Phytochemical Constituents and Antioxidant Enzyme Activity Profiles of Different Barley (Hordeum Vulgare L.) Cultivars at Different Developmental Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chlorophyll and Carotenoid Content in Barley Grass
2.3. Analysis of Total Nitrogen Nutrition in Barley Grass
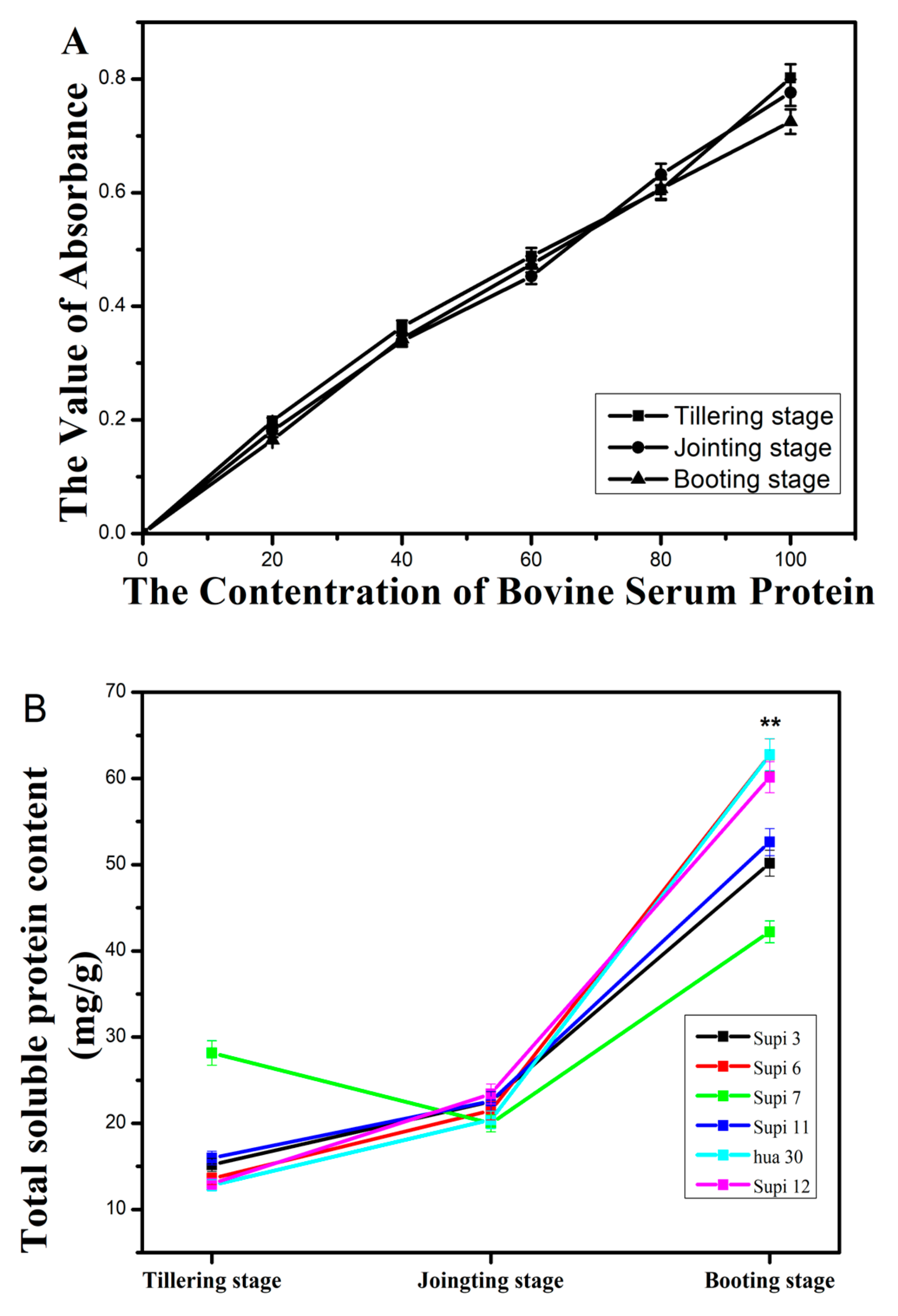
2.4. Measurement of Soluble Protein in Barley Grass
2.5. Determination of Superoxide Dismutase (SOD) Activity
2.6. Determination of Peroxidase (POD) Activity
2.7. Determination of Polyphenol Oxidase (PPO) Activity
2.8. Statistical Analyses
3. Results
3.1. Total Chlorophyll and Carotenoid Content in Barley Grass
3.2. Chlorophyll a/b Ratio in Barley Grass
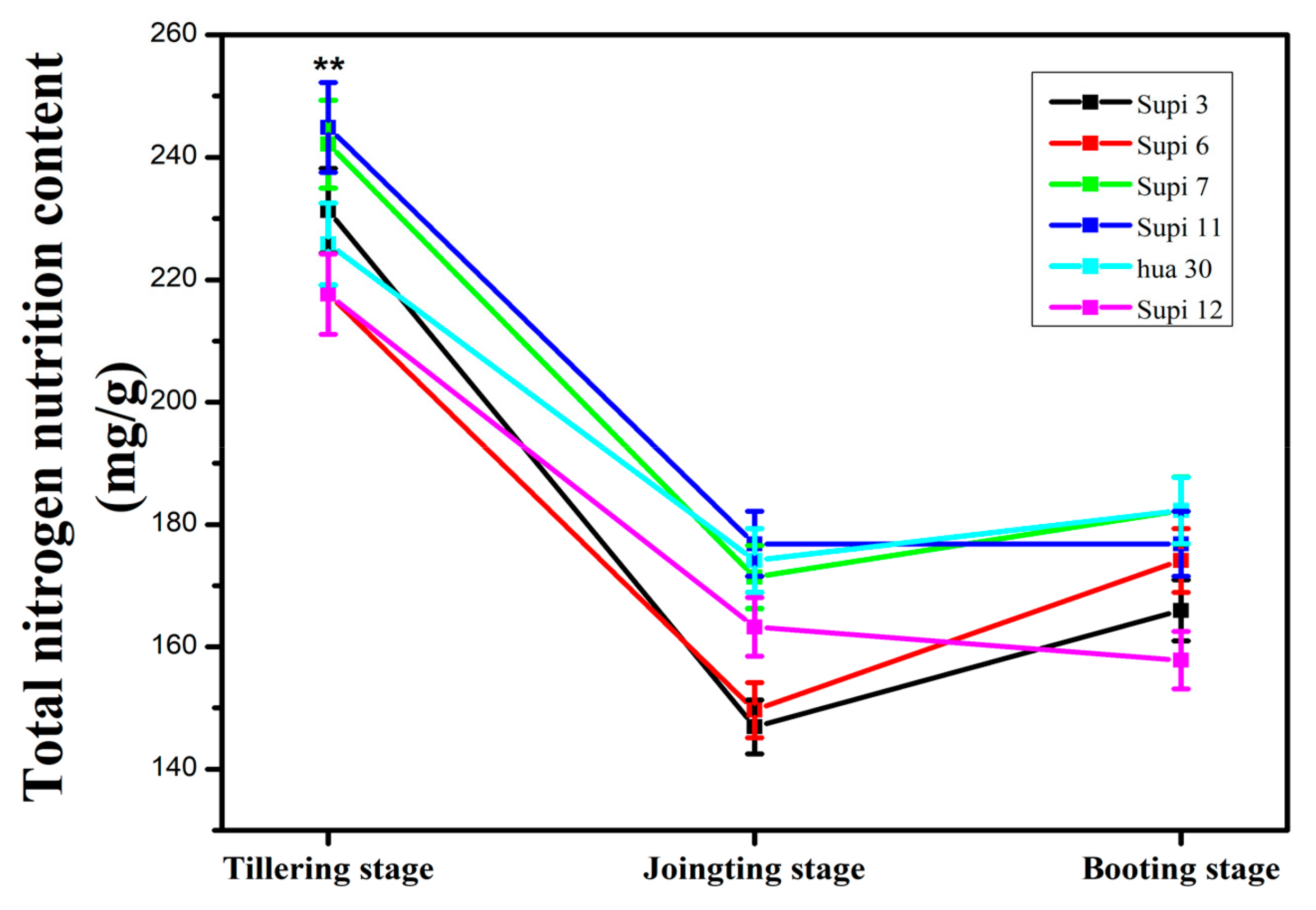
3.3. Total Nitrogen Nutrition (TNN) and Total Soluble Protein (TSP) Content in Barley Grass
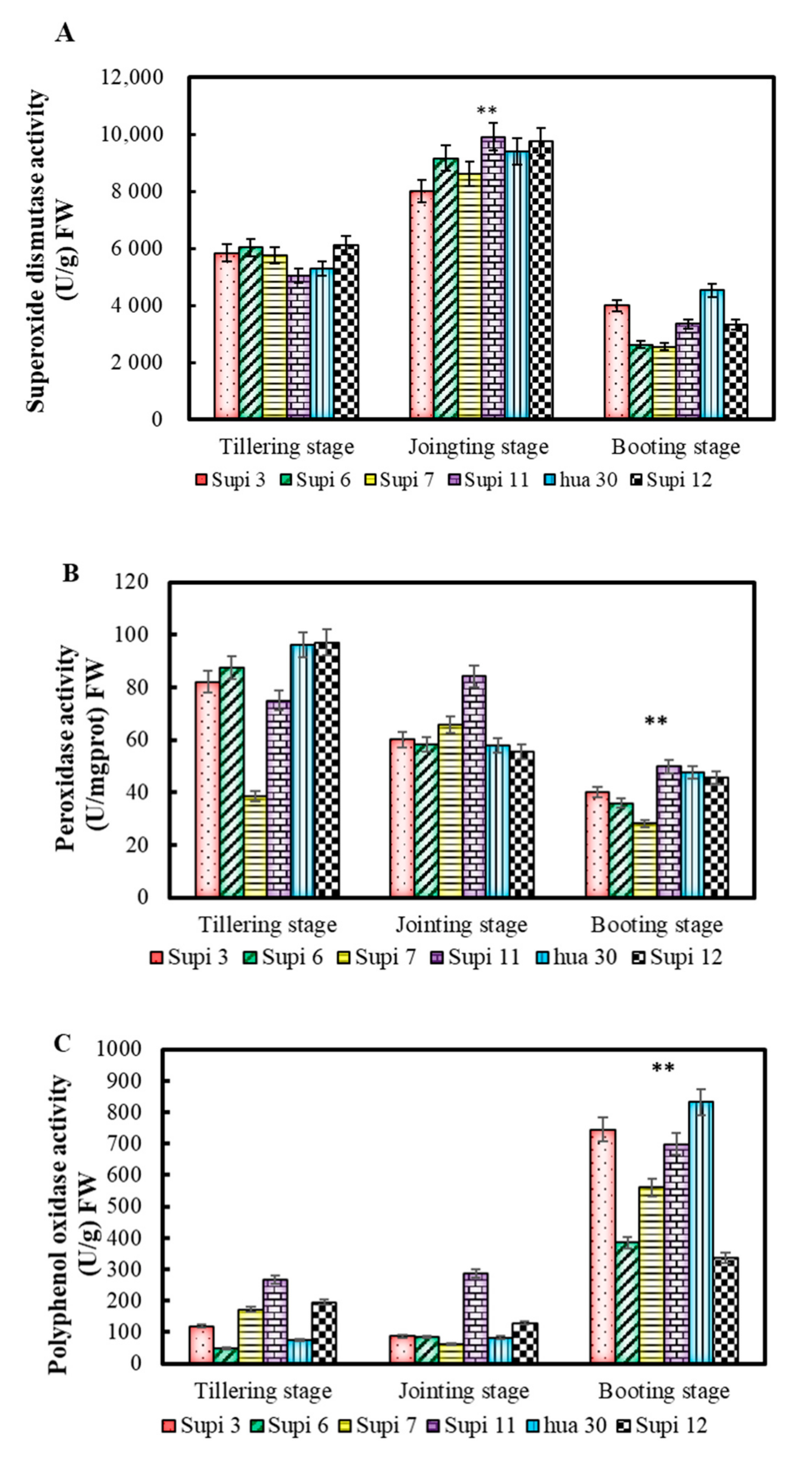
3.4. Antioxidant Enzyme Activities in Barley Grass
3.5. Correlations Among the Quality Parameters
3.6. Principal Component Analysis (PCA) for the Cultivar × Development Interactions for Quality Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thatiparthi, J.; Dodoala, S.; Koganti, B.; Kvsrg, P. Barley grass juice (Hordeum vulgare L.) inhibits obesity and improves lipid profile in high fat diet-induced rat model. J. Ethnopharmacol. 2019, 238, 111843–111855. [Google Scholar] [CrossRef] [PubMed]
- Niroula, A.; Khatri, S.; Timilsina, R.; Khadka, D.; Khadka, A.; Ojha, P. Profile of chlorophylls and carotenoids of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) microgreens. J. Food Sci. Technol. 