Abstract
The present study addresses the effects of Trichoderma-based biostimulants and nitrogen (N) fertilization levels on agronomic performance and functional quality of two important greenhouse leafy vegetables: lettuce and rocket. A factorial analysis of the relative effects of Trichoderma-based biostimulants (Trichoderma harzianum strain T22 and Trichoderma virens strain GV41) and N fertilization levels (sub-optimal, optimal, and supra-optimal) was carried out to evaluate crop productive behavior (marketable and unmarketable yields, leaf dry matter content, and biomass production), nitrogen nutrition (N uptake, apparent N recovery, and nitrogen use efficiency (NUE)) as well as phytochemical qualitative components (antioxidant activity and total polyphenols). The soil plant analysis development (SPAD) index in both leafy vegetables and leaf colorimetry only in rocket were mainly affected by N fertilization levels but not by Trichoderma-based biostimulants. The contribution of native mineral N was 60 and 100 kg N ha−1 of the total uptake in lettuce and rocket, respectively, and N surpluses were observed in both crops, even under optimal fertilization conditions. Trichoderma virens GV41-based biostimulant increased lettuce marketable yield and biomass production, both under optimal and sub-optimal fertilization. In addition, the same treatment increased NUE up to 116% under recommended N fertilization, that was also associated to an increase in phenol content and antioxidant activity. Rocket showed a clear effect of the Trichoderma virens GV41 treatment, only in absence of fertilization, demonstrating an increase in marketable yield and N uptake. Thus, the inoculation of rocket with this Trichoderma biostimulant can be considered as a useful management tool in leafy vegetable cropping systems for the efficient use of residual fertilizers from previous crops, enhancing NUE within the crop rotations. Nevertheless, the application of microbial biostimulant treatments requires good monitoring of soil N fertility in order to avoid an overexploitation of soil N supplying potential.
1. Introduction
Nitrogen (N) is an essential macronutrient, considered as the second main growth factor for plant growth after water [1], that is involved in the synthesis of proteins and other N containing substances [2]. There is a preferential uptake of mineral N in the form of nitrate (NO3−) by plants, even if in some cases ammonium (NH4+) can be adsorbed (i.e., paddy rice) [1]. Nitrate originates from mineralization of native soil organic matter, and in agroecosystems additional inputs come from mineral N fertilizers as well as from decomposition of organic amendments like manure, compost, and crop residues [3].
Optimized N fertilization may have a positive effect on leafy vegetables such as lettuce (Lactuca sativa L.) and rocket (Eruca sativa Mill.) by increasing growth, yield, and nutrient content of these plants [4,5,6]. On the other hand, due to its high mobility, nitrate can threaten groundwater quality [7] since approximately half of all N applied in agricultural fields is leached from the topsoil, transferred downwards by rainfall towards groundwater [8]. This occurs when the fertilizer dose is not appropriate for the crop requirements, leading to N surpluses that are prone to leaching during the rainy season. In addition, excessive N availability can lower the quality of leafy vegetables [9], characterized by a short life cycle such as lettuce and rocket, favoring nitrate accumulation in aboveground tissues [2].
Consequently, more effective N management strategies are required to limit external N inputs in agroecosystems, and avoid environmental and health risks, preserving crop yields [10]. This entails two main strategies aimed at increasing the nitrogen use efficiency (NUE) of crops. The first involves the improvement of the fertilization technique by splitting N inputs [11] or using slow release fertilizers [12] in order to match N availability from fertilizer with N requirements at specific crop stages. The second approach requires an optimization of root architecture and uptake efficiency that can be achieved by adopting appropriate plant breeding [13], as well as the application of plant biostimulants [14]. This last option includes the use of bioactive substances (humic acids, seaweed extracts, protein hydrolysates, and silicon), and/or beneficial microorganisms including mycorrhiza, plant growth promoting rhizobacteria (such as Rhizobium spp., Azotobacter, and Azospirillum) and endophytic fungi such as Trichoderma spp. with a wide range of effects on root activity and specific plant metabolic pathways [15,16,17,18,19,20,21,22,23,24]. Among the most studied beneficial microorganisms, Trichoderma spp. are gaining prominence as microbial plant biostimulants, since they are able to: (i) contrast plant pathogens, mainly phytopathogenic fungi; and (ii) improve tolerance/resistance to abiotic stresses [25,26,27,28,29]. In addition to their activity in overcoming abiotic stresses, some Trichoderma strains, among which those of Trichoderma harzianum, Trichoderma virens, and Trichoderma atroviride, are capable in triggering phytostimulation (i.e., biostimulant action) via a mechanism entailing multifaceted cross-talk communication between root and shoot systems. This includes the transfer of auxins (i.e., indole-3-acetaldehyde, indole-3-carboxaldehyde, and indole-3-ethanol), oligopeptides, volatiles, and other secondary metabolites to the rhizosphere, which augment root branching and nutrient uptake efficiency (P, Fe, Cu, Zn, and Mn) that consequently improve crop performance [23,28,30,31,32,33]. However, the positive effects in terms of plant growth, tolerance to abiotic stresses, and increased NUE, not only rely on the use of an effective Trichoderma strain, but also depend upon several interacting variables including the method of application (on the seed or root and soil), cropping systems (open field versus protected cultivation), fertilizer management, plant species, and pedoclimatic conditions [23,34]. Thus, an optimization of nutrient uptake can be obtained only throughout an appropriate combination of the above-mentioned strategies.
Although research on the biostimulant action of some Trichoderma strains (T. harzianum and Trichoderma asperellum) on eliciting growth and NUE in open field crops such as maize, wheat, and tobacco [30,31,35] have been conducted, little information is available concerning the beneficial effect of Trichoderma on leafy greens [36]. In a former study [36], the authors demonstrated that the positive effect of Trichoderma-based biostimulants was species-dependent and strongly influenced by the quantitative and qualitative composition of eukaryotic populations in the rhizosphere, especially under low input (N) conditions.
