Effects of Water Stress and Modern Biostimulants on Growth and Quality Characteristics of Mint
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Treatments
2.3. Measurements
2.3.1. Morphological and Physiological
2.3.2. Essential Oil and Gas Chromatography/Mass Spectrometry (GC/MS)
2.3.3. Antioxidant Potential
2.4. Statistical Analyses
3. Results
3.1. Morphological Responses
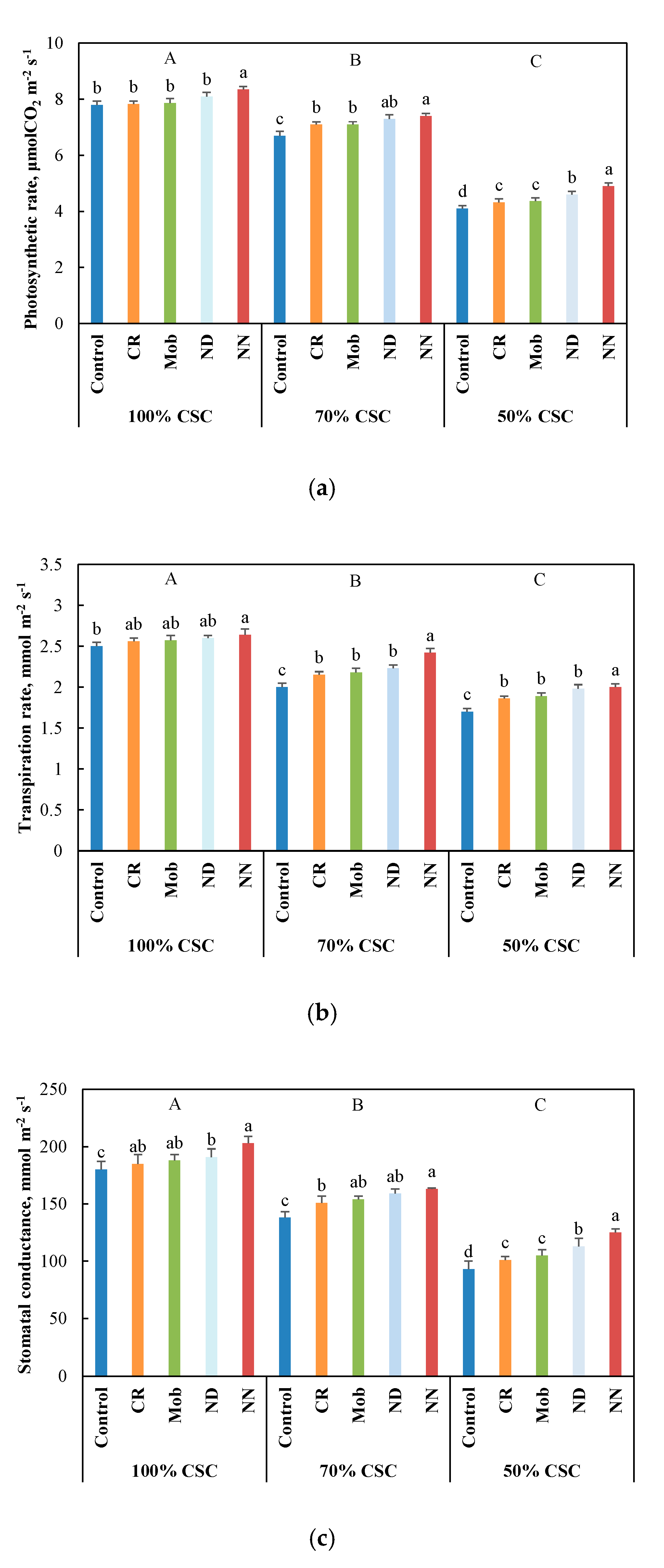
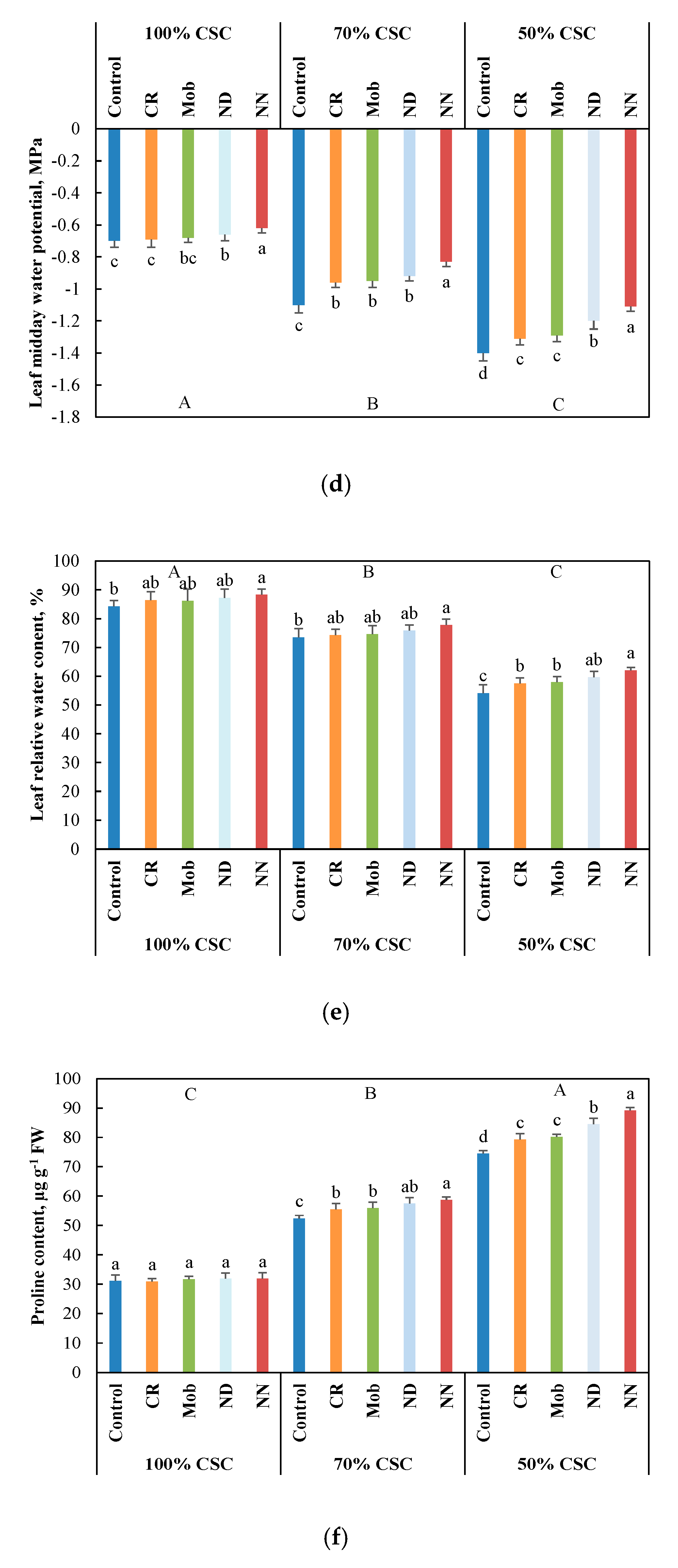
3.2. Physiological and Metabolic Performance
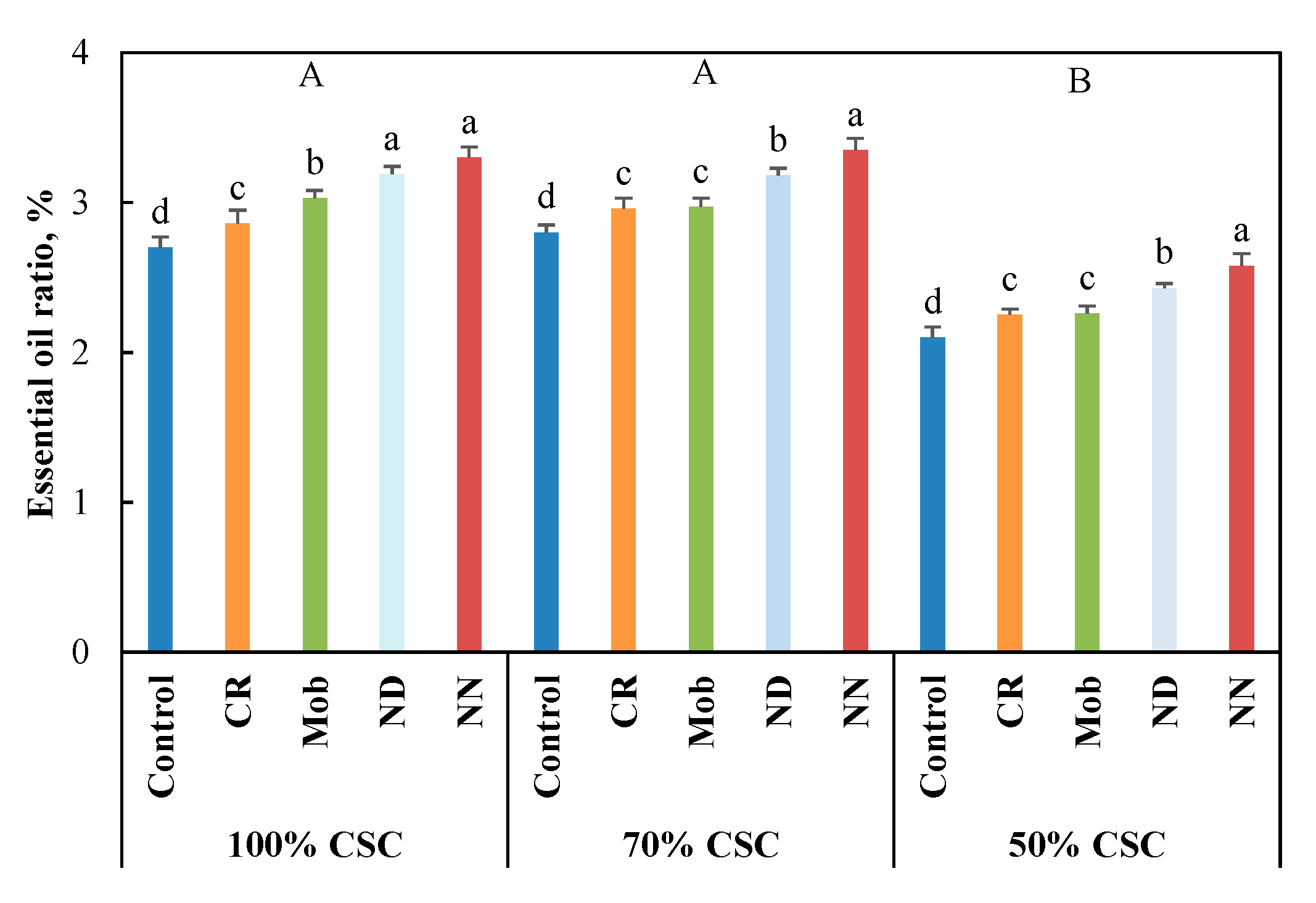
3.3. EO Ratio and Constitutes
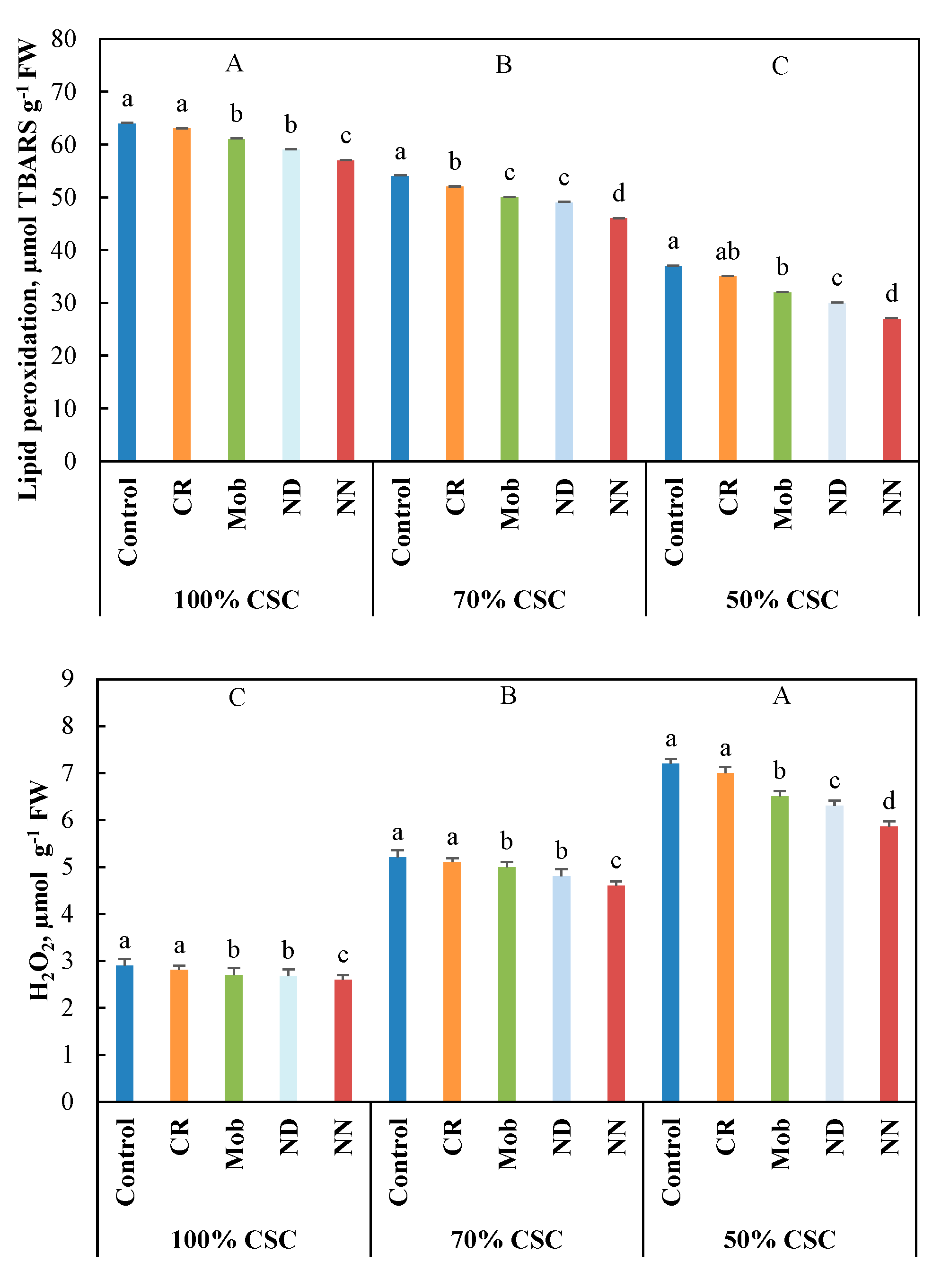
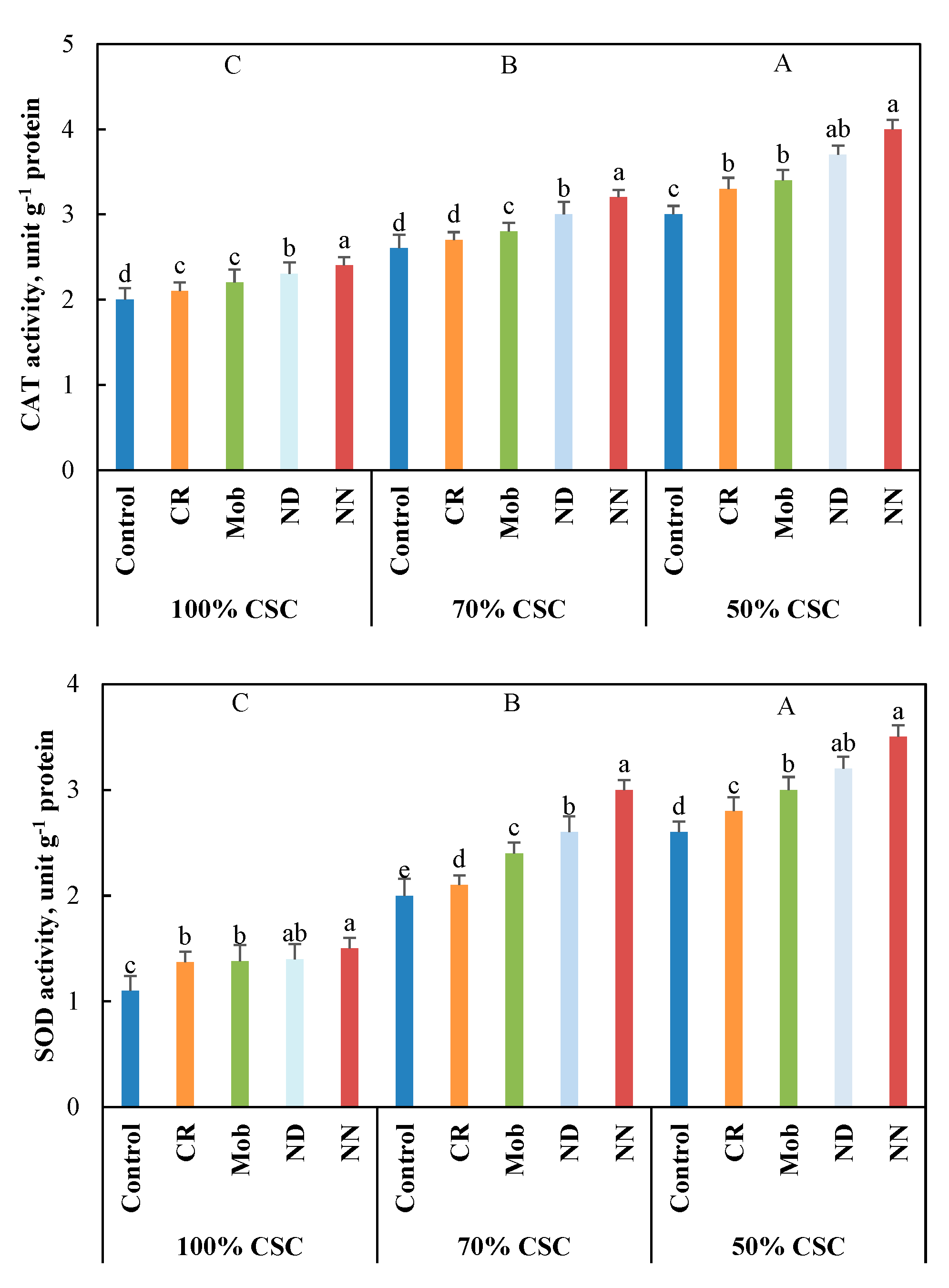
3.4. Antioxidant Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elansary, H.O.; Ashmawy, N.A. Essential oils of mint between benefits and hazards. J. Essent. Oil-Bear. Plants 2013, 16, 429–438. [Google Scholar] [CrossRef]
- Stringaro, A.; Vavala, E.; Colone, M.; Pepi, F.; Mignogna, G.; Garzoli, S.; Cecchetti, S.; Ragno, R.; Angiolella, L. Effects of Mentha suaveolens essential oil alone or in combination with other drugs in candida albicans. Evid. Based Complement. Alternat. Med. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid. Based Complement. Altern. Med. 2016, 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water scarcity and future challenges for food production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Zade, N.S.E.; Sadeghi, A.; Moradi, P. Streptomyces strains alleviate water stress and increase peppermint (Mentha piperita) yield and essential oils. Plant Soil 2019, 434, 441–452. [Google Scholar] [CrossRef]
