Modifying the CROPGRO Safflower Model to Simulate Growth, Seed and Floret Yield under Field Conditions in Southwestern Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Site Characteristics
2.3. Field Experiments
2.4. Data Collection
2.4.1. Non-Destructive
2.4.2. Destructive
2.5. Model Input
2.6. Model Evaluation and Adaptation Based on Literature and Data
2.7. Statistics
3. Results and Discussion
3.1. Model Modification
3.2. Life Cycle and Canopy Development
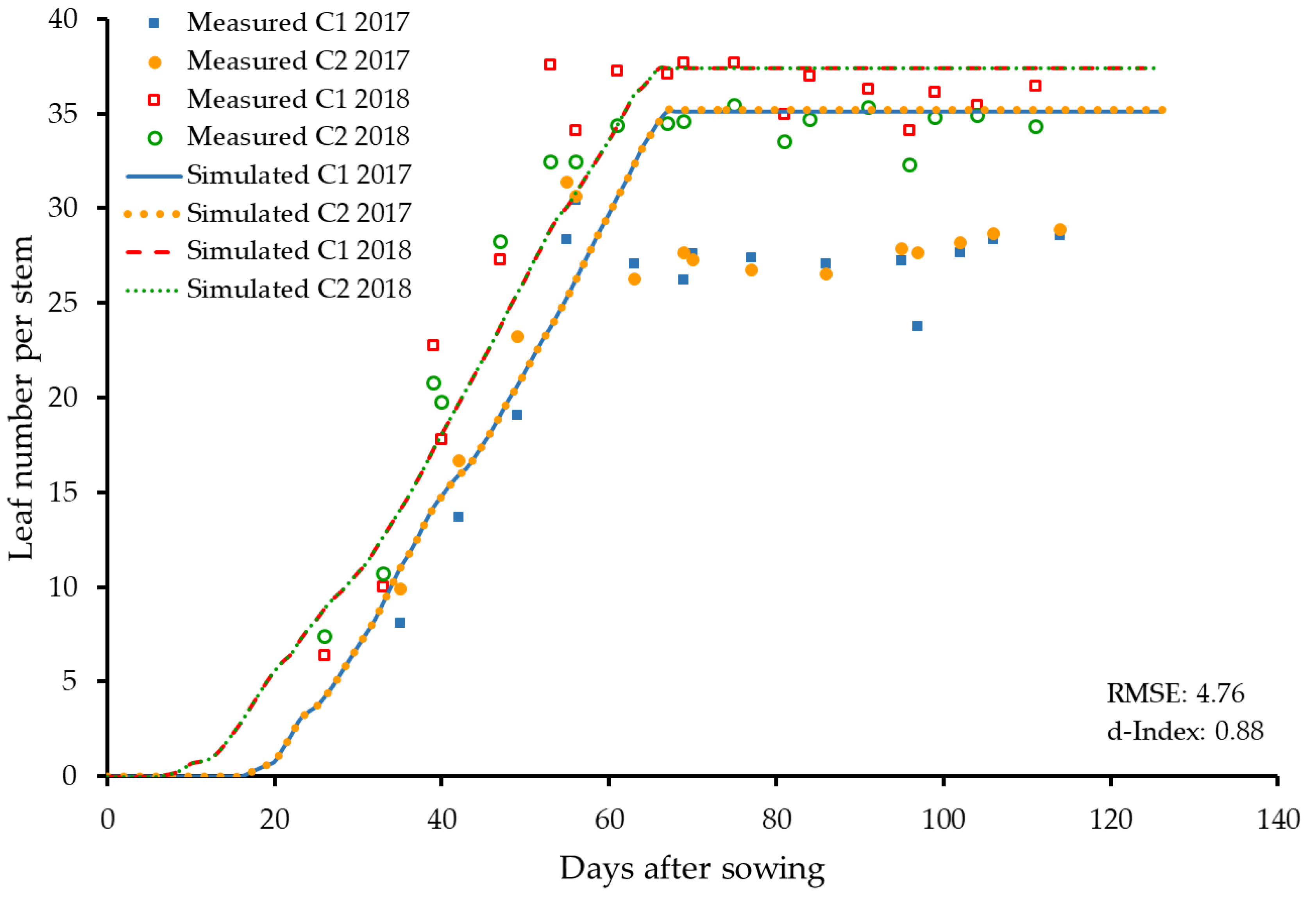
3.2.1. Leaf Number
3.2.2. Height and Width
3.2.3. Time to Flowering
3.2.4. Specific Leaf Area and Leaf Area Index
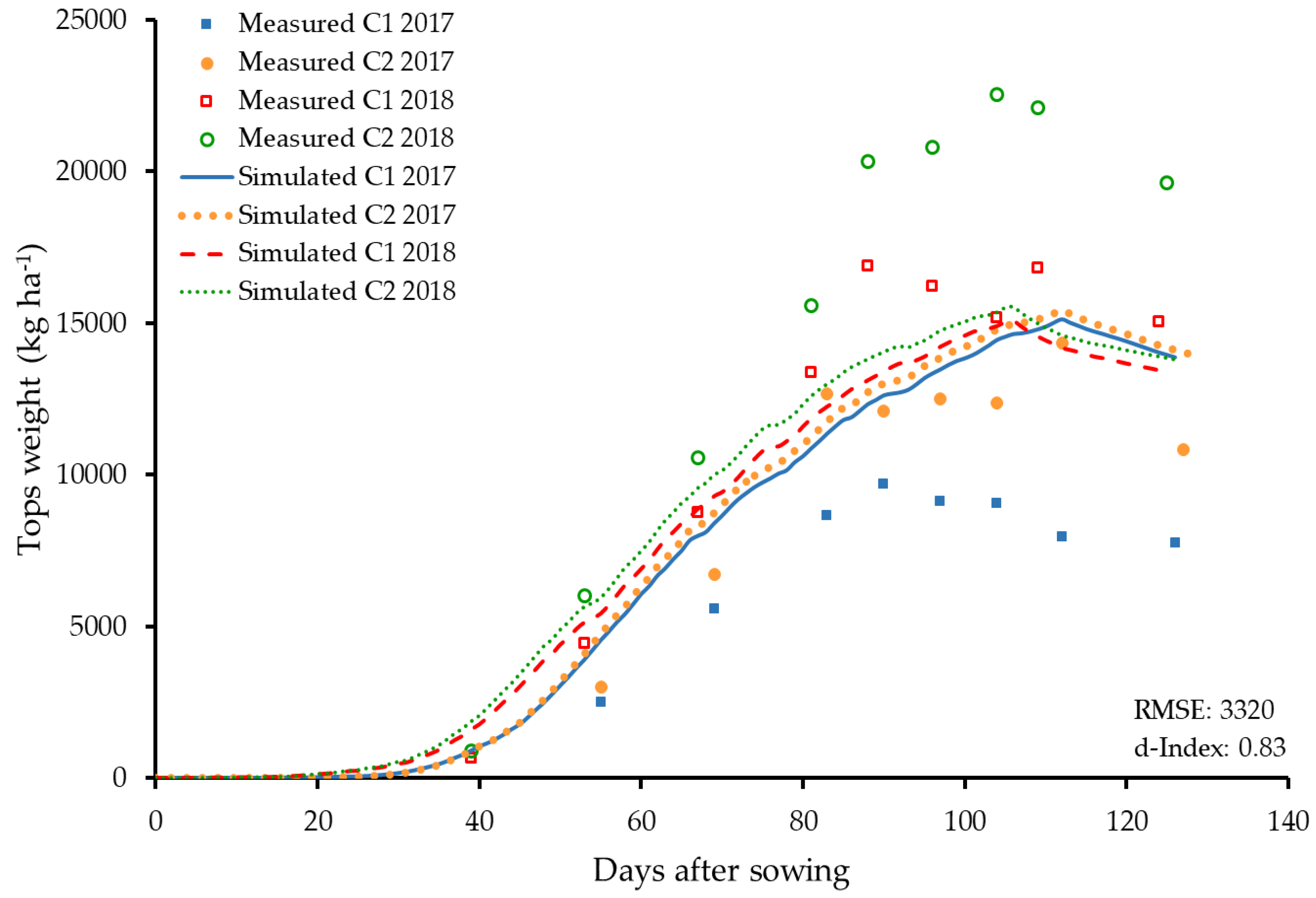
3.3. Dry Matter Accumulation and Partitioning
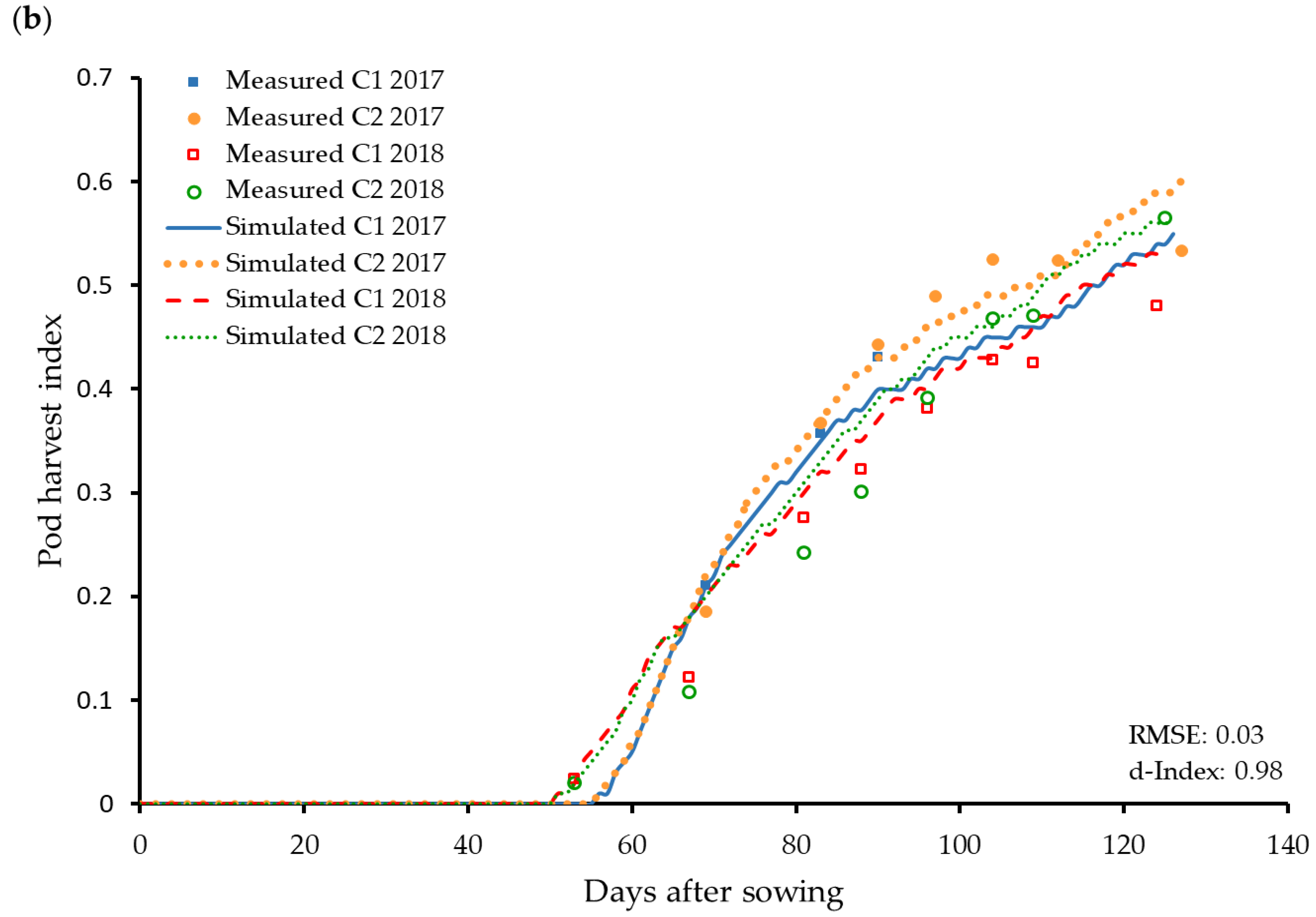
3.4. Reproductive Organs and Yield Influencing Parameters
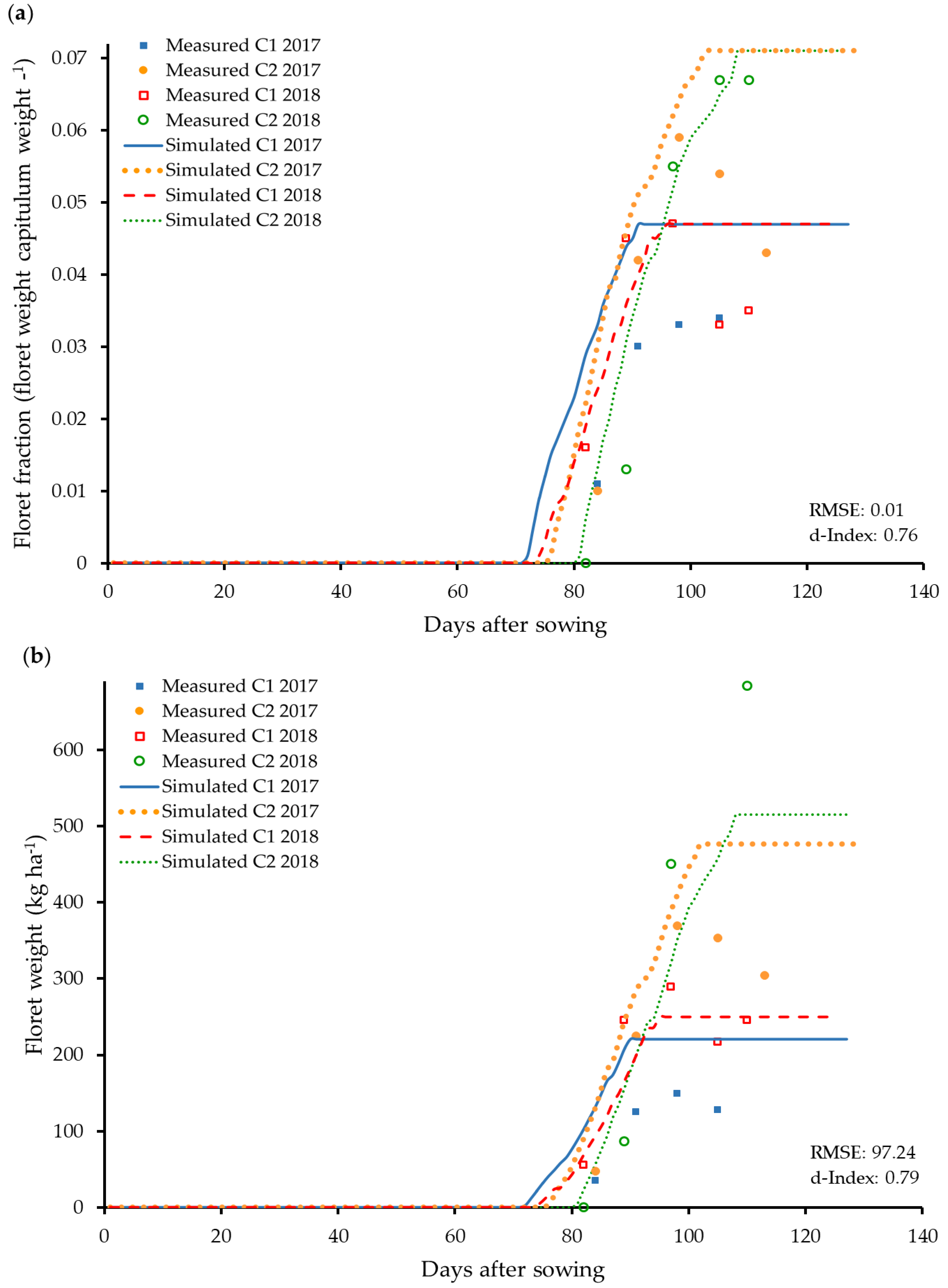
3.5. Modelling of Floret Yield and the Relationship to the Flower Capitulum
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Emongor, V. Safflower (Carthamus tinctorius L.) the underutilized and neglected crop: A review. Asian J. Plant Sci. 2010, 9, 299–306. [Google Scholar] [CrossRef]