In continuation to the above-mentioned work, the present study addresses the effects of Trichoderma-based biostimulants and N fertilization levels on agronomic performance and functional quality of two important greenhouse leafy vegetables: lettuce and rocket.
A factorial analysis of the relative effects of Trichoderma-based biostimulants (T. harzianum strain T22 and T. virens strain GV41) and N fertilization levels (sub-optimal, optimal, and supra-optimal) was conducted to evaluate crop productive behavior (marketable and unmarketable yields, leaf dry matter content, and biomass production), N nutrition (N uptake, apparent N recovery, and NUE) as well as phytochemical qualitative components (antioxidant activity and total polyphenols).
The findings from this study will provide useful information for the development of innovative management practices that can assist farmers in reducing the use of mineral fertilizers, and to develop novel and efficient strategies to stimulate growth and optimize NUE, meeting the new European Union (EU) mandates for agriculture in the future.
2. Materials and Methods
2.1. Experimental Setup and Management
This study was carried out in the 2016 winter-summer growing season, in an unheated polyethylene greenhouse located at the Department of Agricultural Sciences, University of Naples Federico II, Southern Italy (40°49′ N, 14°15′ E; 72 meters above sea level (m a.s.l.)), consisting of two sequential experiments: the first on lettuce (Lactuca sativa L. var. Iceberg cv. ‘Silvinas’; Rijk Zwaan, Bologna, Italy) from 2 February to 31 March, and the second on rocket (Eruca sativa Mill.) from 13 June to 11 July.
The main chemical and physical properties of the soil at the experimental site were: a sandy loam texture (76% sand, 17% silt, 7% clay), a neutral pH (6.9), an electrical conductivity (EC) of 0.6 mS cm−1, an intermediate organic matter (1.25% w/w) and total N (0.11%) content, C:N of 10.8, carbonates at 0.3%, NO3−-N and NH4+-N at 108 and 13 mg kg−1, respectively, Olsen P at 38 mg kg−1, and exchangeable K at 980 mg kg−1.
The following factors were tested within the crop sequence:
Nitrogen fertilization levels (N): sub-optimal (N0), optimal (N1), and supra-optimal (N2) corresponding to 0, 90, and 180 kg N ha−1 for lettuce, and 0, 60, and 120 kg N ha−1 for rocket.
Microbial-based biostimulant treatments (T): Trichoderma harzianum strain T22 (T1), Trichoderma virens strain GV41 (T2), and a non-inoculated control (T0).
N optimal rate of both crops was calculated according to the Integrated Agriculture Techniques (IAT) of Regional Rural Development Programmes (EC No 1698/2005). A randomized-complete block split-plot design with three replicates was arranged to test the nine treatments deriving from the factorial combination of the three N fertilization levels and three microbial-based biostimulant treatments. Fertilization was the main factor and Trichoderma inoculation was the sub-factor accounting to a total of 27 experimental plots, 3.5 m2 each. The expected plant density for lettuce was 11 plants m−2 while for rocket it was 3000 seeds m−2.
Nitrogen total amount was applied as ammonium nitrate (34%) through fertigation in three weekly applications starting for lettuce 10 days after transplanting (DAT) using a drip irrigation system with in-line emitters (distance: 35 cm; emitter flow rate: 3.3 L h−1); while N amount was applied into two identical doses (at 3 and 8 days after seeding, DAS) for rocket and distributed by sprinkler irrigation system. According to the high concentration of native P and K of the soil, no fertilization was carried out for these two macro elements.
The fungal inoculants, Trichoderma harzianum strain T22 (originally from Trianum, Koppert Biological Systems, Rotterdam, the Netherlands) and T. virens strain GV41 were obtained from the collection of the Department of Agricultural Sciences, University of Naples Federico II [36]. A root dip method was used for lettuce at the time of transplant by submerging the roots of the seedlings in the Styrofoam planting trays in the Trichoderma spore suspension (1 × 108 spores mL−1) for 10 min to completely wet the roots. Then, each inoculated plant was removed from the tray, transplanted to pre-bored holes in the soil, and irrigated with 25 mL of the spore suspension. The same root drench application was repeated at 24 DAT with 50 mL plant−1 of the spore suspension. Conversely, a seed-coating treatment was used for the rocket whereby a Trichoderma spore suspension (1 × 108 spores mL−1) was used to inoculate to cover the seed surface, then the treated seeds were air dried and hand seeded to the soil.
2.2. Soil Plant Analysis Development (SPAD) Index, Plants Sampling, and Analysis
Non-destructive measurement of chlorophyll content (SPAD index) was conducted before harvest on 15 undamaged and fully expanded lettuce and rocket leaves of each plot, using a portable chlorophyll meter (SPAD-502, Konica Minolta, Tokyo, Japan). At the end of the growing period, lettuce and rocket fresh yield production was measured on plants harvested in a 1 square meter area (from the center of each plot) to assess total and marketable yields. Lettuce was harvested at 60 DAT, while rocket was harvested at 30 DAS.
Both leafy vegetables (lettuce and rocket) were weighed (fresh weight; fw) and oven dried at 80 °C (about 72 h) until reaching constant dry weight (dw). A sub-sample of dry plant biomass production was finely ground in an electric mill (Wiley mill-IKA, MF10.1, Staufen, Germany) for determination of the total N (Kjeldahl method [37]) and passed through a 0.5 mm sieve for nitrate analysis. Nitrate content in leaf tissues of lettuce and rocket was conducted by extracting 0.25 g of dried plant material in 50 mL of ultrapure water, with incubation in a shaking water bath (ShakeTemp SW22, Julabo, Seelbach, Germany) at 80 °C for 10 min (as described in detail by [38]). After, the extract was centrifuged at 6000 rpm for 10 min (R-10 M, Remi Elektrotechnik Limited, Mumbai, India), then filtered through a 0.20 μm filter paper (Whatman International Ltd., Maidstone, UK). Ion chromatography (ICS-3000, Dionex, Sunnyvale, CA, USA) allowed the nitrate separation. For nitrate determination (g kg−1 dw) an IonPac AG11-HC guard (4 × 50 mm) column and IonPac AS11-HC analytical column (4 × 250 mm) were used.