- Figueroa-Pérez, M.G.; Rocha-Guzmán, N.E.; Pérez-Ramírez, I.F.; Mercado-Silva, E.; Reynoso-Camacho, R. Metabolite profile, antioxidant capacity, and inhibition of digestive enzymes in infusions of peppermint (Mentha piperita) grown under drought stress. J. Agric. Food Chem. 2014, 62, 12027–12033. [Google Scholar] [CrossRef]
- Ekren, S.; Sönmez, Ç.; Özçakal, E.; Kurttaş, Y.S.K.; Bayram, E.; Gürgülü, H. The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). Agric. Water Manag. 2012, 109, 155–161. [Google Scholar] [CrossRef]
- Shormin, T.; Khan, M.A.H.; Alamgir, M. Response of different levels of nitrogen fertilizer and water stress on the growth and yield of Japanese mint (Mentha arvensis L.). Bangladesh J. Sci. Ind. Res. 2009, 44, 137–145. [Google Scholar] [CrossRef]
- Farahani, H.A.; Valadabadi, S.A.; Daneshian, J.; Khalvati, M.A. Evaluation changing of essential oil of balm (Melissa officinalis L.) under water deficit stress conditions. J. Med. Plants Res. 2009, 3, 329–333. [Google Scholar]
- Khorasaninejad, S.; Mousavi, A.; Soltanloo, H.; Hemmati, K.; Khalighi, A. The effect of drought stress on growth parameters, essential oil yield and constituent of Peppermint (Mentha piperita L.). J. Med. Plant Res. 2011, 5, 5360–5365. [Google Scholar]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomila. Int. J. Agric. Biol. 2008, 10, 451–454. [Google Scholar]
- Elansary, H.O.; Yessoufou, K.; Shokralla, S.; Mahmoud, E.A.; Skalicka-Woźniak, K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind. Crops Prod. 2016, 92, 50–56. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.E.; Ali, H.M.; Elshikh, M.S. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef]