- Weiss, E.A. Oilseed Crops, 2nd ed.; Blackwell Science: Oxford, UK, 2000; ISBN 0632052597. [Google Scholar]
- Dajue, L.; Mündel, H.-H. Safflower, Carthamus Tinctorius L.; IPGRI.; IPK: Rome, Italy, 1996; ISBN 9290432977. [Google Scholar]
- Velasco, L.; Fernández-Martínez, J.M. Progress in breeding for modified tocopherol content and composition in safflower. Sesame Safflower Newsl. 2002, 17, 98–102. [Google Scholar]
- Weiss, E.A. Castor, Sesame and Safflower; Hill: London, UK, 1971; ISBN 0249440210. [Google Scholar]
- Corleto, A.; Alba, E.; Polignano, G.B.; Vonghio, G. Safflower: A multipurpose species with unexploited potential and world adaptability. In Proceedings of the IVth International Safflower Conference, Bari, Italy, 2–7 June 1997; Corleto, A., Mündel, H.-H., Eds.; Adruatica Editrice: Bari, Italy, 1997; pp. 23–31. [Google Scholar]
- Ekin, Z. Resurgence of safflower (Carthamus tinctorius L.) utilization: A global view. J. Agron. 2005, 4, 83–87. [Google Scholar]
- Arnold, L.E.; Lofthouse, N.; Hurt, E. Artificial food colors and attention-deficit/hyperactivity symptoms: Conclusions to dye for. Neurotherapeutics 2012, 9, 599–609. [Google Scholar] [CrossRef]
- Bateman, B.; Warner, J.O.; Hutchinson, E.; Dean, T.; Rowlandson, P.; Gant, C.; Grundy, J.; Fitzgerald, C.; Stevenson, J. The effects of a double blind, placebo controlled, artificial food colourings and benzoate preservative challenge on hyperactivity in a general population sample of preschool children. Arch. Dis. Child. 2004, 89, 506–511. [Google Scholar] [CrossRef]
- Křížová, H. Natural dyes: Their past, present, future and sustainability. Recent Dev. Fibrous Mater. Sci. 2015, 12, 59–71. [Google Scholar]
- Future Market Insights. Natural Food Colours Market: Significant Demand for Clean Label and Naturally Sourced Ingredients in Food Products Spurring Revenue Growth: Global Industry Analysis (2013–2017) & Opportunity Assessment (2018–2028). Available online: https://www.futuremarketinsights.com/reports/global-natural-food-colours-market (accessed on 6 May 2019).
