Plant Nutrient Availability and pH of Biochars and Their Fractions, with the Possible Use as a Component in a Growing Media
Abstract
:1. Introduction
2. Materials and Methods
Statistical Methods
3. Results
3.1. Biochar’s Total Elemental Contents and Correlations
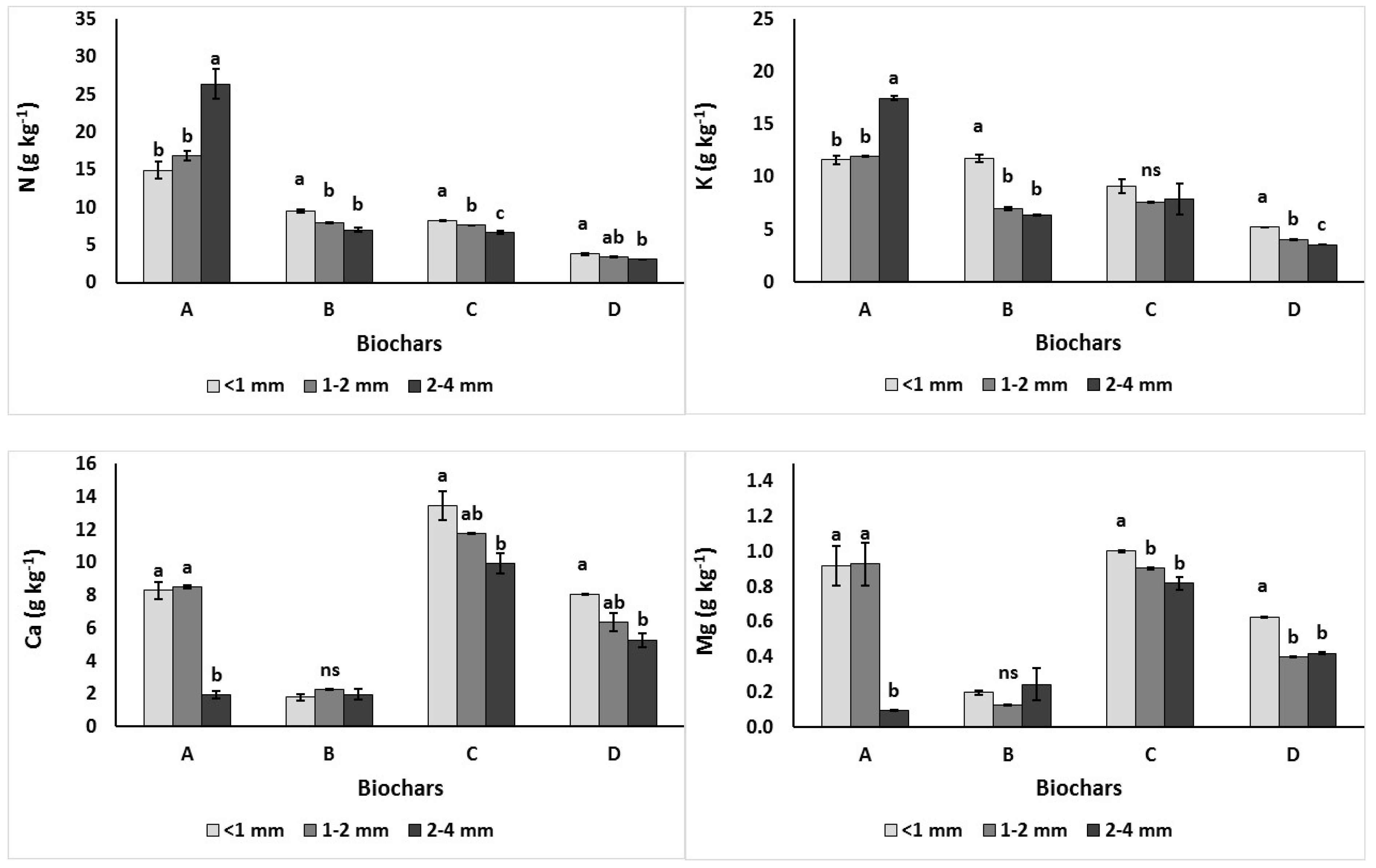
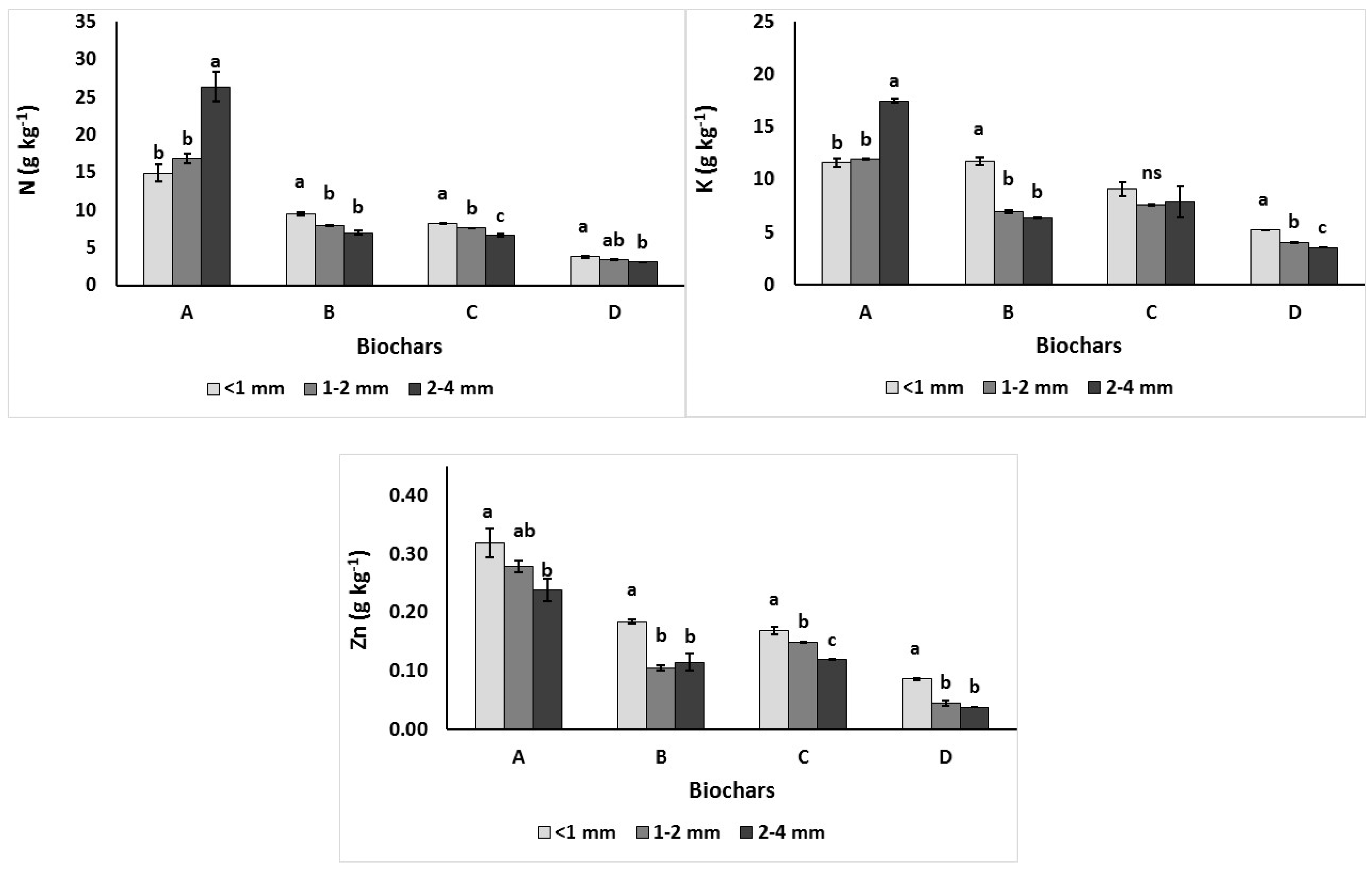
3.1.1. Total Macronutrients N, K, Ca, Mg, P and Na
3.1.2. Total Micronutrients Cu, Mn and Zn
3.2. Biochars’ Extractable Elemental Contents
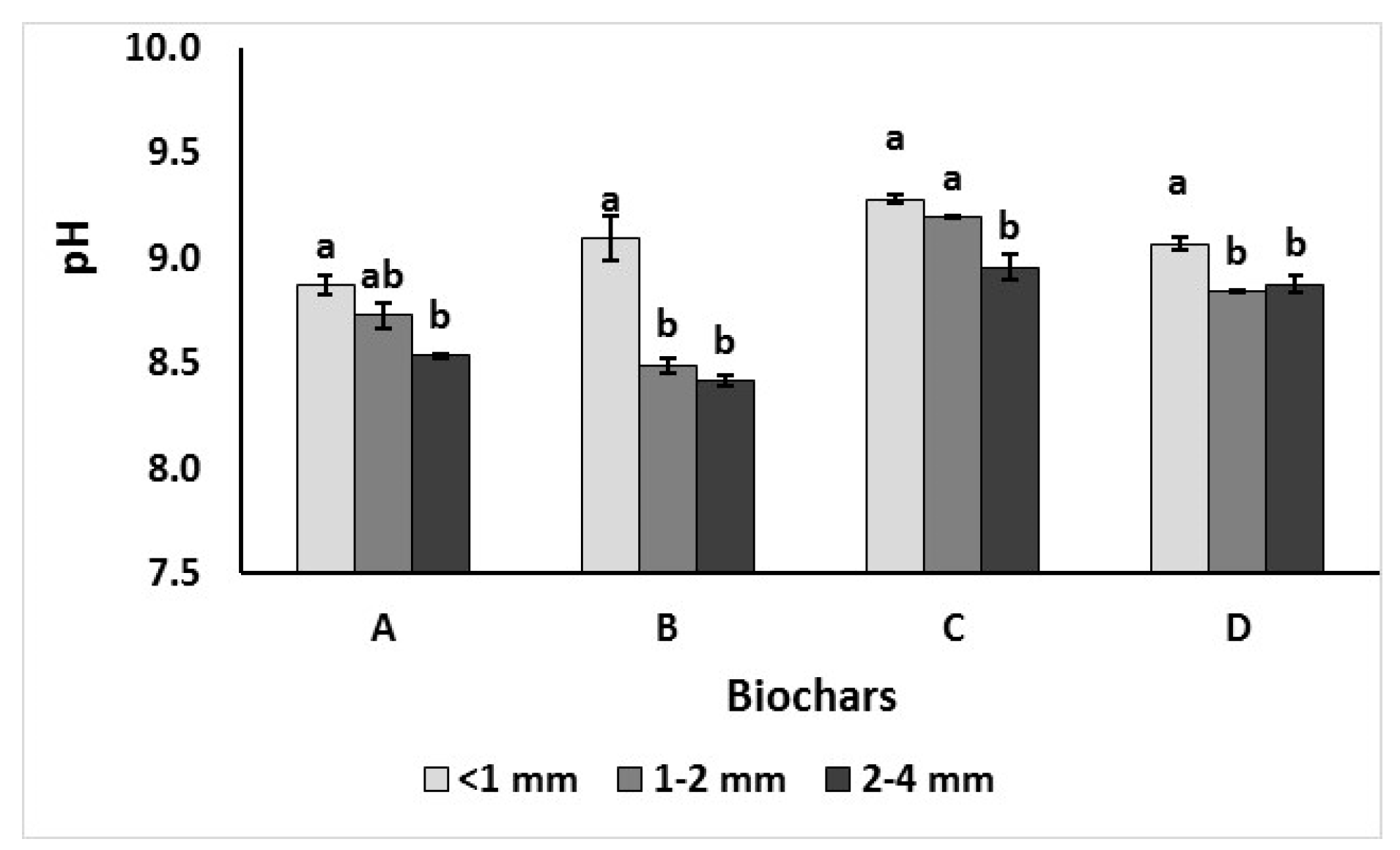
3.2.1. The pH and Extractable Macronutrients N, K, P, Ca, Mg, Na and SO4
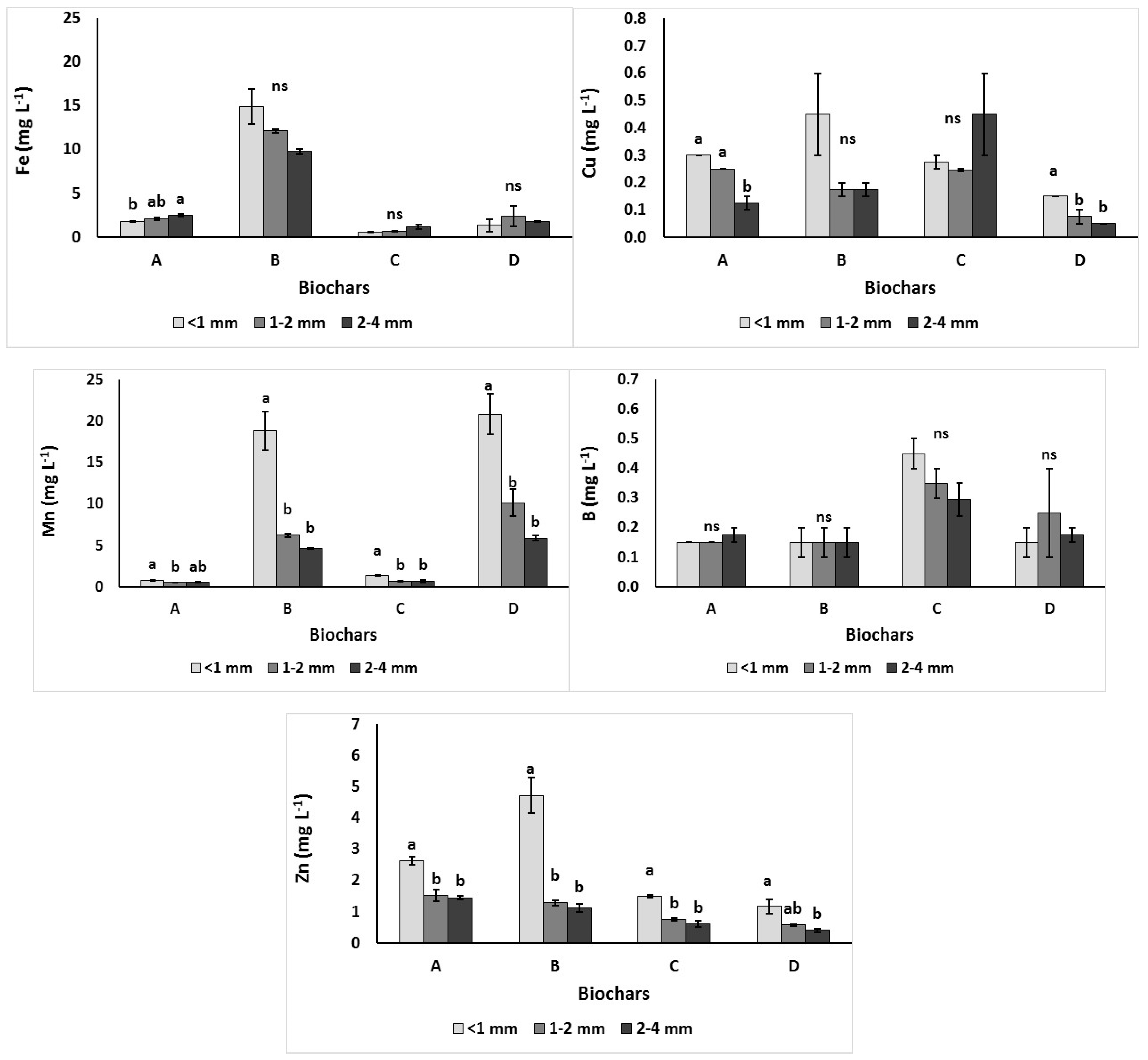
3.2.2. Extractable Micronutrients (Zn, Mn, Fe, B and Cu)
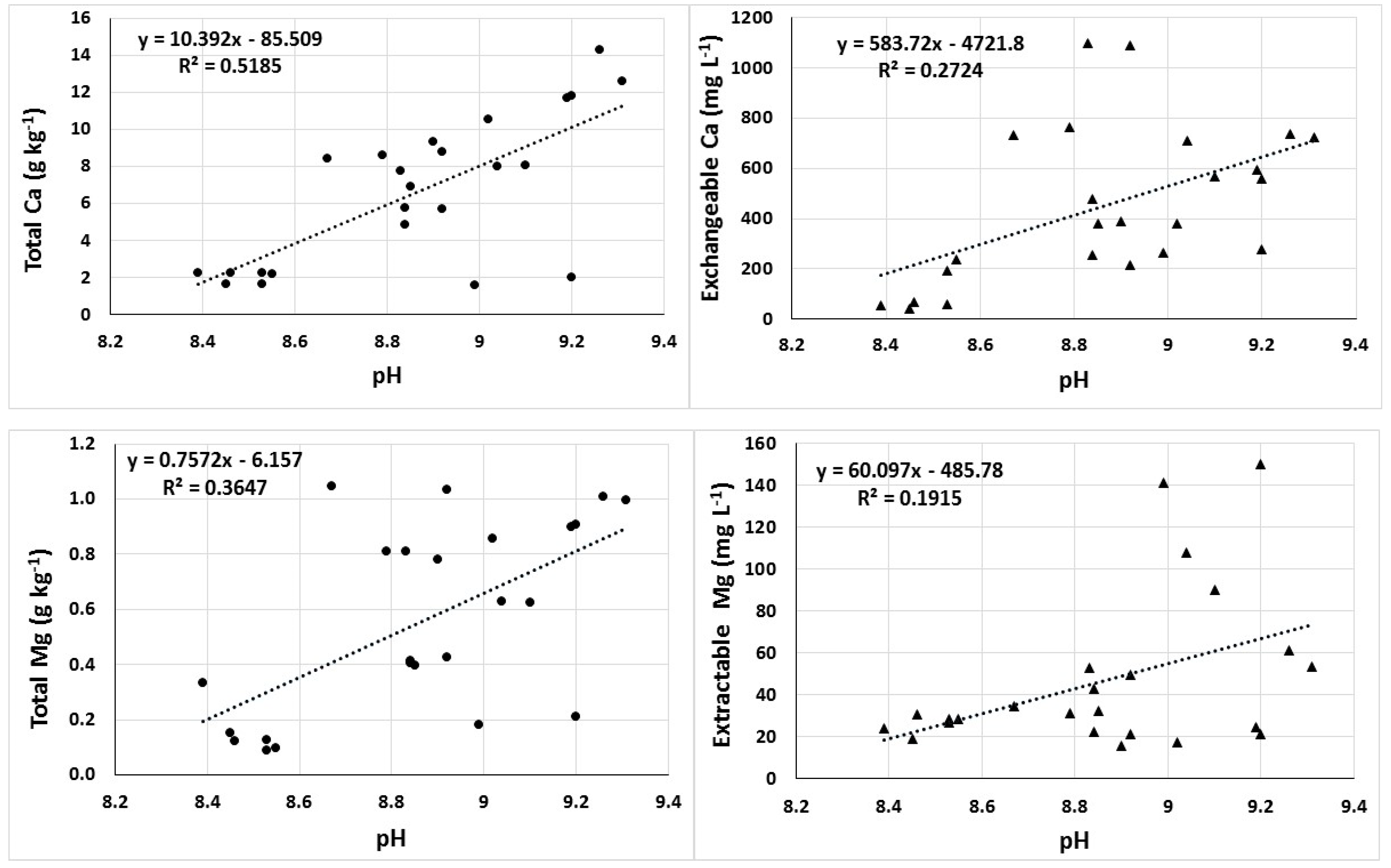
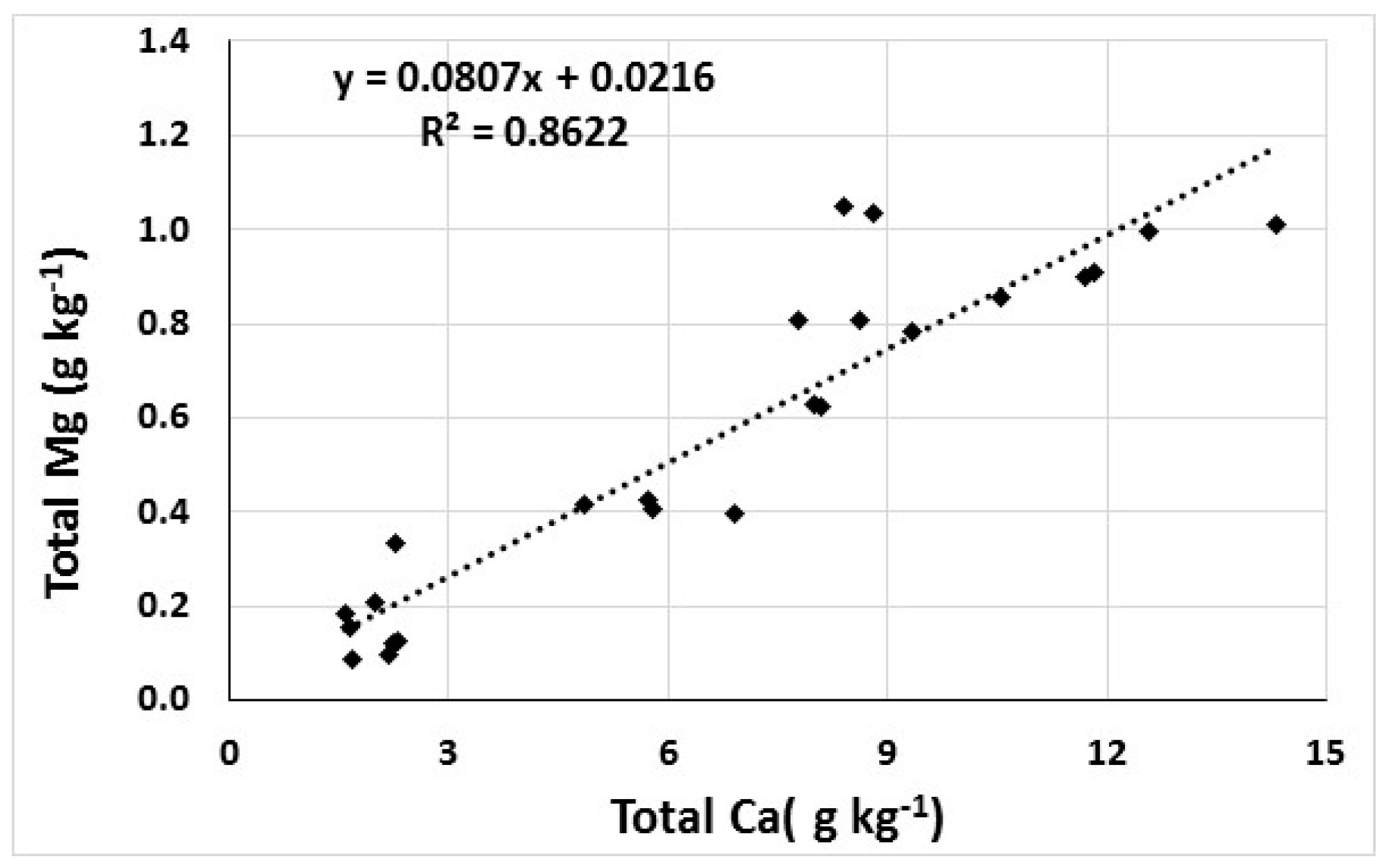
3.3. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Rehrah, D.; Bansode, R.R.; Hassan, O.; Ahmedna, M. Physico-chemical characterization of biochars from solid municipal waste for use in soil amendment. J. Anal. Appl. Pyrolysis 2016, 118, 42–53. [Google Scholar] [CrossRef]
- Xu, C.Y.; Hosseini-Bai, S.; Hao, Y.; Rachaputi, R.C.N.; Wang, H.; Xu, Z.; Wallace, H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. 2015, 22, 6112–6125. [Google Scholar] [CrossRef] [PubMed]
- Kerré, B.; Willaert, B.; Cornelis, Y.; Smolders, E. Long-term presence of charcoal increases maize yield in Belgium due to increased soil water availability. Eur. J. Agron. 2017, 91, 10–15. [Google Scholar] [CrossRef]
