Clipping Forage Sorghum Twice and Nitrogen Topdressing Offer an Option for Dual-Purpose Use for Cover Cropping and Fodder in Mixed Crop/Livestock Farming Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Greenhouse Pot Experiment
2.1.1. Soil Sampling and Preparation
2.1.2. Soil Analysis
2.1.3. Experimental Site, Treatments, and Experimental Design
2.1.4. Agronomic Practices
2.1.5. Data Collection
2.1.6. Data Analysis
2.2. Field Experiment
2.2.1. Experimental Site
2.2.2. Treatments and Design
2.2.3. Agronomic Practices
2.2.4. Data Collection
2.2.5. Data Analysis
3. Results
3.1. Soil Analysis Results
3.2. Greenhouse Experiment
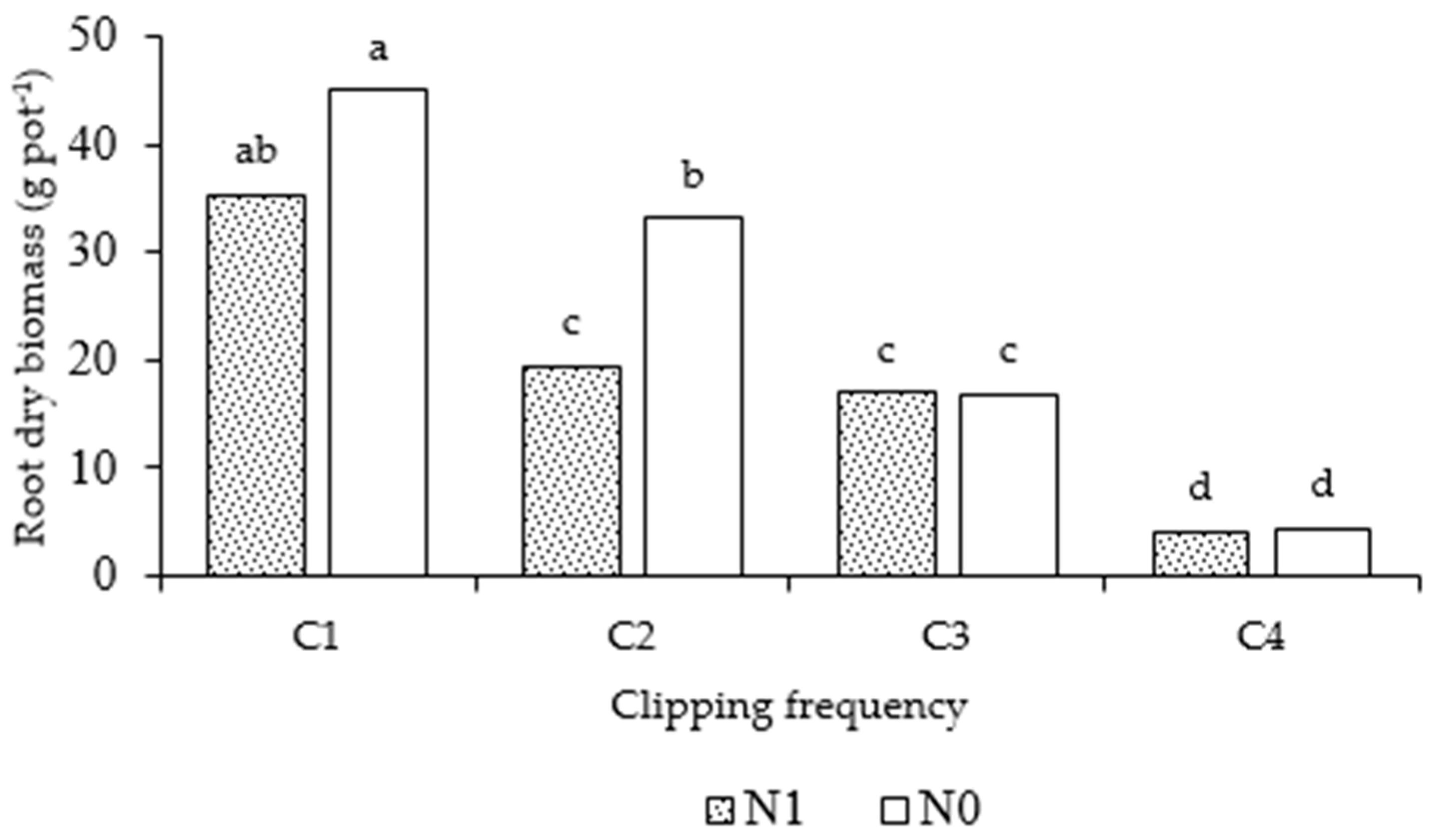
3.2.1. Root Biomass Results
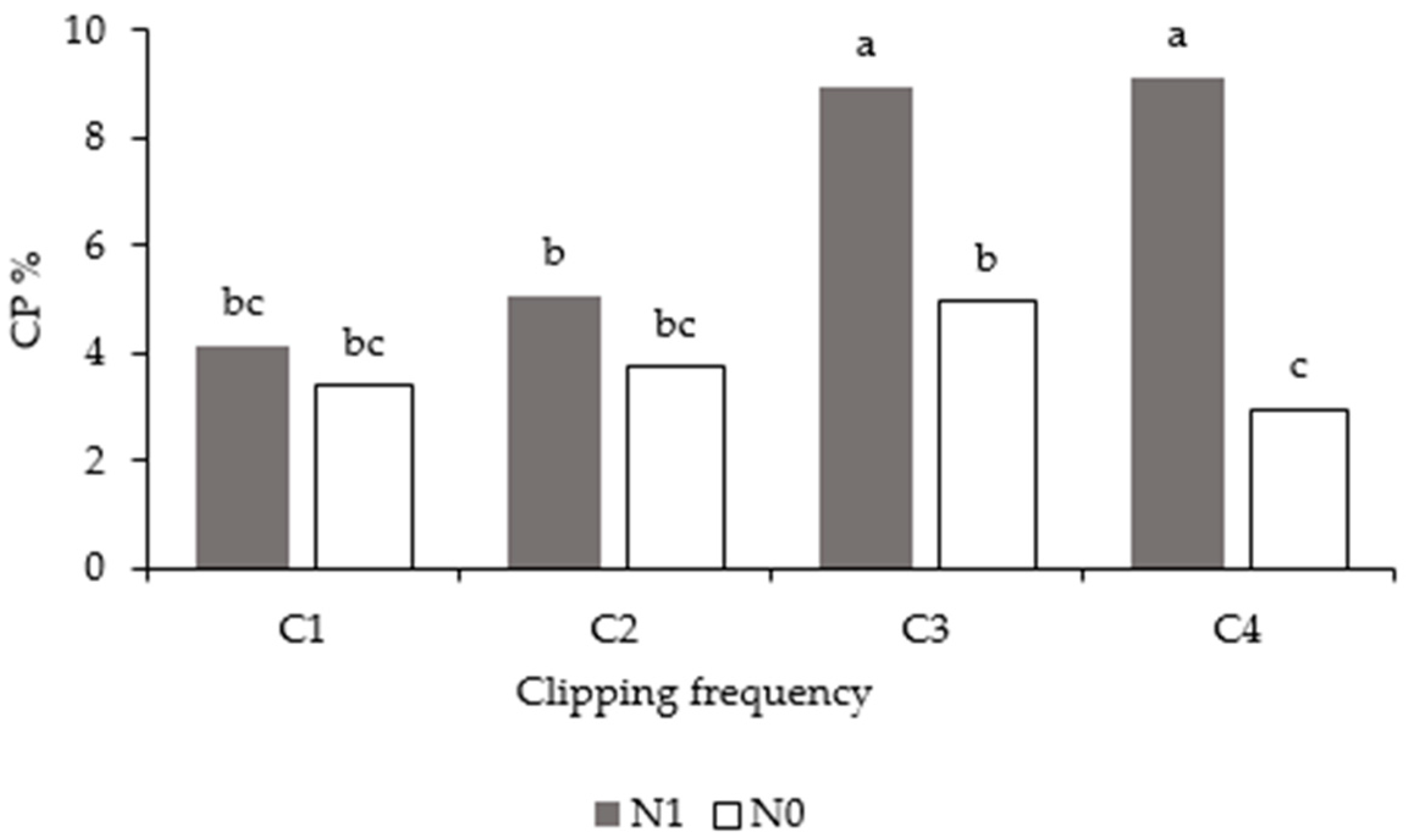
3.2.2. Grazing Quality Measured during the Growing Period
3.2.3. Grazing Quality at Termination
3.3. On-Farm Experiments
3.3.1. Biomass Yield Measured during the Growing Period
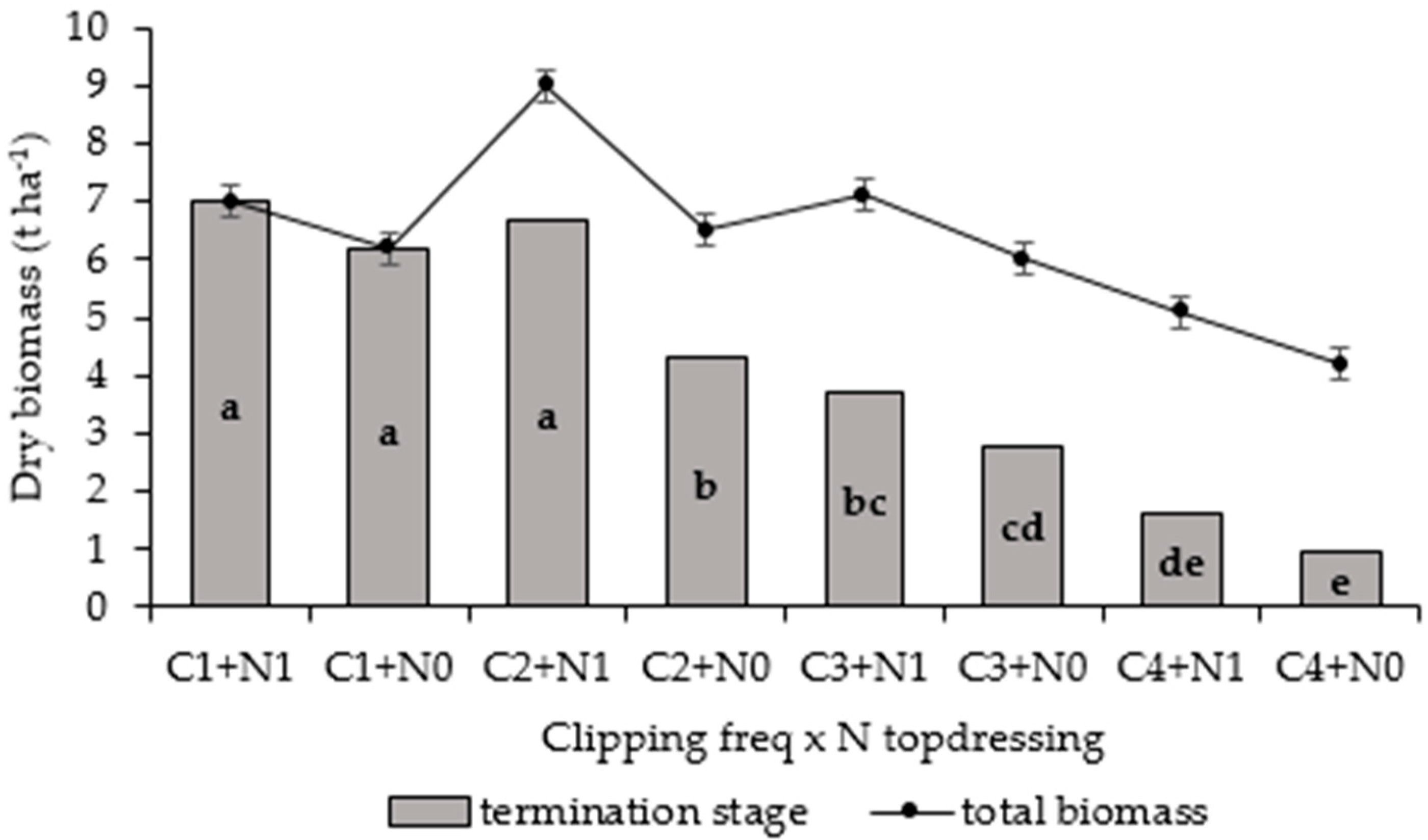
3.3.2. Biomass Yield Harvested
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ighodaro, I.; Yusuf, F. The impact of soil erosion on agricultural potential and performance of Sheshegu community farmers in the Eastern Cape of South Africa. J. Agric. Sci. 2013, 5, 140–147. [Google Scholar] [CrossRef]
- Khapayi, M.; Celliers, P.R. Factors limiting and preventing emerging farmers to progress to commercial agricultural farming in the King William’s Town area of the Eastern Cape Province, South Africa. South Africa Tydskr. Landbouvoorl. S. Afr. J. Agric. Ext. 2016, 44, 25–41. [Google Scholar] [CrossRef]
- Department of Agriculture, Forestry & Fisheries. Trends in the Agricultural Sector 2017; Directorate of Communication Services: Pretoria, South Africa, 2018.