- Grand View Research, I. Natural Food Colors Market Estimates & Trend Analysis by Product (curcumin, Carotenoids, Anthocyanin, Carmine, Chlorophyllin), by Application (Bakery & Confectionery, Beverages, Dairy & Frozen Products, Meat Products), and Segment Forecasts, 2018–2025. Available online: https://www.grandviewresearch.com/industry-analysis/natural-food-colors-market (accessed on 6 May 2019).
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural colorants: Historical, processing and sustainable prospects. Nat. Prod. Bioprospect. 2017, 7, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Dajue, L. Progress research and production in China. In Proceedings of the Third International Safflower Conference, Beijing, China, 14–18 June 1993; Li, D., Henning, H., Eds.; Beijing Botanical Garden, Institute of Botany, Chinese Academy of Sciences: Beijing, China, 1993; pp. 35–46. [Google Scholar]
- Yoon, J.-M.; Cho, M.-H.; Park, J.-E.; Kim, Y.-H.; Hahn, T.-R.; Paik, Y.-S. Thermal stability of the pigments hydroxysafflor yellow A, safflor yellow B, and precarthamin from Safflower (Carthamus tinctorius). J. Food Sci. 2003, 68, 839–843. [Google Scholar] [CrossRef]
- Shin, Y.-S.; Yoo, D.-I. Storage stability and color reproducibility of yellow and red dyes extracted from Carthamus tinctorius L. Text. Coloration Finish. 2012, 24, 165–172. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT-Crop Statistics: Safflower. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 7 May 2019).
- Elfadl, E.; Reinbrecht, C.; Claupein, W. Evaluation of phenotypic variation in a worldwide germplasm collection of safflower (Carthamus tinctorius L.) grown under organic farming conditions in Germany. Genet. Resour. Crop Evol. 2010, 57, 155–170. [Google Scholar] [CrossRef]
- Elfadl, E.Y.; Reinbrecht, C.; Frick, C.; Witzke, S.V.; Rudolphi, S.; Claupein, W.; Esendal, E.; Bergman, J.W.; Kandemir, N.; Johnson, R.C.; et al. Genotype by environment interaction in safflower (Carthamus tinctorius L.) grown under organic farming system in Central Europe. In Proceedings of the VIth International Safflower Conference, Safflower: A Unique Crop for Oil Spices and Health Consequently, a Better Life for You, Istanbul, Turkey, 6–10 June 2005; Esendal, E., Bergman, J.W., Eds.; Engin Maatbacilik Ltd. Scedillati: Istanbul, Turkey, 2005; pp. 39–43. [Google Scholar]
- Elfadl, E.; Reinbrecht, C.; Claupein, W. Safflower (Carthamus tinctorius L.) as a new oil crop in organic farming system: Potential and stability in central Europe. Int. J. AgriSci. 2012, 2, 477–495. [Google Scholar]
- Frick, C.; Hebeisen, T.; Reinbrecht, C. Distelöl aus Schweizer Saflor? AGRARForschung Schweiz 2005, 12, 146–151. [Google Scholar]
- Reinbrecht, C.; Barth, S.; Witzke-Ehbrecht, S.v.; Frick, C.; Elfadl, E.; Kahnt, G.; Becker, H.C.; Claupein, W.; Esendal, E.; Bergman, J.W.; et al. Screening of a world wide safflower collection for adaptation to humid temperate climates and cultivation in organic farming. In Proceedings of the VIth International Safflower Conference, Safflower: A Unique Crop for Oil Spices and Health Consequently, a Better Life for You, Istanbul, Turkey, 6–10 June 2005; pp. 236–242. [Google Scholar]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Boote, K.J.; Jones, J.W.; Pickering, N.B. Potential uses and limitations of crop models. Agron. J. 1996, 88, 704. [Google Scholar] [CrossRef]
- Hoogenboom, G. Contribution of agrometeorology to the simulation of crop production and its applications. Agric. For. Meteorol. 2000, 103, 137–157. [Google Scholar] [CrossRef]
- Boote, K.J.; Mínguez, M.I.; Sau, F. Adapting the CROPGRO legume model to simulate growth of faba bean. Agron. J. 2002, 94, 743–756. [Google Scholar] [CrossRef]
- Singh, S.; Boote, K.J.; Angadi, S.V.; Grover, K.; Begna, S.; Auld, D. Adapting the CROPGRO model to simulate growth and yield of spring safflower in semiarid conditions. Agron. J. 2016, 108, 64. [Google Scholar] [CrossRef]
- Präger, A.; Boote, K.J.; Munz, S.; Graeff-Hönninger, S. Simulating Growth and Development Processes of Quinoa (Chenopodium quinoa Willd.): Adaptation and Evaluation of the CSM-CROPGRO Model. Agronomy 2019, 9, 832. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006. A Framework for International Classification, Correlation and Communication; FAO: Rome, Italy, 2006; ISBN 9251055114. [Google Scholar]