- Gomez-Eyles, J.L.; Sizmur, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. 2011, 159, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cao, X.; Ouyang, X.; Sohi, S.P.; Chen, J. Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere 2016, 145, 336–341. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Pyliotis, I.; Vamvuka, D.; Bartzas, G. Assessment of pistachio shell biochar quality and its potential for adsorption of heavy metals. Waste Biomass Valoriz. 2015, 6, 805–816. [Google Scholar] [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Baronti, S.; Lugato, E.; Genesio, L.; Castaldi, S.; Fornasier, F.; Miglietta, F. Biochar as a strategy to sequester carbon and increase yield in durum wheat. Eur. J. Agron. 2011, 34, 231–238. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Yang, J.E.; Ok, Y.S.; Kim, W., II; Kunhikrishnan, A.; Kim, K.H. Amelioration of horticultural growing media properties through rice hull biochar incorporation. Waste Biomass Valoriz. 2017, 8, 483–492. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type and ratio as a peat additive/partial peat replacement in growing media for cabbage seedling production. Agronomy 2019, 9, 693. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, J.M.; Pasian, C.; Lal, R.; López, R.; Díaz, M.J.; Fernández, M. Morpho-physiological plant quality when biochar and vermicompost are used as growing media replacement in urban horticulture. Urban For. Urban Green. 2018, 34, 175–180. [Google Scholar] [CrossRef]
