A Two-Year Simulated Crop Rotation Confirmed the Differential Infestation of Broomrape Species in China Is Associated with Crop-Based Biostimulants
Abstract
1. Introduction
2. Materials and Methods
2.1. Pot Experiment
2.2. Germination Stimulation of Sunflower and Egyptian Broomrape Seeds with Methanolic Shoot and Root Extracts from the Crops
2.3. Hydroponic Culture and Root Exudates Collection
2.4. Data Processing
2.5. Crops Used in Rotation and their Varietal Recognition
3. Results
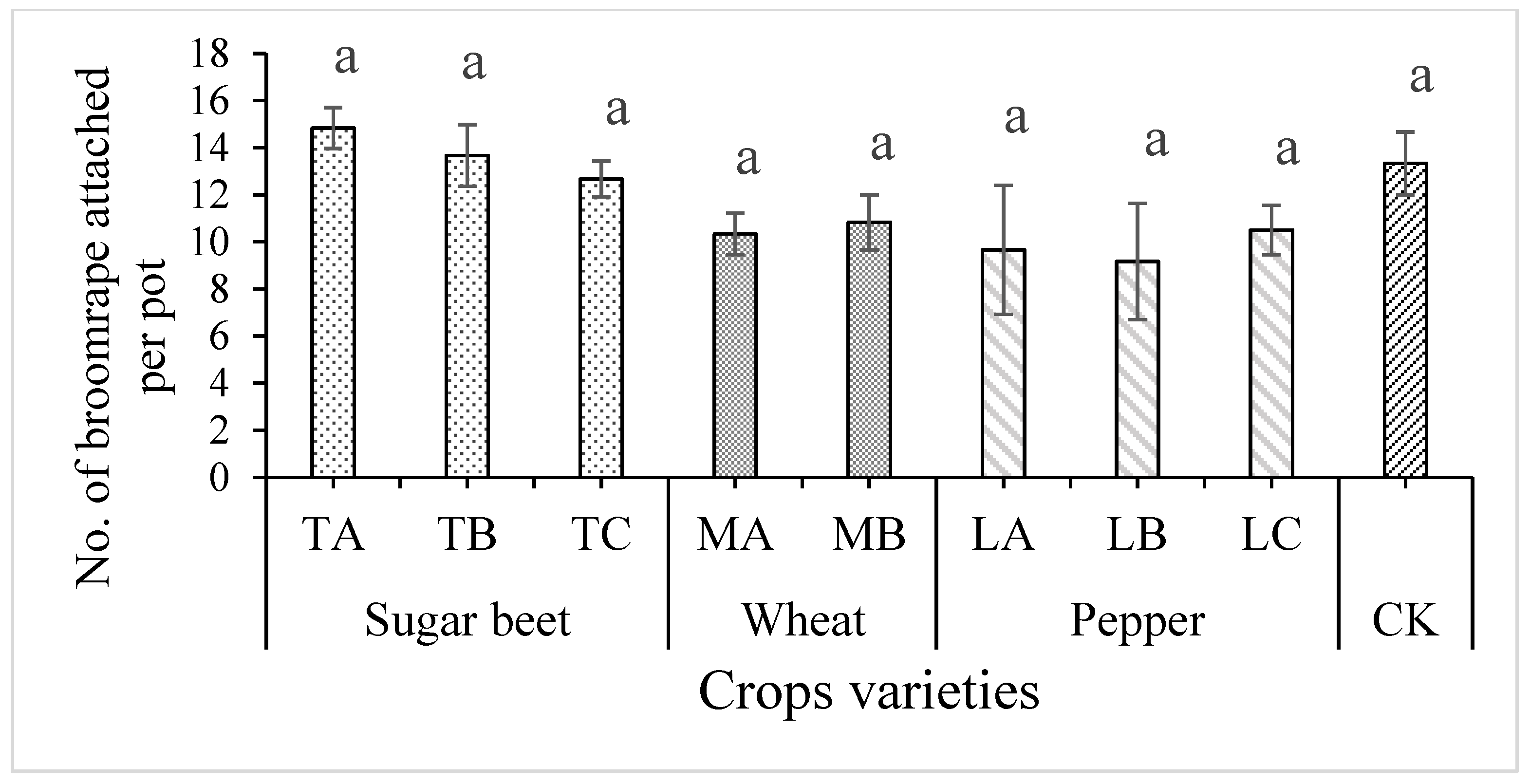
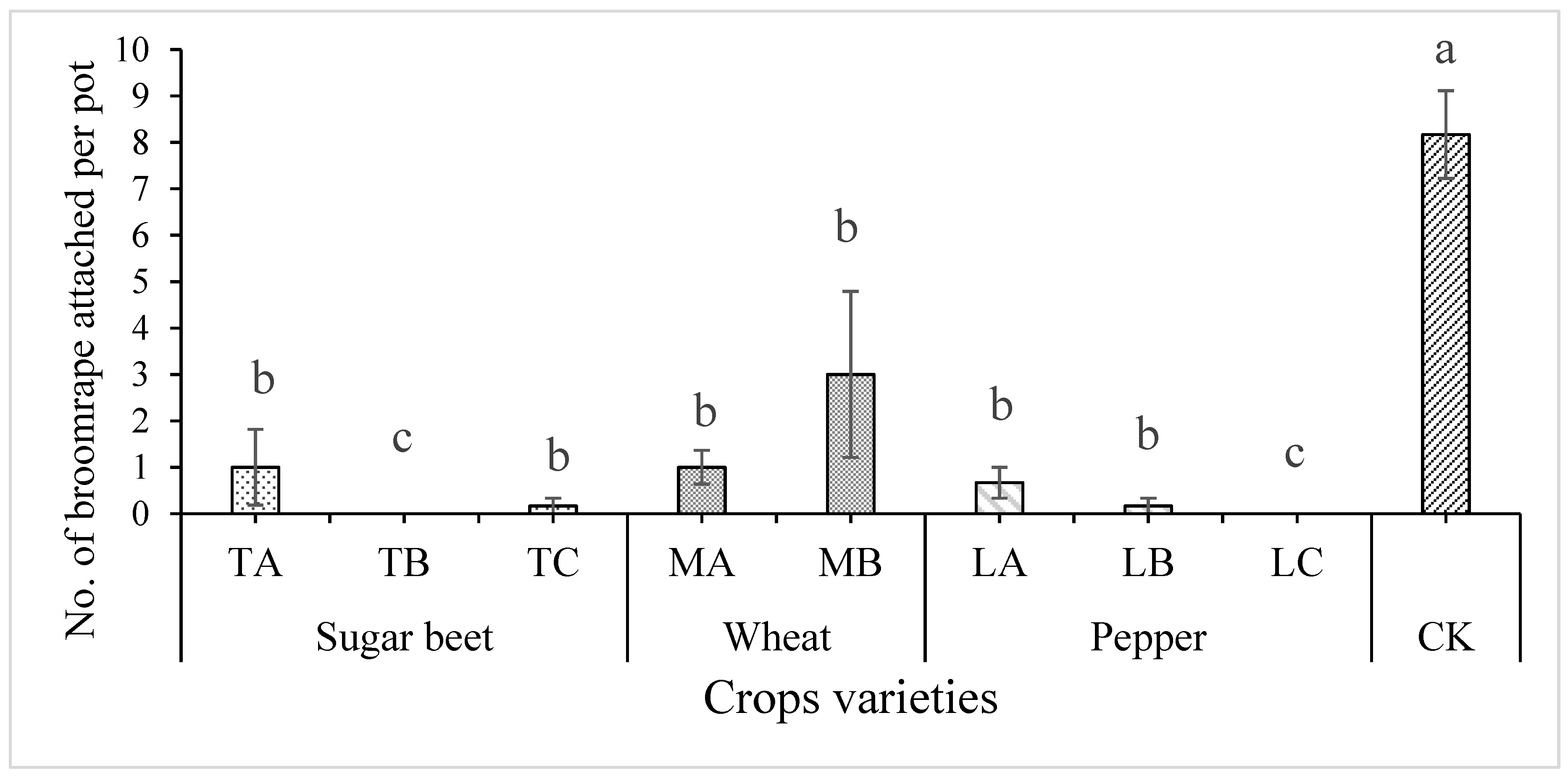
3.1. Effect of Crop Rotation on the Control of Egyptian Broomrape, and Plant Growth of Tomato Plants Grown in Pot Experiment
3.2. Effect of Crop Rotation on the Egyptian Broomrape Parasitism on Tomato Plants
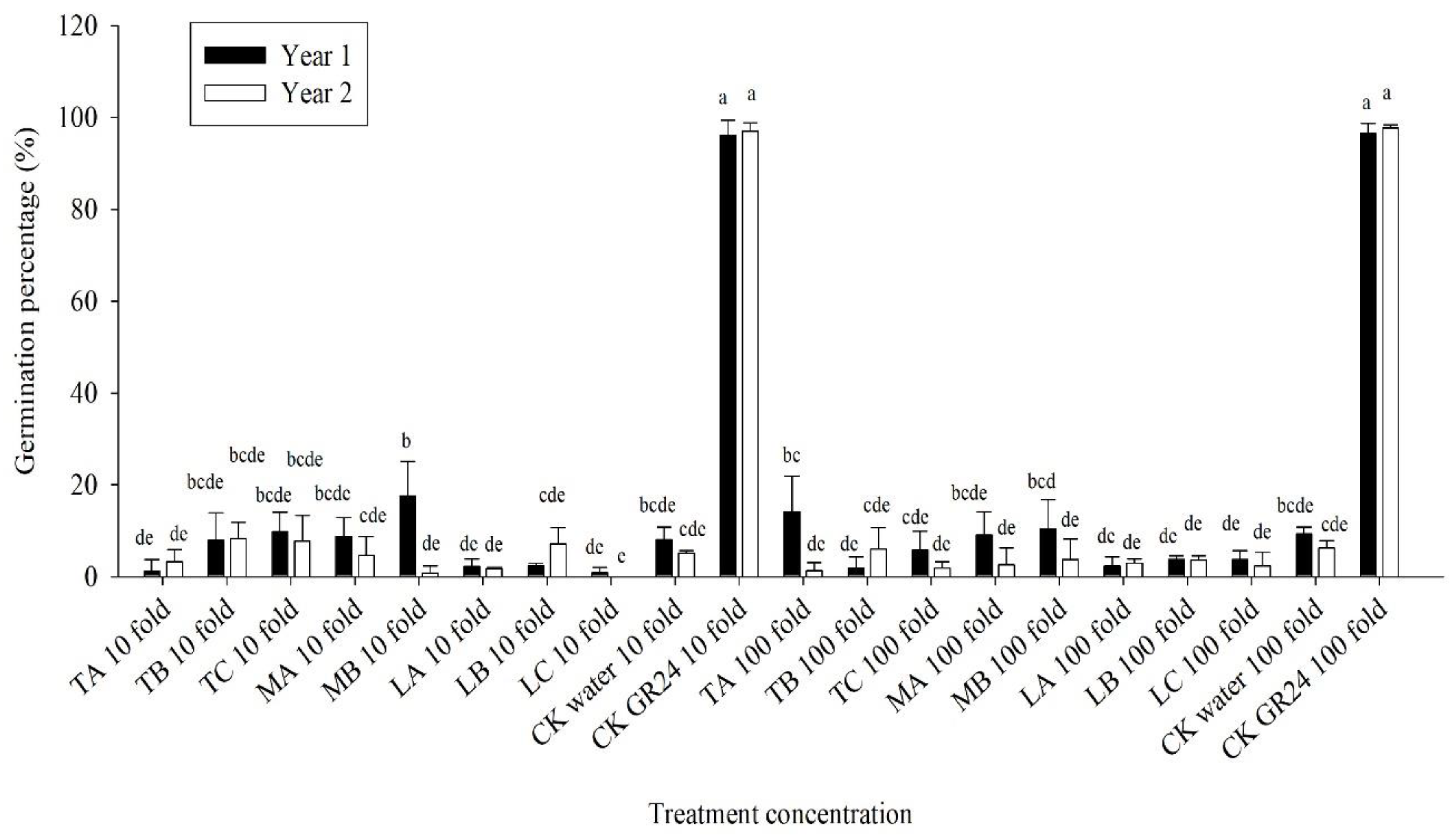
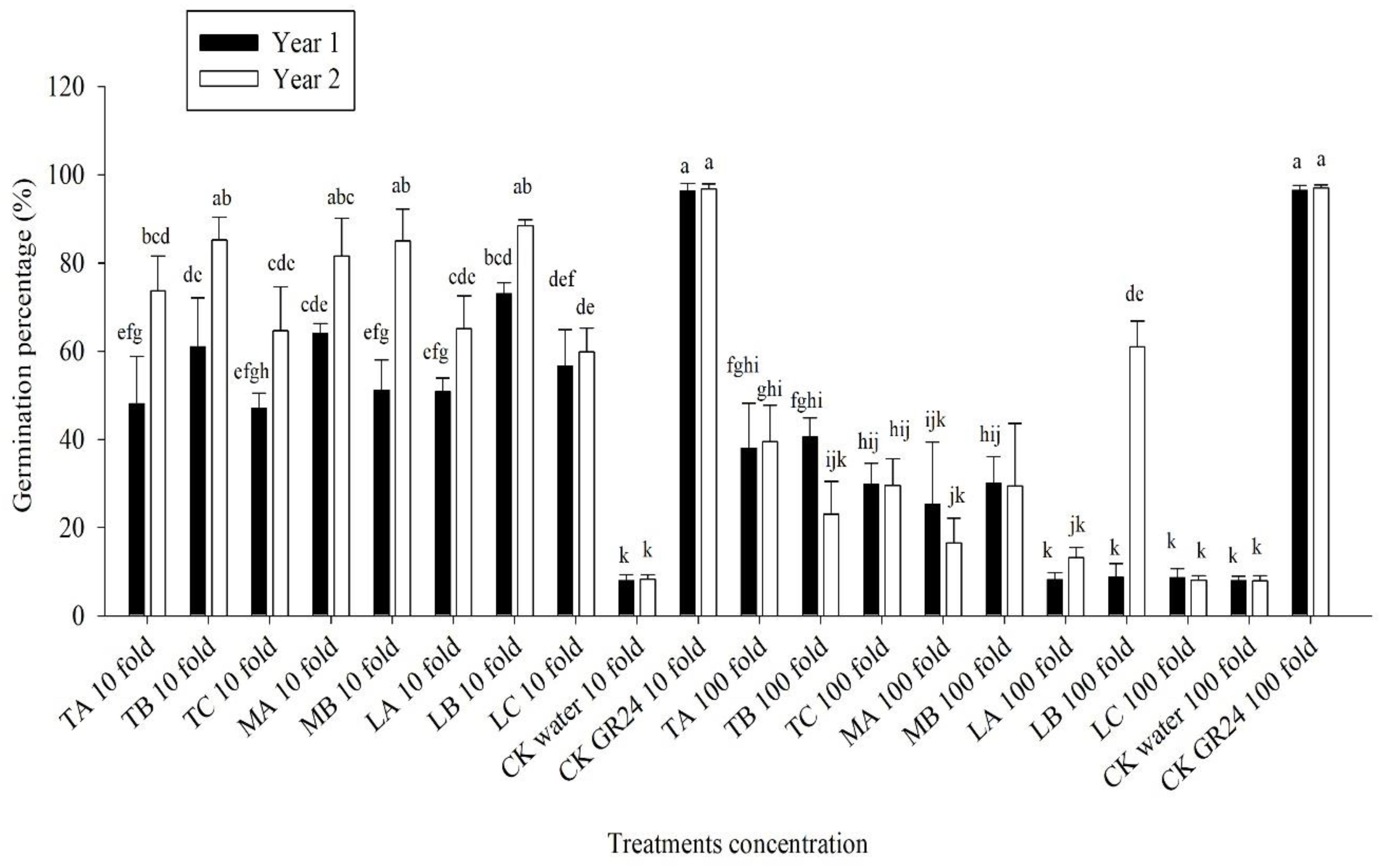
3.3. Effect of Methanolic Shoot Extracts of Various Crops on Broomrape Seed Germination
3.4. Effect of Methanolic Roots Extracts from the Crops on the Germination of Broomrape Seeds
3.5. Broomrape Seeds Germination under the Influence of Roots Exudates Collected from Hydroponic Experiments
4. Discussion
4.1. Differential Response of Broomrape Species Indicates the Allelopathic Diversity of the Crops Biostimulants
4.2. The Differential Germination Indicates a Genetic and Biochemical Evolution in the Broomrape Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parker, C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef]