- Thun, R.; Hoffmann, G. Texturanalyse des Feinbodens. Kombination von Nasssiebung und Pipettmethode nach KÖHN. In Die Untersuchung von Böden, 4th ed.; neubearb. u. erw. Aufl.; Bassler, R., Ed.; VDLUFA-Verl.: Darmstadt, Germany, 2012; ISBN 9783941273139. [Google Scholar]
- International Soil Reference and Information Centre; FAO. Lecture Notes on the Major Soils of the World; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; ISBN 9251046379. [Google Scholar]
- Thun, R.; Hoffmann, G. Bestimmung von mineralischem Stickstoff (Nitrat und Ammonium) in Bodenprofilen (Nmin-Labormethode). In Die Untersuchung Von Böden, 4th ed.; neubearb. u. erw. Aufl.; Bassler, R., Ed.; VDLUFA-Verl.: Darmstadt, Germany, 2012; A 6.1.4.1; ISBN 9783941273139. [Google Scholar]
- Dumas, A. Stickstoffbestimmung nach Dumas. In Die Praxis des Organischen Düngers, 41st ed.; Gattermann, L., Wieland, H., Eds.; DE GRUYTER: Berlin, Germany, 1962; pp. 45–51. ISBN 9783111142210. [Google Scholar]
- Flemmer, A.C.; Franchini, M.C.; Lindström, L.I. Description of safflower (Carthamus tinctorius) phenological growth stages according to the extended BBCH scale. Ann. Appl. Biol. 2015, 166, 331–339. [Google Scholar] [CrossRef]
- Anderson, R.L. Broadleaf weed control in safflower (Carthamus tinctorius) with sulfonylurea herbicides. Weed Technol. 1987, 1, 242–246. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Mündel, H.-H.; Derksen, D.A. Herbicides for weed control in safflower (Carthamus tinctorius). Can. J. Plant Sci. 1990, 70, 237–245. [Google Scholar] [CrossRef]
- Corre-Hellou, G.; Fustec, J.; Crozat, Y. Interspecific competition for soil N and its interaction with N2 fixation, leaf expansion and crop growth in pea–barley intercrops. Plant Soil 2006, 282, 195–208. [Google Scholar] [CrossRef]
- Hunt, L.A.; Boote, K.J. Data for model operation, calibration, and evaluation. In Understanding Options for Agricultural Production; Tsuji, G.Y., Hoogenboom, G., Thornton, P.K., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1998; pp. 9–39. ISBN 9789048149407. [Google Scholar]
- Jagtap, S.S.; Jones, J.W. Adaptation and evaluation of the CROPGRO-soybean model to predict regional yield and production. Agric. Ecosyst. Environ. 2002, 93, 73–85. [Google Scholar] [CrossRef]
- Willmott, C.J.; Ackleson, S.G.; Davis, R.E.; Feddema, J.J.; Klink, K.M.; Legates, D.R.; O’Donnell, J.; Rowe, C.M. Statistics for the evaluation and comparison of models. J. Geophys. Res. 1985, 90, 8995. [Google Scholar] [CrossRef]
- Willmott, C.J. Some comments on the evaluation of model performance. Bull. Amer. Meteor. Soc. 1982, 63, 1309–1313. [Google Scholar] [CrossRef]
- Yakoub, A.; Lloveras, J.; Biau, A.; Lindquist, J.L.; Lizaso, J.I. Testing and improving the maize models in DSSAT: Development, growth, yield, and N uptake. Field Crop. Res. 2017, 212, 95–106. [Google Scholar] [CrossRef]
- Singh, S.; Boote, K.J.; Angadi, S.V.; Grover, K.K. Estimating water balance, evapotranspiration and water use efficiency of spring safflower using the CROPGRO model. Agric. Water Manag. 2017, 185, 137–144. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Porter, C.H.; Shelia, V.; Boote, K.J.; Singh, U.; White, J.W.; Hunt, L.A.; Ogoshi, R.; Lizaso, J.I.; Koo, J.; et al. Decision Support System for Agrotechnology Transfer (DSSAT) Version 4.7; DSSAT Foundation: Gainsville, FL, USA, 2017. [Google Scholar]
- Hoogenboom, G.; Wilkens, P.W.; Tsuji, G.Y. DSSAT Concepts of Calibrating Crop Growth Models. Chaper Three. Systematic Approach and Order for Calibration, 3rd ed.; University of Hawaii: Honolulu, HI, USA, 1999; Volume 4. [Google Scholar]
- Shabana, R.; Abd El-Mohsen, A.A.; Gouda, H.A.H.; Hafez, H.S. Impact of temperature fluctuation on yield and quality traits of different safflower genotypes. Sci. Res. Rev. J. 2013, 1, 74–87. [Google Scholar]
- Balashahri, M.S.; Farhadi, R.; Dehostai, R.M.; Ghadiri, I.; Rahimi, M. Evaluation of cardinal temperatures and germination response to temperature in safflower (Carthamus tinctorius L.) medicinal plant. J. Am. Sci. 2013, 9, 56–60. [Google Scholar]
- Dajue, L.; Mingde, Z.; Ramanatha Rao, V. Characterization and Evaluation of Safflower Germplasm; Geological Pub. House; Beijing Botanical Garden, Institute of Botany, Chinese Academy of Sciences: Beijing, China, 1993; ISBN 9787116013988. [Google Scholar]
- Emongor, V.; Oagile, O. Safflower Production; Impression House Publication: Gaborone, Botswana, 2017; ISBN 978-99968-0-607-0. [Google Scholar]