- Hilioti, Z.; Michailof, C.M.; Valasiadis, D.; Iliopoulou, E.F.; Koidou, V.; Lappas, A.A. Characterization of castor plant-derived biochars and their effects as soil amendments on seedlings. Biomass Bioenergy 2017, 105, 96–106. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M. Effects of biochar on container substrate properties and growth of plants—A review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Kaudal, B.B.; Chen, D.; Madhavan, D.B.; Downie, A.; Weatherley, A. Pyrolysis of urban waste streams: Their potential use as horticultural media. J. Anal. Appl. Pyrolysis 2015, 112, 105–112. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Blok, C.; Van Der Salm, C.; Hofland-Zijlstra, J.; Streminska, M.; Eveleens, B.; Regelink, I.; Fryda, L.; Visser, R. Biochar for horticultural rooting media improvement: Evaluation of Biochar from gasification and slow pyrolysis. Agronomy 2017, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.M.; Pasian, C.; Lal, R.; Lopez, R.; Fernandez, M. Vermicompost and Biochar as growing media replacement for ornamental plant production. J. Appl. Hortic. 2017, 19, 205–214. [Google Scholar]
- Dispenza, V.; De Pasquale, C.; Fascella, G.; Mammano, M.M.; Alonzo, G. Use of biochar as peat substitute for growing substrates of Euphorbia × lomi potted plants. Span. J. Agric. Res. 2016, 14. [Google Scholar] [CrossRef] [Green Version]
- Fryda, L.; Visser, R.; Schmidt, J. Biochar replaces peat in horticulture: Environmental impact assessment of combined biochar & bioenergy production. Detritus 2019, 5, 132–149. [Google Scholar]
- Kaudal, B.B.; Chen, D.; Madhavan, D.B.; Downie, A.; Weatherley, A. An examination of physical and chemical properties of urban biochar for use as growing media substrate. Biomass Bioenergy 2016, 84, 49–58. [Google Scholar] [CrossRef]