- De Zélicourt, A.; Montiel, G.; Pouvreau, J.B.; Thoiron, S.; Delgrange, S.; Simier, P.; Delavault, P. Susceptibility of Phelipanche and Orobanche species to AAL-toxin. Planta 2009, 230, 1047–1055. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, M.; Dong, S.; Ma, Y. Conditioning duration and agents involved in broomrape seeds responding to germination stimulants. Plant Growth Regul. 2017, 81, 221–230. [Google Scholar] [CrossRef]
- Ye, X.; Chen, J.; McErlean, C.S.P.; Zhang, M.; Yu, R.; Ma, Y. The potential of foxtail millet as a trap crop for sunflower broomrape. Acta Physiol. Plant. 2017, 39, 1–11. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Reboud, X.; Gibot-Leclerc, S. Broomrape Weeds. Underground Mechanisms of Parasitism and Associated Strategies for their Control: A Review. Front. Plant Sci. 2016, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. Recognition of root exudates by seeds of broomrape (Orobanche and Phelipanche) species. Ann. Bot. 2009, 103, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, Y.; Yoder, J.I. Differential induction of Orobanche seed germination by Arabidopsis thaliana. Plant Sci. 2001, 160, 951–959. [Google Scholar] [CrossRef]
- Louarn, J.; Carbonne, F.; Delavault, P.; Bécard, G.; Rochange, S. Reduced Germination of Orobanche cumana Seeds in the Presence of Arbuscular Mycorrhizal Fungi or Their Exudates. PLoS ONE 2012, 7, e49273. [Google Scholar] [CrossRef]
- Chen, J.; Wei, J.; Gao, J.M.; Ye, X.X.; McErlean, C.S.P.; Ma, Y.Q. Allelopathic inhibitory effects of Penicillium griseofulvum produced patulin on the seed germination of Orobanche cumana Wallr. and Phelipanche aegyptiaca Pers. Allelopath. J. 2017, 41, 65–80. [Google Scholar] [CrossRef]