- Paul, M.J.; Lawlor, D.W.; Driscoll, S.P. The effect of temperature on photosynthesis and carbon fluxes in sunflower and rape. J. Exp. Bot. 1990, 41, 547–555. [Google Scholar] [CrossRef]
- Mark, U.; Tevini, M. Combination effects of UV-B radiation and temperature on sunflower (Helianthus annuus L., cv. Polstar) and maize (Zea mays L, cv. Zenit 2000) seedlings. J. Plant Physiol. 1996, 148, 49–56. [Google Scholar] [CrossRef]
- Mündel, H.-H.; Blackshaw, R.E.; Byers, J.R.; Huang, H.C.; Johnson, D.L.; Keon, R.; Kubik, J.; McKenzie, R.; Otto, B.; Roth, B.; et al. Safflower Production on the Canadian Prairies: Revisited in 2004; Graphcom Printers Ltd.: Lethbridge, AB, Canada, 2004; ISBN 0662382099. [Google Scholar]
- Johnston, A.M.; Tanaka, D.L.; Miller, P.R.; Brandt, S.A.; Nielsen, D.C.; Lafond, G.P.; Riveland, N.R. Oilseed crops for semiarid cropping systems in the northern Great Plains. Agron. J. 2002, 94, 231–240. [Google Scholar] [CrossRef]
- Armah-Agyeman, G.; Loiland, J.; Karrow, R.; Hang, A. Safflower. In Dryland Cropping Systems; Oregon State University: Corvallis, OR, USA, 2002. [Google Scholar]
- Knowles, P.F. Safflower—Production, processing and utilization. Econ. Bot. 1955, 9, 273–299. [Google Scholar] [CrossRef]
- Kizil, S.; Çakmak, Ö.; Kirici, S.; İnan, M. A comprehensive study on safflower (Carthamus tinctorius L.) in semi-arid conditions. Biotechnol. Biotechnol. Equip. 2008, 22, 947–953. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Siadat, S.A.; Norof, M.S.; Naseri, R. The effects of planting date and row spacing on yield, yield components and associated traits in winter safflower under rain fed conditions. Am. Eurasian J. Agric. Environ. Sci. 2011, 10, 200–206. [Google Scholar]
- Knowles, P.F. Centers of plant diversity and conservation of crop germ plasm: Safflower. Econ. Bot. 1969, 23, 324–329. [Google Scholar] [CrossRef]
- Kumar, U.; Singh, P.; Boote, K.J. Chapter two—Effect of climate change factors on processes of crop growth and development and yield of groundnut (Arachis hypogaea L.). In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 41–69. ISBN 00652113. [Google Scholar]
- Stern, W.R.; Beech, D.F. The growth of safflower (Cathamus tinctorius L.) in a low latitude environment. Aust. J. Agric. Res. 1965, 16, 801. [Google Scholar] [CrossRef]
- Wilkerson, G.G.; Jones, J.W.; Boote, K.J.; Ingram, K.T.; Mishoe, J.W. Modeling soybean growth for crop management. Trans. ASAE 1983, 26, 63–73. [Google Scholar] [CrossRef]
- Kotecha, A.; Zimmerman, L.H. Inheritance and gene action of time of flowering in safflower, Carthamus sp. Agron. Abstr. Madison Am. Soc. Agron. 1977, 62. [Google Scholar]
- Honermeier, B. Saflor. In Ölfrüchte, Faserpflanzen, Arzneipflanzen und Sonderkulturen; Heyland, K.-U., Hanus, H., Keller, E.R., Eds.; Ulmer: Stuttgart, Germany, 2006; pp. 246–252. ISBN 9783800132034. [Google Scholar]
- Elfadl, E.; Reinbrecht, C.; Frick, C.; Claupein, W. Optimization of nitrogen rate and seed density for safflower (Carthamus tinctorius L.) production under low-input farming conditions in temperate climate. Field Crop. Res. 2009, 114, 2–13. [Google Scholar] [CrossRef]
- Azari, A.; Khajehpour, M.R. Effect of planting pattern on development, growth, yield components and seed and petal yields of safflower in summer planting, local variety of Isfahan, Koseh. Person. J. Sci. Technol. Agric. Nat. Resour. 2005, 9, 131–142. [Google Scholar]
- Sharifmoghaddasi, M.R.; Omidi, A.H. Determination of optimum row-spacing and plant density in goldasht safflower variety. Adv. Environ. Biol. 2009, 3, 233–238. [Google Scholar]





| Year | Month | Tmean (°C) | Tmax (°C) | Tmin (°C) | Rainfall (mm) | Solar Radiation (MJ m−2 d−1) |
|---|---|---|---|---|---|---|
| 2017 | April | 7.1 | 21.7 | −4.9 | 29.0 | 15.6 |
| May | 13.6 | 30.4 | −0.1 | 47.0 | 18.9 | |
| June | 18.3 | 32.0 | 5.2 | 72.2 | 23.5 | |
| July | 18.2 | 31.7 | 9.0 | 109.9 | 18.1 | |
| August | 18.1 | 29.7 | 7.0 | 69.3 | 16.2 | |
| 2018 | April | 12.4 | 26.6 | −2.1 | 17.4 | 18.5 |
| May | 14.9 | 26.9 | 2.6 | 75.1 1 | 18.9 | |
| June | 17.4 | 28.0 | 4.3 | 32.5 | 21.3 | |
| July | 19.9 | 32.9 | 9.0 | 32.0 | 21.6 | |
| August | 19.6 | 33.6 | 4.2 | 28.8 | 17.4 |
| Year | Depth (cm) | Corg (%) | Nmin (kg ha−1) | Clay (%) | Sand (%) | Silt (%) |
|---|---|---|---|---|---|---|
| 2017 | 0–30 | 1.2 | 75.0 | 27.1 | 2.7 | 70.2 |
| 30–60 | 0.7 | 37.0 | 27.2 | 2.4 | 70.4 | |
| 60–90 | 0.6 | 11.6 | 33.1 | 3.3 | 63.6 | |
| 2018 | 0–30 | 1.2 | 24.7 | 34.4 | 2.7 | 62.8 |