- Margenot, A.J.; Griffin, D.E.; Alves, B.S.Q.; Rippner, D.A.; Li, C.; Parikh, S.J. Substitution of peat moss with softwood biochar for soil-free marigold growth. Ind. Crops Prod. 2018, 112, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Gruda, N. Current and future perspective of growing media in Europe. Acta Hortic. 2012, 960, 37–43. [Google Scholar] [CrossRef]
- Schmilewski, G. The role of peat in assuring the quality of growing media. Mires Peat 2008, 3, 1–8. [Google Scholar]
- Prins, F. Carbon storage in peat bog areas in Europe. In Proceedings of the Workshop on CO2 Sequestration & Biodiversity, Bratislava, Slovakia, 9–10 November 2009. [Google Scholar]
- Armstrong, H.; Aendekerk, T.G.L. International Substrate Manual: Analysis, Characteristics, Recommendations; International Business Information; Elsevier: Amsterdam, The Netherlands, 2000; ISBN 9054391022. [Google Scholar]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production—A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Eller, F.J.; Moser, B.R.; Jackson, M.A.; Peterson, S.C. Physical and chemical characterization of biochars produced from coppiced wood of thirteen tree species for use in horticultural substrates. Ind. Crops Prod. 2015, 66, 44–51. [Google Scholar] [CrossRef]
- Altland, J.E.; Locke, J.C. Effect of biochar type on macronutrient retention and release from soilless substrate. HortScience 2013, 48, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Solaiman, Z.M.; Murphy, D.V.; Abbott, L.K. Biochars influence seed germination and early growth of seedlings. Plant Soil 2012, 353, 273–287. [Google Scholar] [CrossRef]