2019, 56, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Pu, X.; Yang, J.; Du, J.; Yang, X.; Li, X.; Li, L.; Zhou, Y.; Yang, T. Preventive and therapeutic role of functional ingredients of barley grass for chronic diseases in human beings. Oxid. Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lahouar, L.; El-Bok, S.; Achour, L. Therapeutic potential of young green barley leaves in prevention and treatment of chronic diseases: An overview. Am. J. Chin. Med. 2015, 43, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, T.; Fu, X.; Abbasi, A.M.; Zheng, B.S.; Liu, R.H. Phenolics content, antioxidant and antiproliferative activities of dehulled highland barley (Hordeum vulgare L.). J. Funct. Foods 2015, 19, 439–450. [Google Scholar] [CrossRef]
- Filimon, R.V.; Rotaru, L.; Filimon, R.M. Quantitative investigation of leaf photosynthetic pigments during annual biological cycle of Vitis vinifera L. table grape cultivars. S. Afr. J. Enol. Vitic. 2016, 37, 1–14. [Google Scholar] [CrossRef][Green Version]
- Samanta, T.; Kotamreddy, J.N.R.; Ghosh, B.C.; Mitra, A. Changes in targeted metabolites, enzyme activities and transcripts at different developmental stages of tea leaves: A study for understanding the biochemical basis of tea shoot plucking. Acta. Physiol. Plant. 2017, 39, 11–37. [Google Scholar] [CrossRef]
- Özköse, A.; Arslan, D.; Aysenur, A.C.A.R. The comparison of the chemical composition, sensory, phenolic and antioxidant properties of juices from different, wheatgrass and turfgrass species. Not. Bot. Horti Agrobot. 2016, 44, 499–507. [Google Scholar] [CrossRef]