- Fonteno, W.; Hardin, C.; Brewster, J. Procedures for Determining Physical Properties of Horticultural Substrates Using the NCSU Porometer; Horticultural Substrates Laboratory, North Carolina State University: Raleigh, NC, USA, 1995. [Google Scholar]
- Howell, T.A. Challenges in increasing water use efficiency in irrigated agriculture. In Proceedings of the International Symposium on Water and Land Management for Sustainable Irrigated Agriculture, University of C¸ Ukurova, Adana, Turkey, 4–8 April 2006; pp. 53–63. [Google Scholar]
- Elansary, H.O.; Salem, M.Z.M. Morphological and physiological responses and drought resistance enhancement of ornamental shrubs by trinexapac-ethyl application. Sci. Hortic. 2015, 189, 1–11. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K. Growth regulators and mowing heights enhance the morphological and physiological performance of seaspray turfgrass during drought conditions. Acta Physiol. Plant. 2015, 37, 232. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed extracts enhance salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant Sci. 2017, 8, 830. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, New York, 8th ed.; The Iowa State University Press Publishing: Ames, IA, USA, 1989; p. 503. [Google Scholar]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef]
- Melo, R.O.d.; Oliveira, H.P.d.; Silveira, K.C.; Baldotto, L.E.B.; Baldotto, M.A. Initial performance of maize in response to humic acids and plant growth-promoting bacteria. Revista Ceres 2018, 65, 271–277. [Google Scholar] [CrossRef]
- Schoebitz, M.; López, M.D.; Serrí, H.; Martínez, O.; Zagal, E. Combined application of microbial consortium and humic substances to improve the growth performance of blueberry seedlings. J. Soil Sci. Plant Nutr. 2016, 16, 1010–1023. [Google Scholar] [CrossRef]