- Prasad, M.; Tzortzakis, N.; McDaniel, N. Chemical characterization of biochar and assessment of the nutrient dynamics by means of preliminary plant growth tests. J. Environ. Manag. 2018, 216, 89–95. [Google Scholar] [CrossRef]
- Gascó, G.; Álvarez, M.L.; Paz-Ferreiro, J.; Miguel, G.S.; Méndez, A. Valorization of biochars from pinewood gasification and municipal solid waste torrefaction as peat substitutes. Environ. Sci. Pollut. Res. 2018, 25, 26461–26469. [Google Scholar] [CrossRef]
- Bedussi, F.; Zaccheo, P.; Crippa, L. Pattern of pore water nutrients in planted and non-planted soilless substrates as affected by the addition of biochars from wood gasification. Biol. Fertil. Soils 2015, 51, 625–635. [Google Scholar] [CrossRef]
- Zaccheo, P.; Crippa, L.; Cattivello, C. Liming power of different particle fractions of biochar. Acta Hortic. 2014, 1034, 363–368. [Google Scholar] [CrossRef]
- Steiner, C.; Harttung, T. Biochar as a growing media additive and peat substitute. Solid Earth 2014, 5, 995–999. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic use of peat and charred material in growing media–an option to reduce the pressure on peatlands? J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Méndez, A.; Paz-Ferreiro, J.; Gil, E.; Gascó, G. The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci. Hortic. 2015, 193, 225–230. [Google Scholar] [CrossRef]
- Nemati, M.R.; Simard, F.; Fortin, J.P.; Beaudoin, J. Potential use of biochar in growing media. Vadose Zone J. 2015, 14, 1–8. [Google Scholar] [CrossRef]