| 30–60 | 0.7 | 10.8 | 30.6 | 4.0 | 65.4 | |
| 60–90 | 1.3 | 9.7 | 17.4 | 13.3 | 69.2 |
| CROPGRO Species Parameters | Shape | Default Safflower | Modified Safflower | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tb | Topt1 | Topt2 | Tmax | Tb | Topt1 | Topt2 | Tmax | ||
| Vegetative Development | Lin. 1 | 3.0 | 28.0 | 30.0 | 38.0 | 3.0 | 22.0 | 30.0 | 38.0 |
| Early reproductive development | Lin. 1 | 3.0 | 28.0 | 32.0 | 43.0 | 3.0 | 28.0 | 32.0 | 43.0 |
| Late reproductive development | Lin. 1 | 3.0 | 28.0 | 38.0 | 45.0 | 3.0 | 28.0 | 38.0 | 45.0 |
| Light-saturated leaf photosynthesis (vs. current temperature) | Lin. 1 | 5.0 | 35.0 | 40.0 3 | 45.0 | 4.0 | 35.0 | 40.0 3 | 45.0 |
| Light-saturated leaf photosynthesis (vs. minimum temperature) | Qdr. 2 | 0.0 | 19.0 | - 6 | - 6 | −2.0 | 14.0 | - 6 | - 6 |
| Leaf relative expansion | Lin. 1 | 12.0 4 | 22.0 | - 6 | - 6 | 8.0 5 | 21.0 | - 6 | - 6 |
| Pod addition rate | Qdr. 2 | 14.0 | 21.0 | 26.5 | 40.0 | 9.0 | 24.0 | 26.5 | 40.0 |
| Seed growth rate | Qdr. 2 | 6.0 | 21.0 | 23.5 | 41.0 | 6.0 | 21.0 | 23.5 | 41.0 |
| CROPGRO Ecotype Parameters | Definition of Parameter | Cultivars | |||
|---|---|---|---|---|---|
| Default Safflower Cultivar | Modified Safflower Cultivar | Safflower Cultivars Cultivated in Germany | |||
| PI8311 | PI8311 | C1 | C2 | ||
| PL-EM | Time between planting and emergence (PL-EM)(TD) 1 | 3.60 | 5.0 | 5.0 | 5.0 |
| EM-V1 | Required time from emergence to first true leaf (TD) 1 | 6.0 | 6.0 | 3.0 | 3.0 |
| FL-VS | Time from first flower to last leaf on main stem (PD) 2 | 7.00 | 7.00 | 14.0 | 14.0 |
| TRIFL | Rate appearance of leaves on the main stem (leaves TD−1) 1 | 0.36 | 1.00 | 1.00 | 1.00 |
| RWDTH | Relative width in comparison to the standard width per node defined in the species file | 0.85 | 0.75 | 0.75 | 1.00 |
| RHGHT | Relative height in comparison to the standard height per node defined in the species file | 0.85 | 0.75 | 0.80 | 1.00 |
| CROPGRO Cultivar Parameters | Definition of Parameter | Cultivars | |||
|---|---|---|---|---|---|
| Default Safflower Cultivar | Modified Safflower Cultivar | Safflower Cultivars Cultivated in Germany | |||
| PI8311 | PI8311 | C1 | C2 | ||
| EM-FL | Time from plant emergence to flower appearance (PD) 1 | 17.0 | 16.5 | 10.3 | 10.3 |
| FL-SH | Time between first flower and first pod (PD) 1 | 3.0 | 3.2 | 4.5 | 4.5 |
| FL-SD | Time between first flower and first seed (PD) 1 | 15.0 | 14.7 | 13.0 | 13.0 |
| SD-PM | Time between first seed and physiological maturity (PD) 1 | 30.0 | 30.5 | 28.5 | 28.5 |
| FL-LF | Time between first flower and end of leaf expansion (PD) 1 | 20.25 | 20.25 | 18.00 | 18.00 |
| LFMAX | Maximum leaf photosynthetic rate at 30 °C, 350 ppm CO2, and high light (mg CO2 m2 s−1) | 2.20 | 1.50 | 1.55 | 1.80 |
| SLAVR | Specific leaf area of cultivar under standard growth conditions (cm2 g−1) | 200.0 | 250.0 | 250.0 | 260.0 |
| SIZELF | Maximum size of full leaf (cm2) | 115.0 | 115.0 | 115.0 | 115.0 |
| XFRT | Maximum fraction of daily growth partitioned to seed and shell | 0.55 | 0.64 | 0.67 | 0.76 |
| WTPSD | Maximum weight per seed (g) | 0.040 | 0.054 | 0.064 | 0.052 |
| SFDUR | Seed filling duration for pod cohort (PD) 1 | 29.0 | 29.0 | 30.0 | 30.0 |
| SDPDV | Seeds per pod at standard growth conditions (no. pod−1) | 22.25 | 18.00 | 22.25 | 22.25 |
| PODUR | Duration of pod adding (PD) 1 | 17.0 | 18.0 | 19.0 | 17.0 |
| THRESH | Weight percentage of seeds in pods (%) | 51.3 | 47.0 | 55.0 | 61.0 |
| SDPRO | Potential seed protein (g (protein) g (seed)−1) | 0.14 | 0.14 | 0.14 | 0.14 |
| SDLIP | Potential seed lipid (g (oil) g (seed)−1) | 0.33 | 0.33 | 0.33 | 0.33 |
| CROPGRO Species Parameters | Definition of Parameter | Default Safflower | Modified Safflower |
|---|---|---|---|
| Photosynthesis parameters | |||
| SLWREF | Specific weight at which LFMAX is defined (g cm−2) | 0.0035 | 0.0025 |
| LNREF | Leaf N concentration at which LFMAX is defined (%N) | 4.90 | 4.50 |
| Leaf growth parameters | |||
| FINREF | Specific leaf area of leaves at plant emergence (cm2 g−1) | 150.0 | 200.0 |
| SLAREF | Specific leaf area of the standard reference cultivar at peak early vegetative phase (cm2 g−1) | 270.0 | 260.0 |
| SLAMAX SLAMIN | Upper and lower limit of specific leaf area (response to solar radiation) (cm2 g−1) | 650.0 110.0 | 650.0 260.0 |
| Leaf senescence factors | |||
| SENRTE | Grams of leaf mass that are lost per gram of protein mobilized (g) | 1.00 | 1.10 |