- Abdellatif, I.M.Y.; Abdel-Ati, Y.Y.; Abdel-Mageed, Y.T.; Hassan, M.A.M. Effect of humic acid on growth and productivity of tomato plants under heat stress. J. Hortic. Res. 2017, 25, 59–66. [Google Scholar] [CrossRef]
- Razaq, M.; Zhang, P.; Shen, H.; Salahuddin. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Godlewska, K.; Michalak, I.; Tuhy, Ł; Chojnacka, K. Plant growth biostimulants based on different methods of seaweed extraction with water. Biomed Res. Int 2016, 2016, 5973760. [Google Scholar] [CrossRef]
- Ekinci, M.; Yildirim, E.; Sahin, U. Changes in gas exchange capacity and selected physiological properties of squash seedlings (Cucurbita pepo L.) under well-watered and drought stress conditions AU—Ors, Selda. Arch. Agron. Soil Sci. 2016, 62, 1700–1710. [Google Scholar]
- Kusumi, K.; Hirotsuka, S.; Kumamaru, T.; Iba, K. Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J. Exp. Bot. 2012, 63, 5635–5644. [Google Scholar] [CrossRef]
- Argyrokastritis, I.G.; Papastylianou, P.T.; Alexandris, S. Leaf water potential and crop water stress index variation for full and deficit irrigated cotton in mediterranean conditions. Agric. Agric. Sci. Procedia 2015, 4, 463–470. [Google Scholar] [CrossRef]
- Elansary, H.O.; El-Ansary, D.O. Trinexapac-ethyl application enhanced physiological performance of Prunus persica (L.) Batsch under drought conditions. Bangladesh J. Bot. 2017, 46, 1367–1373. [Google Scholar]
- Elansary, H.O. Basil morphological and physiological performance under trinexapac-ethyl foliar sprays and prolonged irrigation intervals. Acta Physiol. Plant. 2015, 37, 92. [Google Scholar] [CrossRef]
