Am. Coll. Nutr. 2004, 23, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.F.; Penrose, B.; Bell, R.W. Micronutrients limiting pasture production in Australia. Crop Pasture Sci. 2019, 70, 1053–1064. [Google Scholar] [CrossRef]
- Lindström, B.E.M.; Frankow-Lindberg, B.E.; Dahlin, A.S.; Wivstad, M.; Watson, C.A. Micronutrient concentrations in common and novel forage species and varieties grown on two contrasting soils. Grass Forage Sci. 2013, 68, 427–436. [Google Scholar] [CrossRef]
- Reiné, R.; Ascaso, J.; Barrantes, O. Nutritional Quality of Plant Species in Pyrenean Hay Meadows of High Diversity. Agronomy 2020, 10, 883. [Google Scholar] [CrossRef]
- García-Ciudad, A.; Ruano-Ramos, A.; Vázquez de Aldana, B.R.; García-Criado, B. Interannual variations of nutrient concentrations in botanical fractions from extensively managed grasslands. Anim. Feed Sci. Technol. 1997, 66, 257–269. [Google Scholar] [CrossRef]
- Cai, J.; Weiner, J.; Wang, R.; Luo, W.; Zhang, Y.; Liu, H.; Xu, Z.; Li, H.; Zhang, Y.; Jiang, Y. Effects of nitrogen and water addition on trace element stoichiometry in five grassland species. J. Plant Res. 2017, 130, 659–668. [Google Scholar] [CrossRef]
- Laser, H. Effects of liming and nitrogen application on the trace element concentrations of pastures in low mountain range. Plant Soil Environ. 2007, 53, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Høgh-Jensen, H.; Søegaard, K. Robustness in the mineral supply from temporary grasslands. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Pirhofer-Walzl, K.; Søegaard, K.; Høgh-Jensen, H.; Eriksen, J.; Sanderson, M.A.; Rasmussen, J.; Rasmussen, J. Forage herbs improve mineral composition of grassland herbage. Grass Forage Sci. 2011, 66, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Gould, L.; Kendall, N.R. Role of the rumen in copper and thiomolybdate absorption. Nutr. Res. Rev. 2011, 24, 176–182. [Google Scholar] [CrossRef]
- AHDB. Trace Element Supplementation of Beef Cattle and Sheep. 2011. Available online: https://ahdb.org.uk/knowledge-library/brp-trace-element-supplementation-of-beef-cattle-and-sheep (accessed on 1 May 2020).
- AHDB. Nutrient Management Guide (RB209); Section 3 Grass and Forage Crops. 2020. Available online: https://ahdb.org.uk/knowledge-library/nutrient-management-guide-rb209-amendments (accessed on 24 July 2020).
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014; p. 376. [Google Scholar]
- Gray, C.W.; McLaren, R.G. The effect of ryegrass variety on trace metal uptake. N. Z. J. Agric. Res. 2005, 48, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Jarrell, W.M.; Beverly, R.B. The Dilution Effect in Plant Nutrition Studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar] [CrossRef]
- Šmilauer, P.; Šmilauerová, M. Asymmetric relationship between grasses and forbs: Results from a field experiment under nutrient limitation. Grass Forage Sci. 2013, 68, 186–198. [Google Scholar] [CrossRef]
- Lindström, B.E.M.; Frankow-Lindberg, B.E.; Dahlin, A.S.; Watson, C.A.; Wivstad, M. Red clover increases micronutrient concentrations in forage mixtures. Field Crops Res. 2014, 169, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Davis, D.R. Declining Fruit and Vegetable Nutrient Composition: What Is the Evidence? HortScience 2009, 44, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.L.; Dennis, P.G.; Owen, A.G.; van Hees, P.A.W. Organic acid behavior in soils-misconceptions and knowledge gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A. Chapter Four—The Role of Mineral Nutrition on Root Growth of Crop Plants. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 110, pp. 251–331. [Google Scholar]
- Crush, J.R.; Lee, J.M.; Cosgrove, G.P.; Rossi, L.; Chapman, D.F.; Stevens, D.R. Foliar micronutrient concentrations of eight perennial ryegrass (Lolium perenne L.) cultivars grown in four regions in New Zealand. N. Z. J. Agric. Res. 2018, 61, 301–311. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Labreveux, M.; Hall, M.H.; Elwinger, G.F. Nutritive Value of Chicory and English Plantain Forage. Crop Sci. 2003, 43, 1797–1804. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, P.; Wyss, U.; Arrigo, Y.; Hess, H.D. Mineral concentrations of fresh herbage from mixed grassland as influenced by botanical composition, harvest time and growth stage. Anim. Feed Sci. Technol. 2016, 219, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Shellswell, C.H. Is the Rye-Grass Always Greener? An Evidence Review of the Nutritional, Medicinal and Production Value of Species-Rich Grassland (Appendix). Plantlife, 2017. Available online: http://www.magnificentmeadows.org.uk/assets/pdfs/Is_the_rye-grass_always_greener_An_evidence_review.pdf (accessed on 21 January 2020).