- Chen, J.; Xue, Q.H.; McErlean, C.S.P.; Zhi, J.H.; Ma, Y.Q.; Jia, X.T.; Zhang, M.; Ye, X.X. Biocontrol potential of the antagonistic microorganism Streptomyces enissocaesilis against Orobanche cumana. BioControl 2016, 61, 781–791. [Google Scholar] [CrossRef]
- Boutiti, M.Z.; Souissi, T.; Kharrat, M. Evaluation of Fusarium as potential biological control against Orobanche on Faba bean in Tunisia. XII Int. Symp. Biol. Control Weeds 2001, 238–244. [Google Scholar]
- Abebe, G.; Sahile, G.; Abdel-Rahman, M.A.-T. Effect of soil solarization on Orobanche soil seed sank and tomato yield in Central Rift Valley of Ethiopia. World J. Agric. Sci. 2005, 1, 143–147. [Google Scholar]
- Pannacci, E.; Lattanzi, B.; Tei, F. Non-chemical weed management strategies in minor crops: A review. Crop Prot. 2017, 96, 44–58. [Google Scholar] [CrossRef]
- Webber, C.L.; Shrefler, J.W.; Brandenberger, L.P. Organic weed control. Herbic. - Environ. Impact Stud. Manag. Approaches 2003, 185–198. [Google Scholar]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Emeran, A.A.; Rubiales, D. Inter-cropping with berseem clover (Trifolium alexandrinum) reduces infection by Orobanche crenata in legumes. Crop Prot. 2010, 29, 867–871. [Google Scholar] [CrossRef]
- Babaei, S.; Alizadeh, H.; Jahansouz, M.R.; Mashhadi, H.R.; Moeini, M.M. Management of Phelipanche aegyptiaca Pomel. using trap crops in rotation on tomato (Solanum lycopersicom L.). Aust. J. Crop Sci. 2010, 4, 437–442. [Google Scholar]