- Myburg, A.A.; Grattapaglia, D.; Tuskan, G.A.; Hellsten, U.; Hayes, R.D.; Grimwood, J.; Jenkins, J.; Lindquist, E.; Tice, H.; Bauer, D.; et al. The genome of Eucalyptus grandis. Nature 2014, 510, 356. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Seo, H.J.; Min, S.S.; Park, M.; Seol, G.H. The effect of 1,8-cineole inhalation on preoperative anxiety: A randomized clinical trial. J. Evid. Based Complement. Altern. Med. 2014, 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-bdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. J. Biomed Res. Int. 2013, 2013, 9. [Google Scholar]
| Variable | Leaf Number (plant−1) | Leaf Area (cm2 plant−1) | Plant Height (cm) | Root Dry Weight (g) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Levels/Biosti. | Control | CR | Mob | ND | NN | Means | Control | CR | Mob | ND | NN | Means | Control | CR | Mob | ND | NN | Means | Control | CR | Mob | ND | NN | Means |
| 100% CSC | 57.00 e | 62.10 d | 67.20 c | 72.30 b | 77.20 a | 67.16 A | 228.00 e | 247.10 d | 268.80 c | 288.20 b | 307.80 a | 267.98 A | 27.60 e | 29.10 d | 30.20 c | 32.10 b | 35.30 a | 30.86 A | 1.05 e | 1.13 d | 1.19 c | 1.29 b | 1.38 a | 1.21 A |
| 70% CSC | 47.00 e | 51.20 d | 53.10 c | 56.80 b | 59.90 a | 53.60 B | 178.60 e | 193.56 d | 200.78 c | 216.80 b | 228.62 a | 203.67 B | 23.10 e | 24.40 d | 25.70 c | 26.90 b | 27.50 a | 25.52 B | 0.82 d | 1.01 c | 1.04 c | 1.12 b | 1.21 a | 1.02 B |
| 50% CSC | 29.00 d | 34.80 c | 35.90 c | 39.30 b | 43.10 a | 36.42 C | 101.50 d | 121.20 c | 125.65 c | 137.55 b | 151.85 a | 127.55 C | 16.20 d | 17.30 c | 17.80 c | 18.90 b | 20.30 a | 18.10 C | 0.56 d | 0.59c | 0.60 c | 0.64 b | 0.67 a | 0.61 C |