- Kulkarni, S.D.; Tilak, J.C.; Acharya, R.; Rajurkar, N.S.; Devasagayam, T.P.A.; Reddy, A.V.R. Evaluation of the antioxidant activity of wheatgrass (Triticum aestivum L.) as a function of growth under different conditions. Phytother. Res. 2006, 20, 218–227. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids—Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Anantakrishnan, S.V.; Pai, K.V.S. The kjeldahl method of nitrogen determination. Proc. Indian Acad. Sci. Sect. A 1952, 36, 299. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid and chlorophyll pigments in sweet basil grown in the field and greenhouse. Hortscience 2005, 40, 1230–1233. [Google Scholar] [CrossRef]
- Paznochta, L.; Kotíková, Z.; Šulc, M.; Lachman, J.; Orsák, M.; Eliášová, M.; Martinek, P. Free and esterified carotenoids in pigmented wheat, tritordeum and barley grains. Food Chem. 2018, 240, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Ndukwe, O.K.; Edeoga, H.O.; Okwulehie, I.C.; Omosun, G. Variability in the chlorophyll and carotene composition of ten maize (Zea mays) varieties. Eur. J. Pharm. Sci. 2016, 4, 1–6. [Google Scholar]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef]
- Yang, C.M.; Lee, C.N.; Chou, C.H. Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: I. Inhibition of supply-orientation. Bot. Bull. Acad. Sin. 2002, 43, 299–324. [Google Scholar]
- Okpalanma, F.E.; Ojimelukwe, P.C. Evaluation of effects of storage condition and processing on carotenoids, chlorophyll, vitamins and minerals in a water leaf (Talinum triangulare). J. Sci. Food Agric. 2018, 2, 41603–41617. [Google Scholar] [CrossRef]