- Baets, S.; Poesen, J.J.; Meersmans, J.J.; Serlet, L.L. Cover crops and their erosion-reducing effects during concentrated flow erosion. Catena 2011, 85, 237–244. [Google Scholar] [CrossRef]
- Brust, J.; Claupein, W.; Gerhards, R. Growth and weed suppression ability of common and new cover crops in Germany. Crop Prot. 2014, 63, 1–8. [Google Scholar] [CrossRef]
- Turmel, M.; Speratti, A.; Baudron, F.; Verhulst, N.; Govaerts, B. Crop residue management and soil health: A systems analysis. Agric. Syst. 2015, 134, 6–16. [Google Scholar] [CrossRef]
- Choudhary, M.; Rana, K.S.; Meena, M.C.; Bana, R.S.; Jakhar, P.; Ghasal, P.C.; Verma, R.K. Changes in physico-chemical and biological properties of soil under conservation agriculture based pearl millet—Mustard cropping system in rain fed semi-arid region. J. Arch. Agron. Soil Sci. 2018, 65, 911–927. [Google Scholar] [CrossRef]
- Swella, G.B.; Ward, P.R.; Siddique, K.H.M.; Flower, K.C. Combinations of tall standing and horizontal residue affect soil water dynamics in rain-fed conservation agriculture systems. Soil Tillage Res. 2015, 147, 30–38. [Google Scholar] [CrossRef]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed dynamics and conservation agriculture principles: A review. Field Crops Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Ranaivoson, L.; Naudin, K.; Ripoche, A.; Affholder, F.; Rabeharisoa, L.; Corbeels, M. Agro-ecological functions of crop residues under conservation agriculture. A review. Agron. Sustain. Dev. 2017, 37, 26. [Google Scholar] [CrossRef]
- Blezinger, S. Developing Sound Forage Analyses a Program, 1999. Available online: http://www.cattletoday.com/archive/1999/September/Cattle_Today20.shtml (accessed on 27 January 2018).
- Kumar, D.; Chaplot, P.C. Effect of fertility levels on quality of multi-cut forage sorghum genotypes. Forage Res. 2015, 40, 251–253. [Google Scholar]
- Rana, D.S.; Singh, B.; Gupta, K.; Dhaka, A.K.; Pahuja, S.K. Effect of fertility levels on growth, yield and quality of multicut forage sorghum [Sorghum bicolor (l.) Moench] genotypes. Forage Res. 2013, 39, 36–38. [Google Scholar]
- Roy, P.R.S.; Khandaker, Z.H. Effect of phosphorus fertilizer on yield and nutritional value of sorghum (sorghum bicolor) fodder at three cuttings. Bangladesh J. Anim. Sci. 2010, 39, 106–115. [Google Scholar] [CrossRef]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Nirmal, S.S.; Dudhade, D.D.; Solanke, A.V.; Gadakh, S.R.; Bhakare, B.D.; Hasure, R.R.; Gore, S.B. Effect of nitrogen levels on growth and yield of forage sorghum [Sorghum bicolor (l.) moench] varieties. Int. J. Sci. 2016, 5, 2999–3004. [Google Scholar]
- Tang, C.; Yang, X.; Chen, X.; Ameen, A.; Xie, G. Sorghum biomass and quality and soil nitrogen balance response to nitrogen rate on semiarid marginal land. Field Crops Res. 2018, 215, 12–22. [Google Scholar] [CrossRef]
- Almeida, V.O.; Carneiro, R.V.; Carvalho, M.A.M.; Figueiredo-Ribeiro, R.C.L.; Moraes, M.G. Diversity of non-structural carbohydrates in the underground organs of five Iridaceae species from the Cerrado (Brazil). S. Afr. J. Bot. 2015, 96, 105–111. [Google Scholar] [CrossRef]