| Means | 44.33 E | 49.37 D | 52.07 C | 56.13 B | 60.07 A | 169.37 E | 187.29 D | 198.41 C | 214.18 B | 229.42 A | 22.30 E | 23.60 D | 24.57 C | 25.97 B | 27.70 A | 0.81 E | 0.91 D | 0.94 C | 1.02 B | 1.06 A | ||||
| Water levels Biosti. Water levels × Biosti. | *** (LSD0.05 = 0.877) *** (LSD0.05 = 1.132) *** (LSD0.05 = 1.961) | *** (LSD0.05 = 2.502) *** (LSD0.05 = 3.230) *** (LSD0.05 = 5.594) | *** (LSD0.05 = 0.282) *** (LSD0.05 = 0.365) *** (LSD0.05 = 0.631) | *** (LSD0.05 = 0.010) *** (LSD0.05 = 0.013) *** (LSD0.05 = 0.022) | ||||||||||||||||||||
| Variable | Water Applied (mm) | Plant Fresh Weight (g) | Plant Dry Weight (g) | IWUE (kg m™3) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Levels/Biosti. | Control | CR | Mob | ND | NN | Means | Control | CR | Mob | ND | NN | Means | Control | CR | Mob | ND | NN | Means | |
| 100% CSC | 35.91 | 7.38 d | 8.01 c | 8.48 b | 8.47 b | 9.24 a | 8.32 A | 6.64 e | 7.05 d | 7.38 c | 7.79 b | 8.41 a | 7.45 A | 1.85 d | 2.01 c | 2.13 b | 2.12 b | 2.32 a | 2.08 B |
| 70% CSC | 27.00 | 6.02 d | 7.53 c | 7.39 c | 7.81 b | 8.34 a | 7.42 B | 5.12 d | 6.48 c | 6.50 c | 6.87 b | 7.42 a | 6.48 B | 2.01 d | 2.51 c | 2.46 c | 2.60 b | 2.78 a | 2.47 A |
| 50% CSC | 22.14 | 4.69 b | 4.71 b | 4.83 b | 5.18 a | 5.31 a | 4.94 C | 3.75 d | 3.96 c | 4.01 c | 4.25 b | 4.46 a | 4.09 C | 1.91 b | 1.92 b | 1.96 b | 2.11 a | 2.16 a | 2.01 C |
| Means | 6.03 E | 6.75 D | 6.90 C | 7.15 B | 7.63 A | 5.17 E | 5.83 D | 5.96 C | 6.31 B | 6.76 A | 1.92 E | 2.15 D | 2.18 C | 2.28 B | 2.42 A | ||||
| Water levels Biosti. Water levels ×Biosti. | *** (LSD0.05 = 0.086) *** (LSD0.05 = 0.111) *** (LSD0.05 = 0.193) | *** (LSD0.05 = 0.075) *** (LSD0.05 = 0.097) *** (LSD0.05 = 0.168) | *** (LSD0.05 = 0.028) *** (LSD0.05 = 0.036) *** (LSD0.05 = 0.062) | ||||||||||||||||