- Tanner, G.J.; Colgrave, M.L.; Blundell, M.J.; Howitt, C.A.; Bacic, A. Hordein accumulation in developing barley grains. Front. Plant Sci. 2019, 10, 649. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein Determination—Method Matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Loka, D.A.; Fitzsimons, T.R.; Zhou, Z.G.; Oosterhuis, D.M. Potassium deficiency limits reproductive success by altering carbohydrate and protein balances in cotton (Gossypium hirsutum L.). Environ. Exp. Bot. 2018, 145, 87–94. [Google Scholar] [CrossRef]
- Sun, Y.T.; Li, M.; Mitra, S.; Muhammad, R.H.; Debnath, B.; Lu, X.C.; Jian, H.X.; Qiu, D.L. Comparative phytochemical profiles and antioxidant enzyme activity analyses of the southern highbush blueberry (Vaccinium corymbosum) at different developmental stages. Molecules 2018, 23, 2209. [Google Scholar] [CrossRef] [PubMed]
- Jariteh, M.; Ebrahimzadeh, H.; Niknam, V.; Mirmasoumi, M.; Vahdati, K. Developmental changes of protein, proline and some antioxidant enzymes activities in somatic and zygotic embryos of Persian walnut (Juglans regia L.). Plant Cell Tissue Organ Cult. 2015, 122, 101–115. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, X.; Jin, M.; Jiao, L.; Sun, P.; Betancor, M.B.; Tocher, D.R.; Zhou, Q. Modification of nutritional values and flavor qualities of muscle of swimming crab (Portunus trituberculatus): Application of a dietary lipid nutrition strategy. Food Chem. 2020, 308, 125607–125618. [Google Scholar] [CrossRef] [PubMed]

| Growth Stages | Chl t Content (Mg/100 g FW) | |||||
|---|---|---|---|---|---|---|
| Supi3 | Supi6 | Supi7 | Supi11 | Hua30 | Supi12 | |
| Tillering stage | 79.84 ± 2.01 | 55.71 ± 2.41 | 20.39 ± 3.76 | 61.98 ± 2.84 | 54.71 ± 3.25 | 67.46 ± 1.67 |