- Liu, W.; Su, J.; Li, S.; Lang, X.; Huang, X. Non-structural carbohydrates regulated by season and species in the subtropical monsoon broadleaved evergreen forest of Yunnan Province, China. Sci. Rep. 2018, 8, 1083. [Google Scholar] [CrossRef]
- Mapfumo, E.; Naeth, M.A.; Baron, V.S.; Dick, A.C.; Chanasyk, D.S. Grazing impacts on litter and roots: Perennial versus annual grasses. J. Range Manag. 2002, 5, 16–22. [Google Scholar] [CrossRef]
- Soil Classification working group. Soil Classification. In A Taxonomic System for South Africa; Department of Agricultural Development: Pretoria, South Africa, 1991. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources, 2nd ed.; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006; ISBN 92-5-105511-4. [Google Scholar]
- Agri Laboratory Association of Southern Africa (AgriLASA). Soil Handbook; Agri Laboratory Association of Southern Africa: Pretoria, South Africa, 2004. [Google Scholar]
- Mnkeni, P.N.S.; Gichangi, E.M. A Practical Teaching Manual for Plant Analysis; University of Fort Hare, Faculty of Science and Agriculture, Department of Agronomy: Alice, South Africa, 2008. [Google Scholar]
- Mehlich, A. Uniformity of expressing soil test results. A case for calculating results on a volume basis. Commun. Soil Sci. Plant Anal. 1972, 3, 417–424. [Google Scholar] [CrossRef]
- Sher, A.; Hassan, F.; Ali, H.; Hassan, W. Seed rate and nitrogen application effects on production and brix value of forage sorghum cultivars. Grassl. Sci. 2015, 62, 119–127. [Google Scholar] [CrossRef]
- Department of Agriculture, Forestry & Fisheries. Sorghum Production Guideline, 2010. Available online: https://www.nda.agric.za/docs/Brochures/prodGuideSorghum.pdf (accessed on 16 March 2016).
- Mandiringana, O.T.; Mnkeni, P.N.S.; Mkile, Z.; Van Averbeke, W.; Van Ranste, E.; Verplancke, H. Mineralogy and fertility status of selected soils of the Eastern Cape Province, South Africa. Commun. Soil Sci. Plant Anal. 2005, 36, 2431–2446. [Google Scholar] [CrossRef]
- Parra-Londono, S.; Kavka, M.; Samans, B.; Snowdon, R.; Wieckhorst, S.; Uptmoor, R. Sorghum root-system classification in contrasting P environments reveals three main rooting types and root-architecture-related marker–trait associations. Ann. Bot. 2018, 121, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Turnbull, M.H.; Song, J.; Roche, J.; Novak, O.; Späth, J.; Jameson, P.E.; Love, J. Depletion of carbohydrate reserves limits nitrate uptake during early regrowth in Lolium perenne L. J. Exp. Bot. 2017, 68, 1569–1583. [Google Scholar] [CrossRef]
- Sainju, U.M.; Singh, B.P.; Whitehead, W.F. Tillage, cover crops, and nitrogen fertilization effects on cotton and sorghum root biomass, carbon, and nitrogen. Agron. J. 2005, 97, 1279–1290. [Google Scholar] [CrossRef]
- Zuffo, A.M.; Júnior, J.M.Z.; da Silva, J.A.; dos Santos, D.S.; Oliveira, J.B.D.; Zambiazzi, E.V.; Bruzi, A.T.; Vilela, N.J.D.; Vilela, G.L.D.; Ferreira, A.C.G. Morphoagronomic traits of BRS 610 sorghum submitted to artificial defoliation. Afr. J. Agric. Res. 2015, 10, 3798–3803. [Google Scholar]
- Raynor, E.J.; Joern, A.; Nippert, J.B.; Briggs, J.M. Forage decisions underlying restricted space use: Effects of fire and forage maturation on large herbivore nutrient uptake. Ecol. Evol. 2016, 6, 5843–5853. [Google Scholar] [CrossRef]