- Ma, Y.; Jia, J.; An, Y.; Wang, Z.; Mao, J. Potential of some hybrid maize lines to induce germination of sunflower broomrape. Crop Sci. 2013, 53, 260–270. [Google Scholar] [CrossRef]
- Pérez-De-Luque, A.; Rubiales, D.; Cubero, J.I.; Press, M.C.; Scholes, J.; Yoneyama, K.; Takeuchi, Y.; Plakhine, D.; Joel, D.M. Interaction between Orobanche crenata and its host legumes: Unsuccessful haustorial penetration and necrosis of the developing parasite. Ann. Bot. 2005. [Google Scholar] [CrossRef]
- Grenz, J.H.; Iştoc, V.A.; Manschadi, A.M.; Sauerborn, J. Interactions of sunflower (Helianthus annuus) and sunflower broomrape (Orobanche cumana) as affected by sowing date, resource supply and infestation level. Field Crops Res. 2008, 107, 170–179. [Google Scholar] [CrossRef]
- Malo, I.; De Bastiani, M.; Arevalo, P.; Bernacchia, G. Natural extracts from pepper, wild rue and clove can activate defenses against pathogens in tomato plants. Eur. J. Plant Patol. 2017, 149, 89–101. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z.; Meng, H. Transcriptomic insights into the allelopathic effects of the garlic allelochemical diallyl disulfide on tomato roots. Sci. Rep. 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, W.; Dong, S.; Ren, X.; An, Y.; Lang, M. Induction of seed germination in Orobanche spp. by extracts of traditional Chinese medicinal herbs. Sci. China Life Sci. 2012, 55, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Joel, D.M.; Bar, H.; Mayer, A.M.; Plakhine, D.; Ziadne, H.; Westwood, J.H.; Welbaum, G.E. Seed ultrastructure and water absorption pathway of the root-parasitic plant Phelipanche aegyptiaca (Orobanchaceae). Ann. Bot. 2012, 109, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.Q.; Ma, Y.; Wu, H.; Shui, J.F.; Ye, X.; An, Y. Allelopathic stimulatory effects of wheat differing in ploidy levels on Orobanche minor germination. Allelopath. J. 2013, 31, 355–366. [Google Scholar]
- Ma, Y.; Zhang, M.; Li, Y.; Shui, J.; Zhou, Y. Allelopathy of rice (Oryza sativa L.) root exudates and its relations with Orobanche cumana Wallr. and Orobanche minor Sm. germination. J. Plant Interact. 2014, 9, 722–730. [Google Scholar] [CrossRef]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef]
- Eizenberg, H.; Hershenhorn, J.; Achdari, G.; Ephrath, J.E. A thermal time model for predicting parasitism of Orobanche cumana in irrigated sunflower-field validation. Field Crops Res. 2012, 137, 49–55. [Google Scholar] [CrossRef]
- Spallek, T.; Mutuku, M.; Shirasu, K. The genus Striga: A witch profile. Mol. Plant Pathol. 2013, 14, 861–869. [Google Scholar] [CrossRef]
- Martín-Sanz, A.; Malek, J.; Fernández-Martínez, J.M.; Pérez-Vich, B.; Velasco, L. Increased virulence in sunflower broomrape (Orobanche cumana Wallr.) populations from southern Spain is associated with greater genetic diversity. Front. Plant Sci. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Louarn, J.; Boniface, M.-C.; Pouilly, N.; Velasco, L.; Pérez-Vich, B.; Vincourt, P.; Muños, S. Sunflower resistance to broomrape (Orobanche cumana) is controlled by specific QTLs for different parasitism stages. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]

| Crop | Varieties | Label |
|---|---|---|