| Jointing stage | 17.58 ± 5.79 | 11.01 ± 4.87 | 13.04 ± 0.03 | 11.75 ± 0.75 | 11.09 ± 0.63 | 15.94 ± 4.21 |
| Booting stage | 32.72 ± 1.05 | 23.94 ± 1.22 | 13.18 ± 0.22 | 44.29 ± 5.20 | 31.78 ± 1.76 | 46.41 ± 1.98 |
| Growth Stages | Car Content (Mg/100 g FW) | |||||
|---|---|---|---|---|---|---|
| Supi3 | Supi6 | Supi7 | Supi11 | Hua30 | Supi12 | |
| Tillering stage | 11.98 ± 0.40 | 10.50 ± 1.78 | 4.74 ± 0.79 | 14.10 ± 0.83 | 10.09 ± 2.40 | 13.50 ± 2.59 |
| Jointing stage | 2.70 ± 0.26 | 2.13 ± 0.10 | 1.30 ± 0.55 | 2.87 ± 0.68 | 3.45 ± 0.19 | 4.90 ± 0.78 |
| Booting stage | 9.11 ± 0.32 | 8.03 ± 0.29 | 5.72 ± 0.53 | 11.57 ± 0.36 | 7.92 ± 0.18 | 10.68 ± 2.84 |
| Growth Stages | Chl a/b Ratio | |||||
|---|---|---|---|---|---|---|
| Supi3 | Supi6 | Supi7 | Supi11 | Hua30 | Supi12 | |
| Tillering stage | 2.82 ± 0.04 | 2.78 ± 0.06 | 1.75 ± 0.15 | 2.67 ± 0.14 | 2.44 ± 0.31 | 2.61 ± 0.13 |
| Jointing stage | 0.95 ± 0.05 | 0.77 ± 0.27 | 0.68 ± 0.18 | 1.28 ± 0.65 | 1.44 ± 0.33 | 2.02 ± 0.39 |
| Booting stage | 3.41 ± 0.65 | 2.93 ± 0.25 | 2.47 ± 0.81 | 2.77 ± 0.25 | 2.95 ± 0.42 | 3.05 ± 0.28 |
| Items | Chl t | Car | Chl a/b | TNN | TSP | SOD | POD | PPO |
|---|---|---|---|---|---|---|---|---|
| Chl t | 1.00 | |||||||
| Car | 0.91 ** | 1.00 | ||||||
| Chl a/b | 0.63 ** | 0.83 ** | 1.00 | |||||
| TNN | 0.66 ** | 0.57 ** | 0.32 | 1.00 | ||||
| TSP | 0.20 n | 0.09 | 0.49 * | 0.43 n,* | 1.00 | |||
| SOD | 0.40 n,* | 0.63 n,** | 0.80 n,** | 0.22 n | 0.65 n,** | 1.00 | ||
| POD | 0.56 ** | 0.30 | 0.11 n | 0.46 * | 0.76 n,** | 0.40 * | 1.00 | |
| PPO | 0.29 n | 0.26 | 0.58 ** | 0.20 n | 0.81 ** | 0.64 n,** | 0.55 n,** | 1.00 |