- Angst, T.E.; Sohi, S.P. Establishing release dynamics for plant nutrients from biochar. GCB Bioenergy 2013, 5, 221–226. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Y.; He, Y.; Xia, K.; Qiao, S.; Zhang, Q. Polycyclic aromatic hydrocarbons concentration in straw biochar with different particle size. Procedia Environ. Sci. 2016, 31, 91–97. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Liu, Y.; Shao, L.; Zhang, H.; Lü, F. Particle size dependence of the physicochemical properties of biochar. Chemosphere 2018, 212, 385–392. [Google Scholar] [CrossRef]
- Prasad, M.; Maher, M.J. Physical and chemical properties of fractioned peat. Acta Hortic. 1993, 342, 257–263. [Google Scholar] [CrossRef]
- Alt, D.; Peters, I.; Fokken, H. Estimation of phosphorus availability in composts and compost/peat mixtures by different extraction methods. Commun. Soil Sci. Plant Anal. 1994, 25, 2063–2080. [Google Scholar] [CrossRef]
- EN 13651-2002. Soil Improvers and Growing Media—Extraction of Calcium Chloride/DTPA (CAT); British Standards Institution: London, UK, 2002. [Google Scholar]
- EN 13037-2002. Soil Improvers and Growing Media—Determination of pH; British Standards Institution: London, UK, 2002. [Google Scholar]
- EN 13038-2002. Soil Improvers and Growing Media—Determination of Electrical Conductivity; British Standards Institution: London, UK, 2002. [Google Scholar]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crops Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Zainul, A.; Koyro, H.-W.; Huchzermeyer, B.; Gul, B.; Khan, M.A. Impact of a biochar or a compost-biochar mixture on water relation, nutrient uptake and photosynthesis of Phragmites karka. Pedosphere 2017. