Layer-by-Layer Assembly of Polyelectrolyte Multilayer onto PET Fabric for Highly Tunable Dyeing with Water Soluble Dyestuffs
Abstract
:1. Introduction
2. Materials and Methods
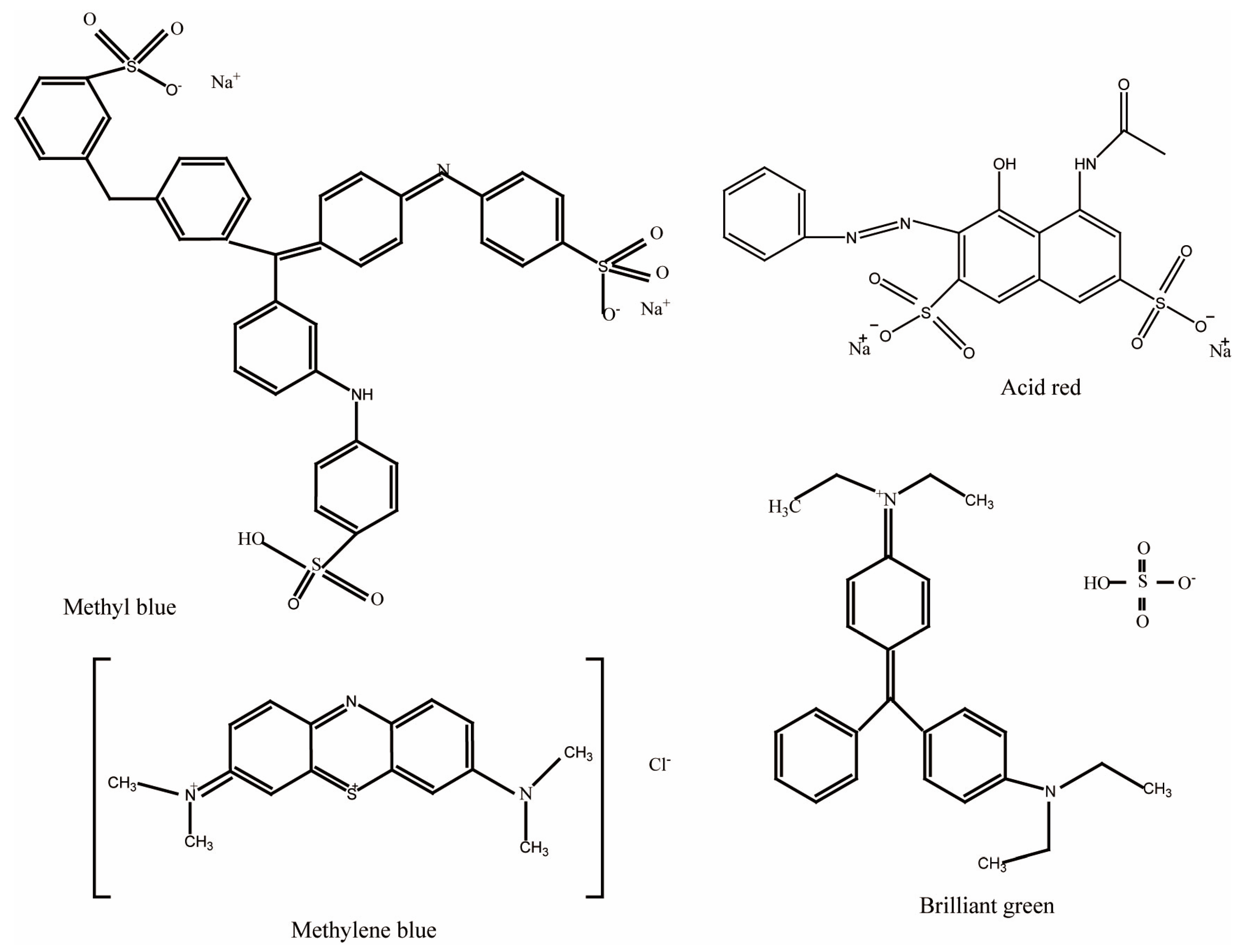
2.1. Materials
2.2. Preparation of Polyelectrolyte Multilayer-Deposited PET Fabric
2.3. Characterization
2.4. Dyeing of Polyelectrolyte Multilayer-Deposited PET Fabric