- Mwangi, P.G.; Gachuiri, C.K.; Mbugua, P.N. Effect of growth stage on fodder yield and quality of dual purpose sorghum. Trop. Drylands 2017, 1, 100–104. [Google Scholar] [CrossRef]
- Eltelib, H.A.; Hamad, M.A.; Ali, E.E. The effect of nitrogen and phosphorus fertilization on growth, yield and quality of forage maize (Zea mays L.). J. Agron. 2006, 5, 515–518. [Google Scholar]
- Reddy, B.V.S.; Reddy, P.S.; Bidingera, F.; Blummel, M. Crop management factors influencing yield and quality of crop residues. Field Crops Res. 2003, 84, 57–77. [Google Scholar] [CrossRef]
- Ferraro, D.O.; Oesterheld, M. Effect of defoliation on grass growth. Quant. Rev. Oikos 2002, 98, 125–133. [Google Scholar]
- Legwaila, G.M.; Sekgwane, S.; Mathowa, T.; Mojeremane, W. Agronomic performance of sorghum after panicle removal. Int. J. Plant Soil Sci. 2016, 10, 1–7. [Google Scholar] [CrossRef]

| 2016–2017 | 2017–2018 | |||
|---|---|---|---|---|
| Month | Rainfall (mm) | Irrigation (mm) | Rainfall (mm) | Irrigation (mm) |
| December | - | - | 48.26 | 50 |
| January | 89.92 | 40 | 54.86 | 30 |
| February | 73.15 | 45 | 53.59 | 20 |
| March | 34.29 | 50 | 57.4 | 20 |
| April | 22.1 | 20 | 62.23 | 0 |
| CP (%) | ADF (%) | NDF (%) | ||||
|---|---|---|---|---|---|---|
| C3 | C4 | C3 | C4 | C3 | C4 | |
| N1 | 7.6a | 6.0a | 28.1a | 30.9a | 55.9a | 48.8b |
| N0 | 4.6b | 4.2b | 27.8a | 29.3a | 56.6a | 56.3a |
| Significance | p < 0.001 | p < 0.001 | ns | Ns | Ns | p < 0.01 |
| LSD (0.05) | 0.6 | 1.6 | 1.5 | 6.1 | 2.8 | 1.6 |
| CV (%) | 7.6 | 9.0 | 6.1 | 5.7 | 8.2 | 6.8 |
| ADF (%) | NDF (%) | |
|---|---|---|
| C1 | 31.9b | 54.8a |
| C2 | 34.1ab | 53.2ab |
| C3 | 35.4a | 51.3b |
| C4 | 34.1ab | 53.6a |
| Significance | p < 0.05 | p < 0.01 |
| LSD (0.05) | 1.97 | 1.61 |
| 2016–2017 Season (t ha−1) | 2017–2018 Season (t ha−1) | |||
|---|---|---|---|---|
| Clipping Frequency | C3 | C4 | C3 | C4 |
| N1 | 1.3a | 0.97a | 1.2a | 1.0a |
| N0 | 1.1a | 0.95a | 1.2a | 0.7a |
| LSD (0.05) | 0.2 | 0.1 | 0.2 | 0.5 |
| Significance | ns | Ns | ns | ns |
| CV (%) | 18 | 13 | 11 | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janhi, K.; Matshaya, Z.; Chiduza, C.; Muzangwa, L. Clipping Forage Sorghum Twice and Nitrogen Topdressing Offer an Option for Dual-Purpose Use for Cover Cropping and Fodder in Mixed Crop/Livestock Farming Systems. Agronomy 2020, 10, 17. https://doi.org/10.3390/agronomy10010017
Janhi K, Matshaya Z, Chiduza C, Muzangwa L. Clipping Forage Sorghum Twice and Nitrogen Topdressing Offer an Option for Dual-Purpose Use for Cover Cropping and Fodder in Mixed Crop/Livestock Farming Systems. Agronomy. 2020; 10(1):17. https://doi.org/10.3390/agronomy10010017
Chicago/Turabian StyleJanhi, Kudzayi, Zimkhitha Matshaya, Cornelius Chiduza, and Lindah Muzangwa. 2020. "Clipping Forage Sorghum Twice and Nitrogen Topdressing Offer an Option for Dual-Purpose Use for Cover Cropping and Fodder in Mixed Crop/Livestock Farming Systems" Agronomy 10, no. 1: 17. https://doi.org/10.3390/agronomy10010017
APA StyleJanhi, K., Matshaya, Z., Chiduza, C., & Muzangwa, L. (2020). Clipping Forage Sorghum Twice and Nitrogen Topdressing Offer an Option for Dual-Purpose Use for Cover Cropping and Fodder in Mixed Crop/Livestock Farming Systems. Agronomy, 10(1), 17. https://doi.org/10.3390/agronomy10010017