| Sugar beet | Ruima | TA |
| 0143 | TB | |
| RG8001 | TC | |
| Pepper | Jinghong | LA |
| Qingdao Xinlilai | LB | |
| Zi jinshan | LC | |
| Wheat | Xinchun 6 | MA |
| Yongliang 15 | MB | |
| Sunflower | Aidatou | |
| Processing tomato | Shifan 33 |
| Treatment | Sunflower Broomrape Germination % | Egyptian Broomrape Germination % | ||
|---|---|---|---|---|
| Rotation effect | ||||
| Year 1 | 65.59 a | 16.18 a | ||
| Year 2 | 59.91 b | 16.07 b | ||
| Concentration effect | ||||
| 10-fold | 68.48 a | 17.00 a | ||
| 100-fold | 57.02 b | 15.25 b | ||
| Treatment effect | ||||
| CK | 8.18 e | 6.98 bc | ||
| CK GR24 | 96.51 a | 96.63 a | ||
| LA | 68.83 bc | 6.27 bc | ||
| LB | 70.97 b | 5.43 c | ||
| LC | 71.19 b | 8.11 bc | ||
| MA | 62.44 bcd | 6.26 bc | ||
| MB | 60.21 cd | 9.13 b | ||
| TA | 64.88 bcd | 7.60 bc | ||
| TB | 59.11 d | 6.00 bc | ||
| TC | 65.15 bcd | 5.53 c | ||
| Interaction effect | ||||
| CK | Year 1 | 10-fold | 8.12 m | 8.17 bc |
| 100-fold | 8.24 m | 8.04 bc | ||
| Year 2 | 10-fold | 8.25 m | 5.79 bc | |
| 100-fold | 8.24 m | 8.15 bc | ||
| CK GR24 | Year 1 | 10-fold | 96.24 ab | 95.01 a |
| 100-fold | 96.24 ab | 96.10 a | ||
| Year 2 | 10-fold | 96.77 a | 97.13 a | |
| 100-fold | 96.77 a | 97.13 a | ||
| LA | Year 1 | 10-fold | 82.28 abcde | 3.96 bc |
| 100-fold | 59.12 fghijkl | 9.09 bc | ||
| Year 2 | 10-fold | 82.96 abc | 5.01 bc | |
| 100-fold | 50.96 ijkl | 7.00 bc | ||
| LB | Year 1 | 10-fold | 84.59 abc | 2.86 c |
| 100-fold | 70.94 cdefghij | 4.99 bc | ||
| Year 2 | 10-fold | 79.16 abcdefg | 6.97 bc | |
| 100-fold | 49.21 jkl | 6.88 bc | ||