2.3. Leaf Colorimetry, Antioxidant Activity, and Total Phenols Analysis
Leaf color of lettuce and rocket was measured before harvest on the upper side using a Minolta CR-300 Chroma Meter (Minolta Camera Co. Ltd., Osaka, Japan). In each plot 10 healthy leaves were measured and averaged to a single value. The International Commission on Illumination ‘Lab’ color space parameters recorded in both experiments were: lightness (L*) and chroma coordinates (a* and b*).
Lettuce and rocket antioxidant activity as well as total phenols were determined on 0.2 g of freeze-dried material and measured by means of a spectrophotometer (Hach DR 2000, Hach Co., Loveland, CO, USA) according to the protocols of [39,40], respectively. Solution absorbances were assessed at 505 and 765 nm for the hydrophilic antioxidant fraction and total polyphenols. Antioxidant capacity and total polyphenols were expressed as mmol ascorbic acid and mg gallic acid per 100 g of dw, respectively.
2.4. Soil Analyses
Soil texture analysis was done using the Boycous hydrometric method. Soil pH and EC were measured on 1:2.5 and 1:5 soil (g) water (mL) suspensions, respectively. Soil nitrogen (total N) concentration was assessed after mineralization with sulfuric acid (96%) in the presence of potassium sulfate and a low concentration of copper by the Kjeldahl method [40]. Mineral N was determined spectrophotometrically (FIAstar 5000 Analyzer, FOSS Analytical, Hillerød, Denmark) on soil extracts. Gas semi-permeable membrane method (ISO11732 procedure) was carried out for NH4-N, while the sulphanilamide-naphtylethylendiamine dihydrochloride method was carried out to analyze NO3− after nitrate to nitrite reduction with a copper–cadmium column (ISO 13395 procedure). Soil C was measured according to the Walkley–Black method and reported as soil organic matter (SOM% = C% × 1.726). Soil P content was measured according to the Olsen method while ammonium acetate method was adopted for exchangeable K. Scheibler calcimeter was used for determination of soil limestone content
2.5. Data Elaboration and Statistical Analysis
Marketable yields were evaluated as fresh weight per hectare (ha), as determined by converting values measured on 1 m2 monitoring plots. Unmarketable yield was calculated as a percentage of the total fresh biomass. Dry biomass production was reported as dry weight per ha and calculated by multiplying total fresh biomass collected in the experimental plots by dry matter content measured after oven-drying of the plant sub-samples (see Section 2.2). Aboveground N uptake, reported as kg ha−1, was calculated by multiplying dry aboveground biomass by the N content (Kjeldahl N plus nitrates) measured according to protocols explained in Section 2.2.
A simplified N balance was assessed according to the following formulation [41]:
where Nin corresponded to N supplied with fertilizers (see Section 2.1) and Nupt corresponded to the aboveground N uptake.
N balance = Nin − Nupt
Apparent N recovery (ANR) from fertilizers was estimated according to the following equation:
where Nin corresponded to N supplied with fertilizers (see Section 2.1) and Nupt corresponded to the aboveground N uptake.
ANR = Nupt/Nin × 100
Nitrogen use efficiency (NUE) of fertilizers was calculated according to Equation (3) [42]:
where Nin corresponded to N supplied with fertilizers (see Section 2.1), Nupt F corresponded to the aboveground N uptake in fertilized plots and Nupt NF corresponded to the aboveground N uptake in non-fertilized plots. This is an indicator of potential loss of N inputs not intercepted by crops.
NUE = (Nupt F − Nupt NF)/Nin × 100
In our data elaboration we calculated two different NUE indices. NUE-C was calculated considering Nupt NF as the average N uptake in non-fertilized and non-inoculated plots (N0-T0 treatment). NUE-T was calculated taking as reference the average uptake of non-fertilized plots within each inoculation type (i.e., N0-T0 for NUE-T calculation of N1-T0 and N2-T0 treatments; N0-T1 for NUE-T calculation of N1-T1 and N2-T1 treatments; N0-T2 for NUE-T calculation of N1-T2 and N2-T2 treatments).
The statistical analyses were all carried out using the software IBM SPSS Statistics 21. All data were subjected to a two-way factorial ANOVA (randomized complete block design) and mean values were separated according to the Least Significant Difference (LSD) test with p < 0.05.
3. Results
3.1. Effect of N Fertilization Levels and Microbial-Based Biostimulants on Lettuce and Rocket Productive Performance
Neither N fertilization level (N) nor Trichoderma-based biostimulant (T) treatments had a significant effect on the percentage of unmarketable yield and leaf dry matter content in both lettuce (avg. 41.8% and 3.7%, respectively) and rocket (avg. 4.5% and 5.1%, respectively) (Table 1). A significant N × T interaction was observed for both lettuce and rocket in terms of marketable yield and total dry biomass production, with a different response between the two tested leafy vegetables (Table 1). When averaged over N fertilization levels or microbial-based biostimulants, lettuce marketable yield increased with the two fertilizations (+44% compared to N0) and with the two inoculations (+19% compared to T0). Optimal N fertilization (N1) associated to T2 (T. virens strain GV41) exhibited the highest marketable yield (56 Mg fw ha−1) and was significantly different from N1-T0 (41 Mg fw ha−1). A tendency to higher values was also associated with N1-T1 treatment (53 Mg fw ha−1), showing intermediate values between N1-T2 and N1-T0 plots. The beneficial effect of Trichoderma inoculation recorded in N1, was not observed under both sub-optimal (N0) and supra-optimal (N2) conditions (Table 1). Nevertheless, an increasing trend was recorded in the absence of fertilization with lower marketable yield for N0-T0 (28.2 Mg fw ha−1) and a higher value for N0-T2 (38.4 Mg fw ha−1). Moreover, dry biomass production significantly varied under different N managements and Trichoderma inoculations. Irrespective of N fertilization levels, T2 increased dry biomass production by 16% as compared to T0, with a significant effect under optimal fertilization (+24% corresponding to 0.7 Mg dw ha−1) as well as in the absence of fertilization (+40% corresponding to 0.6 Mg dw ha−1). In addition, no significant difference on lettuce dry biomass production was recorded between T2 and T1 in the absence of N fertilization (Table 1).