| SENRT2 | Rate of abscision after physiological maturity | 0.20 | 0.10 |
| ICMP | Light compensation point for senescence of lower leaves because of excessive self-shading by the crop canopy (mol PPFD m−2 d−1) | 0.80 | 0.40 |
| PORPT | A stand-in for petiole mass abscised per unit leaf mass abscised (petiole mass leaf mass−1) | 0.58 | 0.01 |
| Evapotranspiration | |||
| KEP | Extinction coefficient for solar radiation for partitioning of potential ET to T | 0.57 | 0.50 |
| CROPGRO Species Parameters | Partitioning-(Fraction), Potential Internode Length (m) and Leaf Senescence at a Given V-Stage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Default safflower values | ||||||||||
| XLEAF | 0.0 | 1.5 | 3.3 | 5.0 | 7.8 | 10.5 | 30.0 | 40.0 | final | |
| YLEAF | 0.43 | 0.44 | 0.45 | 0.44 | 0.39 | 0.35 | 0.34 | 0.34 | ||
| YSTEM | 0.09 | 0.12 | 0.19 | 0.26 | 0.34 | 0.46 | 0.46 | 0.46 | ||
| FRLFF | 0.25 | |||||||||
| FRSTMF | 0.54 | |||||||||
| Modified safflower values | ||||||||||
| XLEAF | 0.0 | 2.0 | 4.0 | 6.5 | 9.8 | 15.0 | 35.0 | 50.0 | final | |
| YLEAF | 0.43 | 0.44 | 0.45 | 0.44 | 0.43 | 0.40 | 0.37 | 0.34 | ||
| YSTEM | 0.09 | 0.12 | 0.19 | 0.26 | 0.30 | 0.41 | 0.43 | 0.46 | ||
| FRLFF | 0.20 | |||||||||
| FRSTMF | 0.63 | |||||||||
| Default safflower values | ||||||||||
| XVSHT | 0.0 | 1.0 | 4.0 | 6.0 | 8.0 | 10.0 | 14.0 | 16.0 | 20.0 | 40.0 |
| YVSHT | 0.030 | 0.053 | 0.063 | 0.066 | 0.069 | 0.066 | 0.062 | 0.051 | 0.034 | 0.006 |
| YVSWH | 0.030 | 0.051 | 0.062 | 0.064 | 0.066 | 0.063 | 0.059 | 0.046 | 0.025 | 0.001 |
| Modified safflower values | ||||||||||
| XVSHT | 0.0 | 1.0 | 4.0 | 6.0 | 8.0 | 10.0 | 14.0 | 18.0 | 26.0 | 40.0 |
| YVSHT | 0.020 | 0.023 | 0.034 | 0.038 | 0.042 | 0.045 | 0.049 | 0.052 | 0.052 | 0.045 |
| YVSWH | 0.020 | 0.021 | 0.030 | 0.030 | 0.030 | 0.028 | 0.023 | 0.021 | 0.014 | 0.001 |
| Default safflower values | ||||||||||
| XSTAGE | 0.0 | 5.0 | 14.0 | 30.0 | ||||||
| SENPOR | 0.0 | 0.0 | 0.12 | 0.12 | ||||||
| XSENMX | 3.0 | 5.0 | 10.0 | 30.0 | ||||||
| SENMAX | 0.0 | 0.2 | 0.6 | 0.6 | ||||||
| Modified safflower values | ||||||||||
| XSTAGE | 0.0 | 5.0 | 18.0 | 40.0 | ||||||
| SENPOR | 0.0 | 0.0 | 0.04 | 0.06 | ||||||
| XSENMX | 3.0 | 5.0 | 18.0 | 40.0 | ||||||
| SENMAX | 0.0 | 0.2 | 0.5 | 0.5 | ||||||
| CROPGRO Species Parameters | Definition of Parameter | Default Safflower | Modified Safflower |
|---|---|---|---|
| PROLF_ | Protein concentrations of leaf tissue | ||
| I = “maximum” | 0.356 | 0.306 | |
| G = “normal growth” | 0.140 | 0.140 | |
| F = “final” | 0.112 | 0.090 | |
| PROST_ | Protein concentrations of stem tissue | ||
| I = “maximum” | 0.150 | 0.150 | |
| G = “normal growth” | 0.100 | 0.100 | |
| F = “final” | 0.035 | 0.035 | |
| PRORT_ | Protein concentrations of root tissue | ||
| I = “maximum” | 0.092 | 0.092 | |
| G = “normal growth” | 0.064 | 0.064 | |
| F = “final” | 0.056 | 0.056 | |
| PROSH_ | Protein concentrations of shell tissue | ||
| I = “maximum” | 0.250 | 0.200 | |
| G = “normal growth” | 0.150 | 0.150 | |
| F = “final” | 0.050 | 0.050 | |
| PCAR_ | Carbohydrate-cellulose concentrations of tissues | ||
| Leaf (LF) | 0.405 | 0.455 | |
| Stem (ST) | 0.572 | 0.572 | |
| Root (RT) | 0.711 | 0.711 | |
| Shell (SH) | 0.500 | 0.550 | |
| Seed (SD) | 0.470 | 0.420 | |
| Nodule (NO) | 0.480 | 0.480 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steberl, K.; Boote, K.J.; Munz, S.; Graeff-Hönninger, S. Modifying the CROPGRO Safflower Model to Simulate Growth, Seed and Floret Yield under Field Conditions in Southwestern Germany. Agronomy 2020, 10, 11. https://doi.org/10.3390/agronomy10010011
Steberl K, Boote KJ, Munz S, Graeff-Hönninger S. Modifying the CROPGRO Safflower Model to Simulate Growth, Seed and Floret Yield under Field Conditions in Southwestern Germany. Agronomy. 2020; 10(1):11. https://doi.org/10.3390/agronomy10010011
Chicago/Turabian StyleSteberl, Kathrin, Kenneth J. Boote, Sebastian Munz, and Simone Graeff-Hönninger. 2020. "Modifying the CROPGRO Safflower Model to Simulate Growth, Seed and Floret Yield under Field Conditions in Southwestern Germany" Agronomy 10, no. 1: 11. https://doi.org/10.3390/agronomy10010011
APA StyleSteberl, K., Boote, K. J., Munz, S., & Graeff-Hönninger, S. (2020). Modifying the CROPGRO Safflower Model to Simulate Growth, Seed and Floret Yield under Field Conditions in Southwestern Germany. Agronomy, 10(1), 11. https://doi.org/10.3390/agronomy10010011