| Variable | 1-Menthone (%) | Isopulegone (%) | Pulegone (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Levels/Biosti. | Control | CR | Mob | ND | NN | Means | Control | CR | Mob | ND | NN | Means | Control | CR | Mob | ND | NN | Means |
| 100% CSC | 2.20 a | 2.10 c | 1.90 c | 1.70 d | 1.50 e | 1.88 A | 5.60 a | 5.50 a,b | 5.40 b | 5.20 c | 4.90 d | 5.32 A | 56.46 a | 54.27 b | 53.51 b | 50.32 c | 46.93 d | 52.30 A |
| 70% CSC | 2.10 a | 2.00 b | 1.80 c | 1.60 d | 1.40 e | 1.78 B | 5.40 a | 5.30 b | 5.20 c | 5.00 d | 4.30 e | 5.04 B | 53.34 a | 50.12 b | 49.68 b | 47.08 c | 43.22 d | 48.69 B |
| 50% CSC | 2.00 a | 2.00 a | 1.70 b | 1.50 c | 1.30 d | 1.70 C | 5.30 a | 5.10 b | 5.00 b | 4.80 c | 4.10 d | 4.86 C | 52.13 a | 49.52 b | 48.31 c | 46.52 d | 42.71 e | 47.84 C |
| Means | 2.10 A | 2.04 B | 1.80 C | 1.60 D | 1.40 E | 5.44 A | 5.30 B | 5.20 C | 5.00 D | 4.43 E | 53.98 A | 51.30 B | 50.50 C | 47.98 D | 44.29 E | |||
| Water levels Biosti. Water levels × Biosti. | *** (LSD0.05 = 0.016) *** (LSD0.05 = 0.021) *** (LSD0.05 = 0.036) | *** (LSD0.05 = 0.046) *** (LSD0.05 = 0.059) *** (LSD0.05 = 0.102) | *** (LSD0.05 = 0.545) *** (LSD0.05 = 0.703) ns | |||||||||||||||
| Variable | α-Pinene (%) | 1,8-Cineol (%) | α- Terpineol (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Levels/Biosti. | Control | CR | Mob | ND | NN | Means | Control | CR | Mob | ND | NN | Means | Control | CR | Mob | ND | NN | Means |
| 100% CSC | 0.90 e | 1.80 d | 3.40 c | 3.60 b | 4.00 a | 2.74 C | 24.28 c | 25.09 c | 25.20 c | 27.31 b | 29.11 a | 26.20 A | 1.70 e | 1.90 d | 2.30 c | 2.50 b | 2.70 a | 2.22 C |