Table 1.
Lettuce and rocket marketable and unmarketable yields, leaf dry matter content, and dry biomass production in relation to N fertilization levels (N0 = 0, N1 = 90, and N2 = 180 kg ha−1 for lettuce and N0 = 0, N1 = 60, and N2 = 120 kg ha−1 for rocket) and microbial-based biostimulants (T0 = non-inoculated control, T1 = T. harzianum strain T22, T2 = T. virens strain GV41).
Rocket average marketable yield increased with N1 fertilization (+17% compared to N0), and no difference occurred in production when the fertilization N dose was doubled (i.e., N2). Moreover, inoculation with T2 exerted a significant effect in non-fertilized plots by increasing marketable yield by 10.8 Mg fw ha−1, increasing yields to the same level of N1 fertilized plots (Table 1). In contrast, the same inoculation treatment significantly reduced marketable yield under supra-optimal (N2) conditions, even though the values were not different from N1-T0 combination. Finally, a significant N × T interaction was observed for rocket dry biomass production. The effect of tested treatments (N fertilization and microbial-based biostimulant) on this parameter was the same as found for the marketable yield, since T2 inoculation significantly increased biomass allocation in leaves in the absence of fertilization, while the beneficial effect of Trichoderma inoculation was absent when rocket plants were grown under both N1 and N2 regimes (Table 1).
3.2. Effect of N Fertilization Levels and Microbial-Based Biostimulants on Lettuce and Rocket N Uptake and Balance and Nitrogen Use Efficiency
As expected, fertilization increased N uptake in lettuce (Table 2), nevertheless, no difference was recorded between the tested N fertilization levels; with an average N removal of 146 kg N ha−1 that corresponded to an apparent N recovery (ANR) of 167% and 80% under N1 and N2 fertilization regimes, respectively.

Table 2.
Lettuce and rocket N uptake, N balance, apparent N recovery (ANR), and nitrogen use efficiency (NUE) in relation to N fertilization levels (N0 = 0, N1 = 90, and N2 = 180 kg ha−1 for lettuce and N0 = 0, N1 = 60, and N2 = 120 kg ha−1 for rocket) and microbial-based biostimulants (T0 = non-inoculated control, T1 = T. harzianum strain T22, T2 = T. virens strain GV41).
Trichoderma virens GV41 (T2) inoculation significantly increased lettuce N uptake under optimal fertilization (+32% compared to N1-T0 treatment), augmenting ANR from 146% to 192%, while a slight increase in N uptake was recorded with T1 inoculation (+12%). A marked effect of both inoculations on lettuce N uptake was recorded in the absence of fertilization (N0), with a 34% and 51% increase for T1 and T2, respectively. Conversely, no effect of either Trichoderma treatments was recorded under supra-optimal fertilization regimes (N2; Table 2).
NUE-T values did not differ among treatments, with an average value of 50%, while NUE-C showed a significant N × T interaction. No effect of either microbial inoculations was recorded under N2 fertilization, whereas under optimal fertilization (N1) T2 raised NUE-C values from 70% to 116%, in comparison to T1 treatment (Table 2).
Nitrogen uptake of rocket (Table 2) was at the same level of lettuce under both fertilization regimes, while N uptake in non-fertilized plots was higher in rocket (121 kg N ha−1) than in lettuce (87 kg N ha−1). For both Trichoderma inoculations, there was a tendency towards lower values recorded under N1 fertilization, whereas inoculation with T2 applied in the absence of fertilization raised N uptake to levels not different from the fertilized plots and higher than N2-T1. Moreover, apparent N recovery was not different between the two fertilization regimes, with values ranging from 201% to 250% in N1 and from 96% to 106% in N2 treatment. Rocket N fertilizer use efficiency decreased when the N fertilization dose was doubled (N2) (from 22% to 1% for NUE-T; from 55% to 17% for NUE-C), and average values were lower than those recorded for lettuce (NUE-C equal to 67% and 36%, and NUE-T equal to 51% and 12% for lettuce and rocket, respectively). Rocket NUE-C values did not show differences between the microbial treatments, with an average value of 36%, while average NUE-T values were highest with T0 and T1 and were even negative with T2. Finally, the same pattern was recorded under N1 fertilization, while N uptake values T0 and T1 were not different under N2 and higher than T2 (Table 2).
3.3. SPAD Index, Leaf Colorimetry, Antioxidant Activity, and Total Phenols Content
SPAD (soil plant analysis development) index in both leafy vegetables was significantly affected by N fertilization levels, without the N × T interaction (Table 3). When averaged over microbial-based biostimulants, N application positively influenced the non-destructive measurement of chlorophyll content (i.e., SPAD index) showing the highest values for N2 in both crops (Table 3). Furthermore, in lettuce, an increasing trend was also recorded for N1 showing an intermediate value between N2 and non-fertilized plots. Among the physical properties, the visual appearance, particularly leaf color, is a primary criterion used in consumers’ product selection [43]. In the current study, neither N fertilization level nor Trichoderma-based biostimulants had a significant effect on the leaf colorimetric Commission internationale de l’éclairage (CIELAB) parameters in lettuce (Table 3). On the other hand, augmenting N applications resulted in lighter green rocket leaves, with the highest values recorded in the N2 treatment (Table 3). Finally, augmenting N fertilization levels from 0 to 120 kg ha−1 decreased both chroma coordinate a* in the N2 treatment, with no significant differences between the N0 and N1 fertilization levels (Table 3).