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Calvelo Pereira, R.; Kaal, J.; Camps Arbestain, M.; Pardo Lorenzo, R.; Aitkenhead, W.; Hedley, M.; Macías, F.; Hindmarsh, J.; Maciá-Agulló, J.A. Contribution to characterisation of biochar to estimate the labile fraction of carbon. Org. Geochem. 2011, 42, 1331–1342. [Google Scholar] [CrossRef]
- Gaskin, C.; Steiner, K.; Harris, K.C.; Das, B. Bibens Effect of Low-Temperature Pyrolysis Conditions on Biochar for Agricultural Use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov Vladimir, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Gruda, N.; Caron, J.; Prasad, M.; Maher, M. Growing Media. In Encyclopedia of Soil Sciences; Lal, R., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 1053–1058. ISBN 9781498738903. [Google Scholar]
- Jackson, B.E.; Wright, R.D.; Gruda, N. Container medium pH in a pine tree substrate amended with peatmoss and dolomitic limestone affects plant growth. HortScience 2009, 44, 1983–1987. [Google Scholar] [CrossRef] [Green Version]



| Feedstock | Trade Name | pH | EC (µS cm−1) | NH4-N (mg L−1) | NO3-N (mg L−1) | N (mg L−1) | P (mg L−1) | K (mg L−1) | BD 1 (g L−1) | BET 2 (m3 g−1) | Pore Vol 3 (cc g−2) | Pore Vol 3 (cc mL−1) | Pore Size 4 (Å) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biochar A | Cereal husk/paper fibre | DU | 9.56 ± 0.09 a | 653 ± 7.5 a | 1 ± 0.0 a | 1.0 ± 0.0 a | 2.0 ± 0.0 a | 8.5 ± 1.5 b | 891 ± 9.5 c | 232 | 81.3 | 0.00592 | 0.00254 | 29.04 |