| Items | Chl t | Car | Chl a/b | TNN | TSP | SOD | POD | PPO |
|---|---|---|---|---|---|---|---|---|
| Cultivar | 0.056 | 0.033 | 0.352 | 0.586 | 0.999 | 0.989 | 0.169 | 0.408 |
| Developmental stage (D) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| C × D | <0.001 | <0.001 | 0.032 | 0.041 | <0.001 | 0.007 | <0.001 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.-N.; Feng, G.-N.; Gao, Y.; Shen, Y.-X.; Li, H.-S.; Gu, Y.; Luan, H.-Y. Phytochemical Constituents and Antioxidant Enzyme Activity Profiles of Different Barley (Hordeum Vulgare L.) Cultivars at Different Developmental Stages. Agronomy 2020, 10, 37. https://doi.org/10.3390/agronomy10010037
Deng L-N, Feng G-N, Gao Y, Shen Y-X, Li H-S, Gu Y, Luan H-Y. Phytochemical Constituents and Antioxidant Enzyme Activity Profiles of Different Barley (Hordeum Vulgare L.) Cultivars at Different Developmental Stages. Agronomy. 2020; 10(1):37. https://doi.org/10.3390/agronomy10010037
Chicago/Turabian StyleDeng, Li-Na, Gong-Neng Feng, Yue Gao, Yu-Xiang Shen, Hong-Shan Li, Yue Gu, and Hai-Ye Luan. 2020. "Phytochemical Constituents and Antioxidant Enzyme Activity Profiles of Different Barley (Hordeum Vulgare L.) Cultivars at Different Developmental Stages" Agronomy 10, no. 1: 37. https://doi.org/10.3390/agronomy10010037
APA StyleDeng, L.-N., Feng, G.-N., Gao, Y., Shen, Y.-X., Li, H.-S., Gu, Y., & Luan, H.-Y. (2020). Phytochemical Constituents and Antioxidant Enzyme Activity Profiles of Different Barley (Hordeum Vulgare L.) Cultivars at Different Developmental Stages. Agronomy, 10(1), 37. https://doi.org/10.3390/agronomy10010037