Table 3.
Lettuce and rocket soil plant analysis development (SPAD) index and hunter color parameters (L*, a*, and b*) in relation to N fertilization levels (N0 = 0, N1 = 90, and N2 = 180 kg ha−1 for lettuce and N0 = 0, N1 = 60, and N2 = 120 kg ha−1 for rocket) and microbial-based biostimulants (T0 = non-inoculated control, T1 = T. harzianum strain T22, T2 = T. virens strain GV41).
A tendency to higher total phenols content in lettuce (Figure 1a) was recorded with both fertilizations for non-inoculated plants even if a significant increase (+21%) was recorded only for N1-T2 treatment (+21% compared to N0-T2) showing also the highest value compared to all the tested treatments. In general, T. virens strain GV41-based biostimulant increased total phenols in lettuce leaves only in the N1 condition, where T2 produced values that were significantly higher than T0.
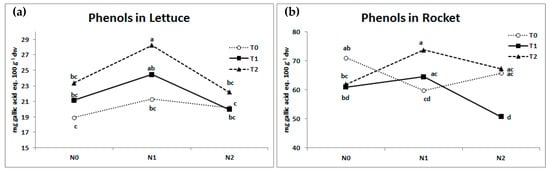
Figure 1.
Total phenols content in (a) lettuce and (b) rocket in relation to N fertilization levels (N0 = 0, N1 = 90, and N2 = 180 kg ha−1 for lettuce and N0 = 0, N1 = 60, and N2 = 120 kg ha−1 for rocket) and microbial-based biostimulants (T0 = non-inoculated control, T1 = T. harzianum strain T22, T2 = T. virens strain GV41). Mean values with the same letter were not different according to the LSD test (p < 0.05).
Rocket showed a very different and inconsistent pattern in total phenols (Figure 1b). Measured values were significantly higher than those in lettuce, regardless of fertilization and inoculation treatments (average values of 63.9 and 22.0 mg gallic acid eq. 100 g−1 dw for rocket and lettuce, respectively). The N1 fertilization treatment was anomalous (Figure 1b), demonstrating a significant reduction in total phenol accumulations with non-inoculated plants (−16%), while the same fertilization regime significantly increased total phenols accumulation with T2 treatment (+19%). There was a general tendency to lower phenols in the absence of N fertilization by both T1 and T2, whereas under N2 fertilization only T1 was observed to significantly reduce the total phenols accumulation.
Antioxidant activity in lettuce was only affected by the T2 microbial-based biostimulant (Figure 2) but not influenced by N fertilization levels or the N × T interaction (data not shown). When averaged over N fertilization levels T. virens strain GV41 (T2) inoculation elicited a significant increase (+15%) of antioxidant activity compared to T0 and T1. Finally, the effect of the two tested factors on antioxidant activity of rocket was not significant with values ranging between 1.63 and 1.78 (mmol ascorbate eq. 100 g−1) dw.
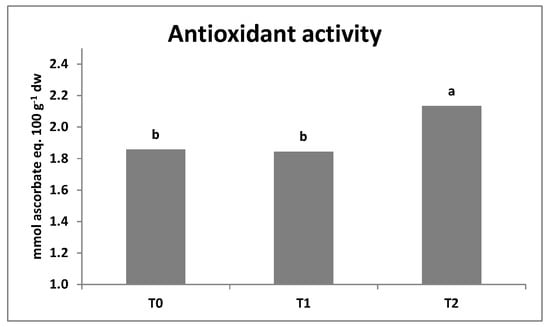
Figure 2.
Mean effect of microbial-based biostimulants on antioxidant activity in lettuce (T0 = non-inoculated control, T1 = T. harzianum strain T22, T2 = T. virens strain GV41). Mean values with the same letter were not different according to the LSD test (p < 0.05).
4. Discussion
Lettuce and rocket responses to tested treatments (N fertilization levels and microbial-based biostimulants) were clearly different (Table 1), both in terms of biomass accumulation and marketable yield. According to the average effect of treatments, lettuce was more responsive to both N fertilization (higher dry biomass production and yield with N1 and N2) and microbial-based biostimulant (higher dry biomass production with T2 and yield with both T1 and T2), while rocket showed only a significant increase of marketable yield due to fertilization. The interactive effect of tested factors on biomass allocation clearly demonstrated a discrepancy in lettuce and rocket behavior. Indeed, even if T. virens GV41 (T2) increased dry biomass production of both crops in the absence of N fertilizers, only lettuce showed an analogous effect with T. harzianum T22 (T1) and an increased biomass production in T2 inoculated plants under optimal fertilization (N1).
Marketable yield confirmed this pattern, showing a significant effect of N0-T2 for rocket whereby the values were comparable to the best treatments. In addition, a tendency to higher values for inoculated lettuce under the fertilized regimes was recorded. Surprisingly, T2 reduced rocket marketable weight when fertilizer rate was doubled (N2), as confirmed by an increase in unmarketable fraction. This differential response in the tested crops was previously discussed by [36], suggesting that the inoculation strategies (root dip for lettuce and seed coating for rocket), as well as the duration of the cropping cycle (57 and 28 days for lettuce and rocket, respectively) could have an effect on Trichoderma colonization in the plant rhizosphere. The same authors suggested that rocket, belonging to Brassicaceae species, produces many glucosinolates that have an antimicrobial effect and, therefore, could negatively influence Trichoderma colonization in the rhizosphere. An additional hypothesis regarding the poor rocket response to inoculation under T2 treatment, could also be associated to a reduction of the growth cycle due to microbial biostimulation. The effect of microbial-biostimulants on crop senescence (early flowering) has been reported for other vegetables such as zucchini [44] and eggplant [45]. A similar effect was recorded by [46] on banana trees (cv. Grand Naine) treated with Trichoderma-based biostimulants that anticipated flowering. No information has been found for leafy vegetables on this topic according to the current scientific literature, nevertheless the significant reduction of rocket marketable yield with N2-T2 treatment and the tendency to lower values in other inoculated treatments under optimal and excessive fertilization regime, could be due to an early senescence promoted by the inoculation.