- Judson, G.J.; McFarlane, J.D. Mineral disorders in grazing livestock and the usefulness of soil and plant analysis in the assessment of these disorders. Aust. J. Exp. Agric. 1998, 38, 707–723. [Google Scholar] [CrossRef]
- Grace, C.; Lynch, M.B.; Sheridan, H.; Lott, S.; Fritch, R.; Boland, T.M. Grazing multispecies swards improves ewe and lamb performance. Anim. Int. J. Anim. Biosci. 2019, 13, 1721–1729. [Google Scholar] [CrossRef]
- Bryant, R.H.; Miller, M.E.; Greenwood, S.L.; Edwards, G.R. Milk yield and nitrogen excretion of dairy cows grazing binary and multispecies pastures. Grass Forage Sci. 2017, 72, 806–817. [Google Scholar] [CrossRef]
- Jing, J.; Søegaard, K.; Cong, W.-F.; Eriksen, J. Species Diversity Effects on Productivity, Persistence and Quality of Multispecies Swards in a Four-Year Experiment. PLoS ONE 2017, 12, e0169208. [Google Scholar] [CrossRef]
- Stroud, J.L.; Li, H.F.; Lopez-Bellido, F.J.; Broadley, M.R.; Foot, I.; Fairweather-Tait, S.J.; Hart, D.J.; Hurst, R.; Knott, P.; Mowat, H.; et al. Impact of sulphur fertilisation on crop response to selenium fertilisation. Plant Soil 2010, 332, 31–40. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of Nutrient Antagonism and Synergism on Yield and Fertilizer Use Efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef] [Green Version]
- Dias, R.S.; López, S.; Montanholi, Y.R.; Smith, B.; Haas, L.S.; Miller, S.P.; France, J. A meta-analysis of the effects of dietary copper, molybdenum, and sulfur on plasma and liver copper, weight gain, and feed conversion in growing-finishing cattle. J. Anim. Sci. 2013, 91, 5714–5723. [Google Scholar] [CrossRef]
- Gooneratne, S.R.; Laarveld, B.; Pathirana, K.K.; Christensen, D.A. Effects of dietary Cu, Mo and S on urinary Cu and Zn excretion in Simmental and Angus cattle. Res. Vet. Sci. 2011, 91, e116–e120. [Google Scholar] [CrossRef]
- Ergon, Å.; Kirwan, L.; Bleken, M.A.; Skjelvåg, A.O.; Collins, R.P.; Rognli, O.A. Species interactions in a grassland mixture under low nitrogen fertilization and two cutting frequencies: 1. dry-matter yield and dynamics of species composition. Grass Forage Sci. 2016, 71, 667–682. [Google Scholar] [CrossRef] [Green Version]
- Brophy, C.; Finn, J.A.; Lüscher, A.; Suter, M.; Kirwan, L.; Sebastià, M.-T.; Helgadóttir, Á.; Baadshaug, O.H.; Bélanger, G.; Black, A.; et al. Major shifts in species’ relative abundance in grassland mixtures alongside positive effects of species diversity in yield: A continental-scale experiment. J. Ecol. 2017, 105, 1210–1222. [Google Scholar] [CrossRef]
- Li, G.D.; Lodge, G.M.; Moore, G.A.; Craig, A.D.; Dear, B.S.; Boschma, S.P.; Albertsen, T.O.; Miller, S.M.; Harden, S.; Hayes, R.C.; et al. Evaluation of perennial pasture legumes and herbs to identify species with high herbage production and persistence in mixed farming zones in southern Australia. Aust. J. Exp. Agric. 2008, 48, 449–466. [Google Scholar] [CrossRef]
- Darch, T.; Dunn, R.M.; Guy, A.; Hawkins, J.M.B.; Ash, M.; Frimpong, K.A.; Blackwell, M.S.A. Fertilizer produced from abattoir waste can contribute to phosphorus sustainability, and biofortify crops with minerals. PLoS ONE 2019, 14, e0221647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindström, B.E.M.; Frankow-Lindberg, B.E.; Dahlin, A.S.; Wivstad, M.; Watson, C.A. Micronutrient concentrations in relation to phenological development of red clover (Trifolium pratense L.), perennial ryegrass (Lolium perenne L.) and timothy (Phleum pratense L.). Grass Forage Sci. 2014, 69, 276–284. [Google Scholar] [CrossRef]
| Latin Name | Commercial | Wild |
|---|---|---|
| Grasses | ||
| Alopecurus geniculatus * | Wild | |
| Alopecurus pratensis * | Zuberska | |
| Anthoxanthum odoratum * | Commercial | Wild |
| Dactylis glomerata * | Sparta | Wild |