| Biochar B | Bamboo | n/a | 8.84 ± 0.08 b | 571 ± 12 b | 1 ± 0.0 a | 0.5 ± 0.5 a | 1.5 ± 0.5 a | 23.0 ± 2.0 a | 1158 ± 13.0 a | 296 | 4.7 | 0.00021 | 0.00006 | 17.90 |
| Biochar C | Wood screenings | Verora | 9.54 ± 0.10 a | 410 ± 16 c | 1 ± 0.0 a | 1.0 ± 0.0 a | 2.0 ± 0.0 a | 10.5 ± 1.5 b | 659 ± 4.5 d | 258 | 62.6 | 0.0045 | 0.0017 | 28.81 |
| Biochar D | Forest wood | Carbon Terra | 9.51 ± 0.04 a | 637 ± 5.5 a | 1 ± 0.0 a | 0.5 ± 0.5 a | 1.5 ± 0.5 a | 5.5 ± 1.5 b | 990 ± 7.0 b | 389 | 243.0 | 0.146 | 0.056 | 24.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant Nutrient Availability and pH of Biochars and Their Fractions, with the Possible Use as a Component in a Growing Media. Agronomy 2020, 10, 10. https://doi.org/10.3390/agronomy10010010
Prasad M, Chrysargyris A, McDaniel N, Kavanagh A, Gruda NS, Tzortzakis N. Plant Nutrient Availability and pH of Biochars and Their Fractions, with the Possible Use as a Component in a Growing Media. Agronomy. 2020; 10(1):10. https://doi.org/10.3390/agronomy10010010
Chicago/Turabian StylePrasad, Munoo, Antonios Chrysargyris, Nicola McDaniel, Anna Kavanagh, Nazim S. Gruda, and Nikolaos Tzortzakis. 2020. "Plant Nutrient Availability and pH of Biochars and Their Fractions, with the Possible Use as a Component in a Growing Media" Agronomy 10, no. 1: 10. https://doi.org/10.3390/agronomy10010010
APA StylePrasad, M., Chrysargyris, A., McDaniel, N., Kavanagh, A., Gruda, N. S., & Tzortzakis, N. (2020). Plant Nutrient Availability and pH of Biochars and Their Fractions, with the Possible Use as a Component in a Growing Media. Agronomy, 10(1), 10. https://doi.org/10.3390/agronomy10010010