Similar to the effects of inoculation and fertilization on biomass production, our results highlighted the higher N uptaking efficiency of rocket, even without N fertilization (68 vs. 101 kg N ha−1 in N0-T0 for lettuce and rocket, respectively; Table 2). It is well known that Brassicaceae species, such as rocket, are potentially toxic elements (PTE) and N hyperaccumulator plants even used for phytoremediation of contaminated polluted sites [47,48] as well as N catch crops to limit nitrate leaching [49]. This could explain the low effect of both fertilizers and Trichoderma-based biostimulants on rocket N uptake. Inoculation with T. virens GV41 increased N uptake of both lettuce and rocket in non-fertilized plots, with values not different than N1-T0, even if values were higher than N2-T1 treatment. The marked effect of Trichoderma inoculation under abiotic stress conditions such as nutrient deficiency and low water availability has also been recognized by other authors for vegetable crops [50].
ANR values under optimal fertilization (N1) were significantly greater than 100% in both crops (167% and 224%, average values for lettuce and rocket, respectively), which implies that the sole N input from fertilizers was not sufficient to satisfy crop N requirements. For lettuce under N1 management, almost 60 kg N ha−1 uptaken by plants (67% of N input) was soil native N, while for rocket soil contribution to crop N nutrition corresponded to 74 kg N ha−1 (124% of N input). Values of native N adsorbed by crops were of the same order of magnitude as N uptake in control plots (N0-T0), for both lettuce (68 kg N ha−1) and rocket (101 kg N ha−1), but were significantly lower than those measured in plants inoculated with T2 (103 and 161 kg N ha−1, for lettuce and rocket, respectively) in absence of N fertilization. This indicates that soil mineralization flush was better intercepted by crops when inoculated with T. virens GV41 (T2), suggesting an increased N uptake in inoculated roots. According to this evidence, T2 values under non-fertilized conditions can be likely assumed as plant-available N from soil organic N mineralization and added to the N input–N output calculation to complete the N balance. According to this calculation the complete N balance under N1 fertilization regime was 43 and 87 kg N ha−1 for lettuce and rocket, highlighting an N surplus to be considered as residual N fertility for an accurate planning of fertilization of the whole cropping cycle. For N2 treatments, very high N surpluses of about 140 and 158 kg N ha−1 were recorded for lettuce and rocket respectively, highlighting that this fertilization strategy is meaningless from both an environmental and economic point of view.
Our findings also indicate that T. virens GV41 inoculation can be a useful tool to minimize N additional inputs to the cropping system, which permits a reduction of production costs to farmers and of N surpluses prone to be volatilized as N2O or NH3 or leached in the watertable [51]. Ideally a reduction of mineral N inputs below the optimal dose coupled with T. virens GV41 inoculation can be considered to achieve this goal, but this option could lead to nutrient mining due to an overexploitation of soil reservoirs in the long period. This is because mineral fertilizers represent a short-term strategy aimed at supplying nutrients to plants, without taking into account N deficits balanced by native N mineralization. A systemic approach to the fertility management should consider a partial or a total substitution of mineral fertilizers with slow available N sources, as compost and digestate [41,52], in order to balance the N deficits and increase the soil N supplying potential from organic N fraction. This choice could be appropriate for lettuce inoculated with T. virens GV41 that showed a higher NUE-C with T2 (116%) compared to the other inoculations (70% and 87%, for T0 and T1, respectively) under optimal N management (N1). Otherwise, in our experimental conditions, the best practice for rocket should be to avoid plant inoculation under fertilized managements since N1-T2 effects showed very low NUE-C (32%) and NUE-T (−66%) values. The management of soil organic fertility of the whole cropping system can minimize direct N fertilization to rocket, which could take advantage of an increased native N mineralization when plant uptake is enhanced by T2 inoculation.
As previously discussed, the estimated available native mineral N, equivalent to N uptake under T2 non-fertilized plots, in our experiment corresponded to a cumulative value of 264 kg N ha−1 for the entire crop sequence (85 days). This value was significantly higher than that estimated for our soil using the Remy et al. [53] equation, which determines an annual N availability of 100 kg N ha−1 yr−1 [36]. This evidence indicates that under greenhouse conditions N mineralization rates can be more than doubled due to higher soil moisture content, maintained close to water holding capacity by irrigation, and due to higher temperatures compared to outdoor conditions (i.e., open field). Lei and McDonald [54] effectively described the interactive effects of soil moisture and temperature on N mineralization in a high tunnel farming system. They suggested that a fertilization strategy aimed at increasing N self-supplying soil under greenhouse must be targeted to both crop N requirements and expected mineralization rates under specific climatic conditions. Thus, when organic amendments are applied, mineralization rates of these organic N sources as well as of the non-fertilized soil should be carefully investigated and determined in controlled laboratory incubation tests [55] prior to applications in real field conditions.
The application of microbial-based biostimulants is able to modify plant primary and secondary metabolism [19,23] leading to the synthesis and accumulation of antioxidant molecules (i.e., secondary metabolites) which are important for the human diet. This was the case in the current experiment, whereby lettuce and rocket plants treated with GV41 under optimal N (N1) positively modulated the synthesis and accumulation of total polyphenols (Figure 1), which have been reported to have greater antioxidant activity than vitamin C [56]. Possible putative mechanisms by which the strain T. virens GV41 stimulates phytochemical production could be (i) the activation of key enzymes leading to the production of flavonoids as well as the amino acids, phenylalanine and tyrosine, precursors of total phenols in the phenylpropanoid pathway, and (ii) the higher assimilation and translocation of P and especially N leading to a higher production of phenylalanine ammonia-lyase (PAL), a key enzyme involved in the production of polyphenols [23]. Furthermore, the improvement of antioxidant capacity, a functional quality parameter of food with Trichoderma inoculation was species-dependent, since the increase in antioxidant activity content was only observed in lettuce plants (Figure 2). Therefore, our results highlight the need to conduct further investigations on this argument to unravel the physiological and molecular mechanism(s) behind the synthesis and accumulation of these secondary metabolisms in edible leafy produce. Finally, future research should also focus on designing ideotype Trichoderma strains and identifying the best combination of crop species × Trichoderma strains for the production of healthy and nutrient-dense foods [33].