| Festuca arundinacea | Debussy | |
| Festulolium loliaceum | AberNiche | |
| Holcus lanatus | Wild | |
| Lolium perenne * | Nifty | Wild |
| Phleum pratense | Promesse | |
| Poa trivialis | Dasas | |
| Legumes | ||
| Lotus corniculatus * | Leo | Wild |
| Medicago lupulina * | Virgo | Wild |
| Melilotus officinalis * | Commercial | Wild |
| Trifolium dubium | Wild | |
| Trifolium hybridum | Dawn | |
| Trifolium pratense * | Merula & Altaswede | Essex |
| Forbs | ||
| Achillea millefolium * | Commercial | Wild |
| Centaurea nigra | Wild | |
| Cichorium intybus | Puna II | |
| Plantago lanceolata * | Endurance | Wild |
| Sanguisorba minor * | Commercial | Wild |
| Plant Type | Botanical Group | Type−Botanical Group Interaction | |
|---|---|---|---|
| I | 0.804 | <0.001 | 0.051 |
| Cu | 0.001 | ||
| Co | 0.005 | ||
| Se | 0.029 | ||
| Mo | 0.003 | ||
| Zn | <0.001 | ||
| Mn | <0.001 | ||
| Fe | 0.389 | <0.001 | 0.219 |
| S | <0.001 |
| Mineral | Botanical Group | Cultivated | Confidence Interval | Wild | Confidence Interval |
|---|---|---|---|---|---|
| Cu | Forb | 8.10 c | 7.940–8.264 | 6.68 d | 6.520–6.844 |
| Grass | 6.02 de | 5.924–6.116 | 5.30 e | 5.152–5.452 | |
| Legume | 12.4 a | 12.26–12.48 | 10.3 b | 10.09–10.47 | |
| Co | Forb | 0.160 b | 0.1537–0.1656 | 0.162 b | 0.1563–0.1684 |
| Grass | 0.120 c | 0.1174–0.1227 | 0.130 c | 0.1256–0.1346 | |
| Legume | 0.223 a | 0.2173–0.2286 | 0.179 b | 0.1715–0.1873 | |
| Se | Forb | 0.141 a | 0.1362–0.1466 | 0.131 ab | 0.1265–0.1362 |
| Grass | 0.103 c | 0.1003–0.1050 | 0.116 bc | 0.1121–0.1196 | |
| Legume | 0.121 b | 0.1182–0.1247 | 0.101 c | 0.09787–0.1052 | |
| Mo | Forb | 1.72 b | 1.657–1.791 | 1.64 b | 1.576–1.704 |
| Grass | 4.62 a | 4.524–4.709 | 5.25 a | 5.079–5.435 | |
| Legume | 5.03 a | 4.901–5.163 | 4.20 a | 4.036–4.370 | |
| Zn | Forb | 23.9 c | 23.17–24.61 | 16.5 d | 16.03–17.03 |
| Grass | 34.0 bc | 33.37–34.57 | 37.5 bc | 36.46–38.56 | |
| Legume | 58.5 a | 57.29–59.69 | 38.7 b | 37.36–40.14 | |
| Mn | Forb | 127 c | 123.3–131.6 | 106 d | 102.5–109.4 |
| Grass | 234 b | 229.2–238.7 | 403 a | 390.7–415.1 | |
| Legume | 126 c | 123.3–129.2 | 103 d | 99.02–106.7 | |
| S | Forb | 3410 a | 3315–3512 | 2300 b | 2236–2369 |
| Grass | 3340 a | 3282–3403 | 3240 a | 3157–3332 | |
| Legume | 2400 b | 2350–2449 | 2580 b | 2498–2670 | |
| Mean | Confidence interval | ||||
| I | Forb | 2.03 a | 1.985–2.068 | ||
| Grass | 0.630 b | 0.6205–0.6390 | |||
| Legume | 0.621 b | 0.6104–0.6321 | |||
| Fe | Forb | 92.3 b | 90.24–94.32 | ||
| Grass | 59.0 c | 58.18–59.87 | |||
| Legume | 136 a | 133.5–138.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darch, T.; McGrath, S.P.; Lee, M.R.F.; Beaumont, D.A.; Blackwell, M.S.A.; Horrocks, C.A.; Evans, J.; Storkey, J. The Mineral Composition of Wild-Type and Cultivated Varieties of Pasture Species. Agronomy 2020, 10, 1463. https://doi.org/10.3390/agronomy10101463
Darch T, McGrath SP, Lee MRF, Beaumont DA, Blackwell MSA, Horrocks CA, Evans J, Storkey J. The Mineral Composition of Wild-Type and Cultivated Varieties of Pasture Species. Agronomy. 2020; 10(10):1463. https://doi.org/10.3390/agronomy10101463
Chicago/Turabian StyleDarch, Tegan, Steve P. McGrath, Michael R. F. Lee, Deborah A. Beaumont, Martin S. A. Blackwell, Claire A. Horrocks, Jessica Evans, and Jonathan Storkey. 2020. "The Mineral Composition of Wild-Type and Cultivated Varieties of Pasture Species" Agronomy 10, no. 10: 1463. https://doi.org/10.3390/agronomy10101463
APA StyleDarch, T., McGrath, S. P., Lee, M. R. F., Beaumont, D. A., Blackwell, M. S. A., Horrocks, C. A., Evans, J., & Storkey, J. (2020). The Mineral Composition of Wild-Type and Cultivated Varieties of Pasture Species. Agronomy, 10(10), 1463. https://doi.org/10.3390/agronomy10101463