| LC | Year 1 | 10-fold | 82.35 abcde | 11.28 b |
| 100-fold | 58.64 fghijkl | 10.17 bc | ||
| Year 2 | 10-fold | 83.76 abc | 4.86 bc | |
| 100-fold | 60.00 defghijkl | 6.13 bc | ||
| MA | Year 1 | 10-fold | 59.71 efghijkl | 4.43 bc |
| 100-fold | 74.07 abcdefgh | 8.15 bc | ||
| Year 2 | 10-fold | 64.59 cdefghijkl | 8.57 bc | |
| 100-fold | 51.38 hijkl | 10.80 b | ||
| MB | Year 1 | 10-fold | 62.32 cdefghijkl | 9.67 bc |
| 100-fold | 80.49 abcdef | 10.26 bc | ||
| Year 2 | 10-fold | 55.03 hijkl | 7.89 bc | |
| 100-fold | 43.00 l | 8.71 bc | ||
| TA | Year 1 | 10-fold | 72.79 cdefghi | 6.33 bc |
| 100-fold | 67.13 cdefghijk | 9.51 bc | ||
| Year 2 | 10-fold | 62.61 cdefghijkl | 5.31 bc | |
| 100-fold | 57.02 ghijkl | 9.24 bc | ||
| TB | Year 1 | 10-fold | 66.63 cdefghijk | 6.16 bc |
| 100-fold | 51.31 hijkl | 8.81 bc | ||
| Year 2 | 10-fold | 65.32 cdefghijkl | 6.38 bc | |
| 100-fold | 53.19 hijkl | 8.99 bc | ||
| TC | Year 1 | 10-fold | 73.43 bcdefghi | 4.02 bc |
| 100-fold | 57.27 ghijkl | 5.21 bc | ||
| Year 2 | 10-fold | 82.72 abcd | 4.04 bc | |
| 100-fold | 47.22 kl | 8.85 bc | ||
| Crops | Varieties | Egyptian Broomrape | Sunflower Broomrape | ||||||
|---|---|---|---|---|---|---|---|---|---|
| First | Second | First | Second | ||||||
| EP | WP | EP | WP | EP | WP | EP | WP | ||
| Sugar beet | TA | 0.0 b | 0.0 | 0.0 a | 0.0 | 21.4 e | 3.3 ab | 6.3 de | 2.7 a |
| TB | 0.0 b | 0.0 | 0.0 a | 0.0 | 14.0 f | 8.3 a | 4.2 e | 6.0 a | |
| TC | 0.0 b | 0.0 | 0.0 a | 0.0 | 37.0 d | 7.8 a | 7.4 de | 4.1 a | |
| Pepper | LA | 0.0 b | 0.0 | 0.0 a | 0.0 | 70.2 b | 4.6 ab | 60.3 b | 4.6 a |