5. Conclusions
Managing greenhouse crop nutrition under Mediterranean conditions requires a deep understanding of N suppling potential of soils, to regulate N inputs and limit N surpluses. In our experiment, N surpluses occurred even under optimal N fertilization management (43 and 87 kg N ha−1), meaning that recommended N doses locally identified for tested crops are sized only to ensure optimal productive levels, without considering soil N mineralization as a possible input for crop nutrition.
Native mineral N can represent an important source for the nutrition of leafy crops, as proven in our experiment, contributing with 60 and 100 kg N ha−1 to total uptake of lettuce and rocket, respectively. Thus, a modification of the paradigm of fertilization, with the introduction of practices aimed at increasing N supply potential, as an organic fertilization of soils, can reduce the need for mineral N inputs. This approach makes sense to minimize N inputs for the cultivation of short and medium vegetable crop cycles, whose N uptake requires good synchronization with available native and fertilizer nitrogen.
On the other hand, the application of microbial-based biostimulants through Trichoderma inoculation is recognized as a tool to improve NUE of leafy crops with several economic (yield increase, reduction of costs due to N fertilizers), nutritional, and environmental benefits. A clear positive effect of Trichoderma virens GV41-based biostimulants was identified for lettuce under our experimental conditions, demonstrating an increase in NUE up to 116% under recommended N fertilization, thus suggesting that a further reduction of N inputs in the production of this crop is possible. It must be pointed out that an increase of N root uptake potential, coupled with a reduction of N inputs, is a feasible option only when soil native N fertility is preserved. Intensive greenhouse/protected vegetable systems are prone to a severe reduction in soil fertility due to the high tillage frequency as well as to the absence of well-planned crop rotations. Once more, the sole option to counterbalance soil fertility decline in these systems is to increase soil organic fertility. Trichoderma virens GV41 inoculation of lettuce was associated with a clear increase of phenols content and antioxidant activity, indicating that this crop’s biostimulation can promote growth and functional quality of this produce. Our findings indicate that rocket can take advantage of Trichoderma virens GV41 inoculations when there is an absence/lack of fertilizer inputs, increasing both marketable yield and N uptake. Therefore, inoculation with microbial biostimulants to rocket can be considered in leafy vegetable cropping systems as a tool to efficiently utilize residual N fertility from previous crop production. Trichoderma-based biostimulants prove an effective tool for enhancing NUE of leafy cropping systems, and their application provides a good opportunity/strategy for careful management of soil N fertility in order to avoid an overexploitation of soil N supplying potential.
Author Contributions
Experiment conceptualization, N.F. and Y.R.; biostimulant preparation and crop inoculation, S.L.W.; crop management and samplings, E.C., D.V., and N.F.; laboratory analyses, D.V.; data analyses, N.F. and D.V.; manuscript writing D.V., N.F., and Y.R.; manuscript review M.F. and S.L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank Roberto Maiello and Sabrina Nocerino for their support in laboratory work. We would also thank Vincenzo Cenvinzo for his support in crop management and sampling. Concluding, our recognition goes to Giovanna Ameno for her support in bibliographic research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kant, S. Understanding nitrate uptake, signaling and remobilisation for improving plant nitrogen use efficiency. Semin. Cell Dev. Biol. 2018, 74, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Awaad, M.S.; Badr, R.A.; Badr, M.A.; Abd-elrahman, A.H. Effects of different nitrogen and potassium sources on lettuce (Lactuca sativa L.) yield in a sandy soil. Eurasian J. Soil Sci. 2016, 5, 299–306. [Google Scholar] [CrossRef][Green Version]
- Weightman, R.M.; Hudson, E.M. Noxious or nutritious? Progress in controlling nitrate as a contaminant in leafy crop species. Food Energy Secur. 2013, 2, 141–156. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Growth and yield of garden rocket (Eruca sativa Mill.) affected by nitrogen and potassium fertilization. Acta Sci. Pol. Hortic. 2009, 8, 23–33. [Google Scholar]
- Bozkurt, S.M.; Mansuroğlu, G.S.; Kara, M.; Önder, S. Responses of lettuce to irrigation levels and nitrogen forms. Afr. J. Agric. Res. 2009, 4, 1171–1178. [Google Scholar]
- Gülser, F.; Sönmez, F.; Boysan, S. Effect of calcium nitrate and humic acid applications on growth and yield criteria of pepper seedling under salt stress. J. Environ. Biol. 2010, 31, 873–876. [Google Scholar]
- European Council. Directive of the Council of December 12, 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources (1991/676/EEC); European Commission: Brussels, Belgium, 1991. [Google Scholar]
- Davidson, E.A.; David, M.B.; Galloway, J.N.; Goodale, C.L.; Haeuber, R.; Harrison, J.A.; Howarth, R.W.; Jaynes, D.B.; Lowrance, R.R.; Nolan, B.T.; et al. Excess Nitrogen in the U.S. Environment: Trends, Risks, and Solutions; Issues in Ecology; Ecological Society of America: Washington, DC, USA, 2012. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Lyu, X.; Wang, T.; Ma, Z.M.; Zhao, C.Y.; Siddiquee, K.H.M.; Ju, X.T. Enhanced efficiency nitrogen fertilizers maintain yields and mitigate global warming potential in an intensified spring wheat system. Field Crops Res. 2019, 244, 107624. [Google Scholar] [CrossRef]