| 70% CSC | 1.50 e | 2.50 d | 3.80 b | 3.70 c | 4.20 a | 3.14 B | 25.62 c | 26.62 c | 26.70 c | 28.61 b | 35.71 a | 28.65 B | 1.90 e | 2.10 d | 2.40 c | 2.70 b | 3.10 a | 2.44 B |
| 50% CSC | 2.10 e | 3.10 d | 3.70 c | 4.10 b | 4.30 a | 3.46 A | 26.82 c | 27.79 c | 27.92 c | 29.82 b | 36.11 a | 29.70 C | 2.10 e | 2.30 d | 2.50 c | 3.00 b | 3.40 a | 2.66 A |
| Means | 1.50 E | 2.47 D | 3.63 C | 3.80 B | 4.17 A | 25.57 D | 26.50 C | 26.61 C | 28.58 B | 33.64 A | 1.90 E | 2.10 D | 2.40 C | 2.73 B | 3.07 A | |||
| Water levels Biosti. Water levels × Biosti. | *** (LSD0.05 = 0.047) *** (LSD0.05 = 0.061) *** (LSD0.05 = 0.105) | *** (LSD0.05 = 0.544) *** (LSD0.05 = 0.702) *** (LSD0.05 = 1.216) | *** (LSD0.05 = 0.018) *** (LSD0.05 = 0.024) *** (LSD0.05 = 0.041) | |||||||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O. Elansary, H.; Mahmoud, E.A.; El-Ansary, D.O.; Mattar, M.A. Effects of Water Stress and Modern Biostimulants on Growth and Quality Characteristics of Mint. Agronomy 2020, 10, 6. https://doi.org/10.3390/agronomy10010006
O. Elansary H, Mahmoud EA, El-Ansary DO, Mattar MA. Effects of Water Stress and Modern Biostimulants on Growth and Quality Characteristics of Mint. Agronomy. 2020; 10(1):6. https://doi.org/10.3390/agronomy10010006
Chicago/Turabian StyleO. Elansary, Hosam, Eman A. Mahmoud, Diaa O. El-Ansary, and Mohamed A. Mattar. 2020. "Effects of Water Stress and Modern Biostimulants on Growth and Quality Characteristics of Mint" Agronomy 10, no. 1: 6. https://doi.org/10.3390/agronomy10010006
APA StyleO. Elansary, H., Mahmoud, E. A., El-Ansary, D. O., & Mattar, M. A. (2020). Effects of Water Stress and Modern Biostimulants on Growth and Quality Characteristics of Mint. Agronomy, 10(1), 6. https://doi.org/10.3390/agronomy10010006