| LB | 0.0 b | 0.0 | 0.0 a | 0.0 | 74.3 b | 3.7 ab | 25.8 c | 0.8 a | |
| Wheat | LC | 0.6 b | 0.0 | 0.5 a | 0.0 | 56.9 c | 0.0 b | 11.4 d | 0.0 a |
| MA | 3.2 a | 0.0 | 1.0 a | 0.0 | 87.3 a | 0.0 b | 77.0 a | 0.0 a | |
| MB | 2.1 a | 0.0 | 1.1 a | 0.0 | 59.4 c | 0.0 b | 10.5 de | 0.0 a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, S.; Wang, K.; Liu, B.; Wang, Y.; Chen, F.; Li, P.; Hayat, K.; Ma, Y. A Two-Year Simulated Crop Rotation Confirmed the Differential Infestation of Broomrape Species in China Is Associated with Crop-Based Biostimulants. Agronomy 2020, 10, 18. https://doi.org/10.3390/agronomy10010018
Hayat S, Wang K, Liu B, Wang Y, Chen F, Li P, Hayat K, Ma Y. A Two-Year Simulated Crop Rotation Confirmed the Differential Infestation of Broomrape Species in China Is Associated with Crop-Based Biostimulants. Agronomy. 2020; 10(1):18. https://doi.org/10.3390/agronomy10010018
Chicago/Turabian StyleHayat, Sikandar, Kai Wang, Bo Liu, Yue Wang, Fangjie Chen, Pufang Li, Kashif Hayat, and Yongqing Ma. 2020. "A Two-Year Simulated Crop Rotation Confirmed the Differential Infestation of Broomrape Species in China Is Associated with Crop-Based Biostimulants" Agronomy 10, no. 1: 18. https://doi.org/10.3390/agronomy10010018
APA StyleHayat, S., Wang, K., Liu, B., Wang, Y., Chen, F., Li, P., Hayat, K., & Ma, Y. (2020). A Two-Year Simulated Crop Rotation Confirmed the Differential Infestation of Broomrape Species in China Is Associated with Crop-Based Biostimulants. Agronomy, 10(1), 18. https://doi.org/10.3390/agronomy10010018