- Walsh, O.; Raun, W.; Klatt, A.; Solie, J. Effect of delayed nitrogen fertilization on maize (Zea mays L.) grain yields and nitrogen use efficiency. J. Plant Nutr. 2012, 35, 538–555. [Google Scholar] [CrossRef]
- Mi, W.H.; Gao, Q.; Xia, S.Q.; Zhao, H.T.; Wu, L.H.; Mao, W.; Hu, Z.P.; Liu, Y.L. Medium-term effects of different types of N fertilizer on yield, apparent N recovery, and soil chemical properties of a double rice cropping system. Field Crops Res. 2019, 234, 87–94. [Google Scholar] [CrossRef]
- Mi, G.; Chen, F.; Yuan, L.; Zhang, F. Ideotype root system architecture for maize to achieve high yield and resource use efficiency in intensive cropping systems. Adv. Agron. 2016, 139, 73–97. [Google Scholar]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Youssef, R. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-throughput plant phenotyping for developing novel biostimulants: From lab to field or from field to lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Saia, S.; Colla, G.; Raimondi, G.; Di Stasio, E.; Cardarelli, M.; Bonini, P.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. An endophytic fungi-based biostimulant modulated lettuce yield, physiological and functional quality responses to both moderate and severe water limitation. Sci. Hortic. 2019, 256, 108595. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multi level properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L. Trichoderma: A Multi-Purpose Tool for Integrated Pest Management. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 345–353. [Google Scholar]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Lombardi, N.; D’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Woo, S.L.; Bonanomi, G.; Lorito, M. Application of Trichoderma strains and metabolites enhances soybean productivity and nutrient content. J. Agric. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef]
- Harman, G.R. Myths and dogmas of biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Huang, S.; Yang, X.; Wang, B.; Li, X.; Ma, Y. Nitrogen efficiency in long-term wheat–maize cropping systems under diverse field sites in China. Field Crops Res. 2010, 118, 145–151. [Google Scholar] [CrossRef]
- Singh, B.N.; Dwivedi, P.; Sarma, B.K.; Singh, G.S.; Singh, H.B. Trichoderma asperellum T42 reprograms tobacco for enhanced nitrogen utilization efficiency and plant growth when fed with N nutrients. Front. Plant Sci. 2018, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis; Black, C.A., Evans, D.D., White, I.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Fagnano, M.; Adamo, P.; Zampella, M.V.; Fiorentino, N. Environmental and agronomic impact of fertilization with composted organic fraction from municipal solid waste. Agric. Ecosyst. Environ. 2011, 141, 100–107. [Google Scholar] [CrossRef]
- Ladha, J.K.; Pathak, H.J.; Krupnik, T.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.M.; Schwarz, D. Vegetable grafting: The implications of a growing agronomic imperative for vegetable fruit quality and nutritive value. Front. Plant Sci. 2017, 8, 741. [Google Scholar] [CrossRef]
- Gázquez, J.C.; Meca, D.E.; López, J.C.; Baeza, E.J.; Pérez-Parra, J.J. Comparison of different strategies for fruit set in greenhouse zucchini (Cucurbita pepo L.). Acta Hortic. 2012, 927, 187–194. [Google Scholar] [CrossRef]
- Pohl, A.; Grabowska, A.; Kalisz, A.; Sękara, A. The eggplant yield and fruit composition as affected by genetic factor and biostimulant application. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 929–938. [Google Scholar] [CrossRef]
- Nayyer, M.A.; Tripathi, V.K.; Kumar, S.; Lal, D.; Tiwari, B. Influence of integrated nutrient management, growth, yield, and quality of tissue cultured banana (Musa x paradisiaca) cv grand naine. Indian J. Agric. Sci. 2014, 84, 680–683. [Google Scholar]
- Clemente, R.; Walker, D.J.; Bernal, M.P. Uptake of heavy metals and As by Brassica juncea grown in a contaminated soil in Aznalcollar (Spain): The effect of soil amendments. Environ. Pollut. 2005, 138, 46–58. [Google Scholar] [CrossRef]
- Fiorentino, N.; Mori, M.; Cenvinzo, V.; Duri, L.G.; Gioia, L.; Visconti, D.; Fagnano, M. Assisted phytoremediation for restoring soil fertility in contaminated and degraded land. Ital. J. Agron. 2018, 13, 34–44. [Google Scholar]
- Justes, E.; Beaudoin, N.; Bertuzzi, P.; Charles, R.; Constantin, J.; Dürr, C.; Hermon, C.; Joannon, A.; Le Bas, C.; Mary, B.; et al. The use of cover crops to reduce nitrate leaching: Effect on the water and nitrogen balance and other ecosystem services. In Proceedings-International Fertiliser Society; International Fertiliser Society: Cambrige, UK, 2012; pp. 4–43. [Google Scholar]
- Lucini, L.; Colla, G.; Miras Morebo, M.B.; Bernardo, L.; Cardarelli, M.; Terzi, V.; Bonini, P.; Rouphael, Y. Inoculation of Rhizoglomus irregulare or Trichoderma atroviride differentially modulates metabolite profiling of wheat root exudates. Phytochemistry 2019, 157, 158–167. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Stinner, W.; Deuker, A.; Leithold, G. Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr. Cycl. Agroecosyst. 2008, 82, 209–232. [Google Scholar] [CrossRef]
- Remy, J.C.; Marin-Lafleche, A. L’analyse de terre: Realisation d’un programme d’interpretation automatique. Ann. Agron. 1974, 25, 607–632. [Google Scholar]
- Lei, L.; Mc Donald, L.M. Soil moisture and temperature effects on nitrogen mineralization in a high tunnel farming system. Commun. Soil Sci. Plant Anal. 2019, 50, 2140–2150. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Bertora, B.; Pepe, O.; Moschetti, G.; Grignani, C.; Fagnano, M. Changes in soil mineral N content and abundances of bacterial communities involved in N reactions under laboratory conditions as predictors of soil N availability to maize under field conditions. Biol. Fertil. Soils 2016, 52, 523–537. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).