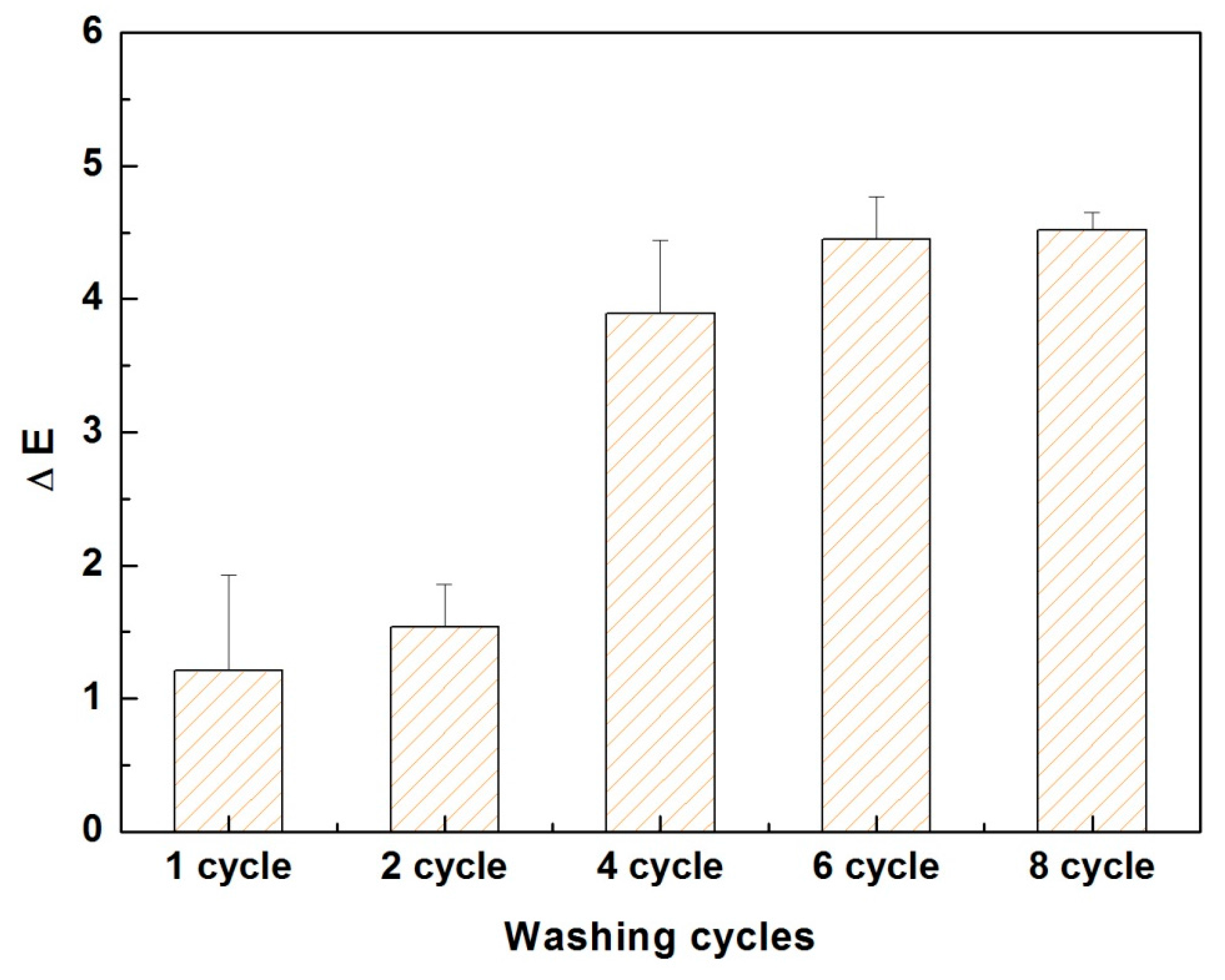
2.5. Colorfastness Test to Washing
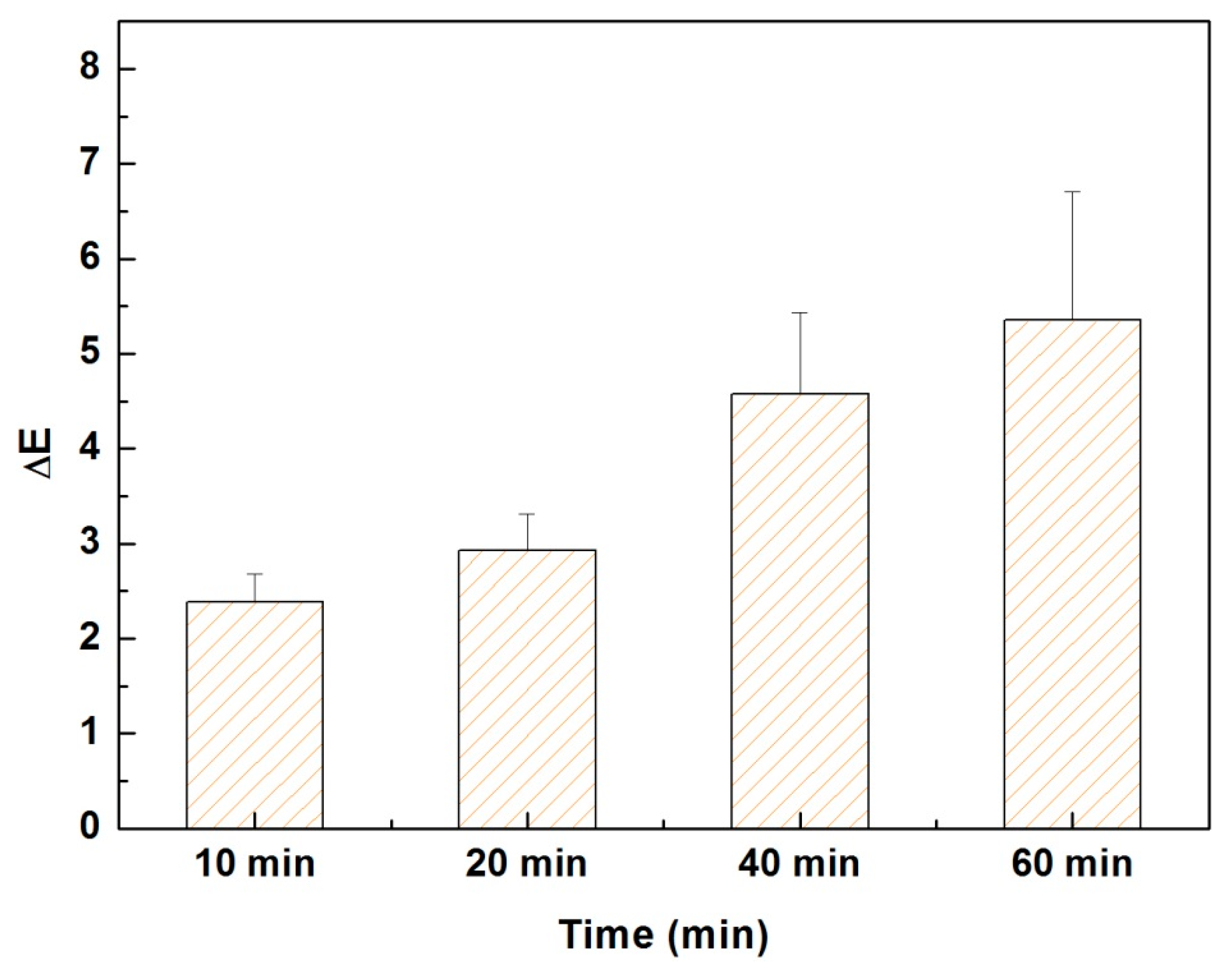
2.6. Measurement of Fastness to Light
3. Results and Discussion
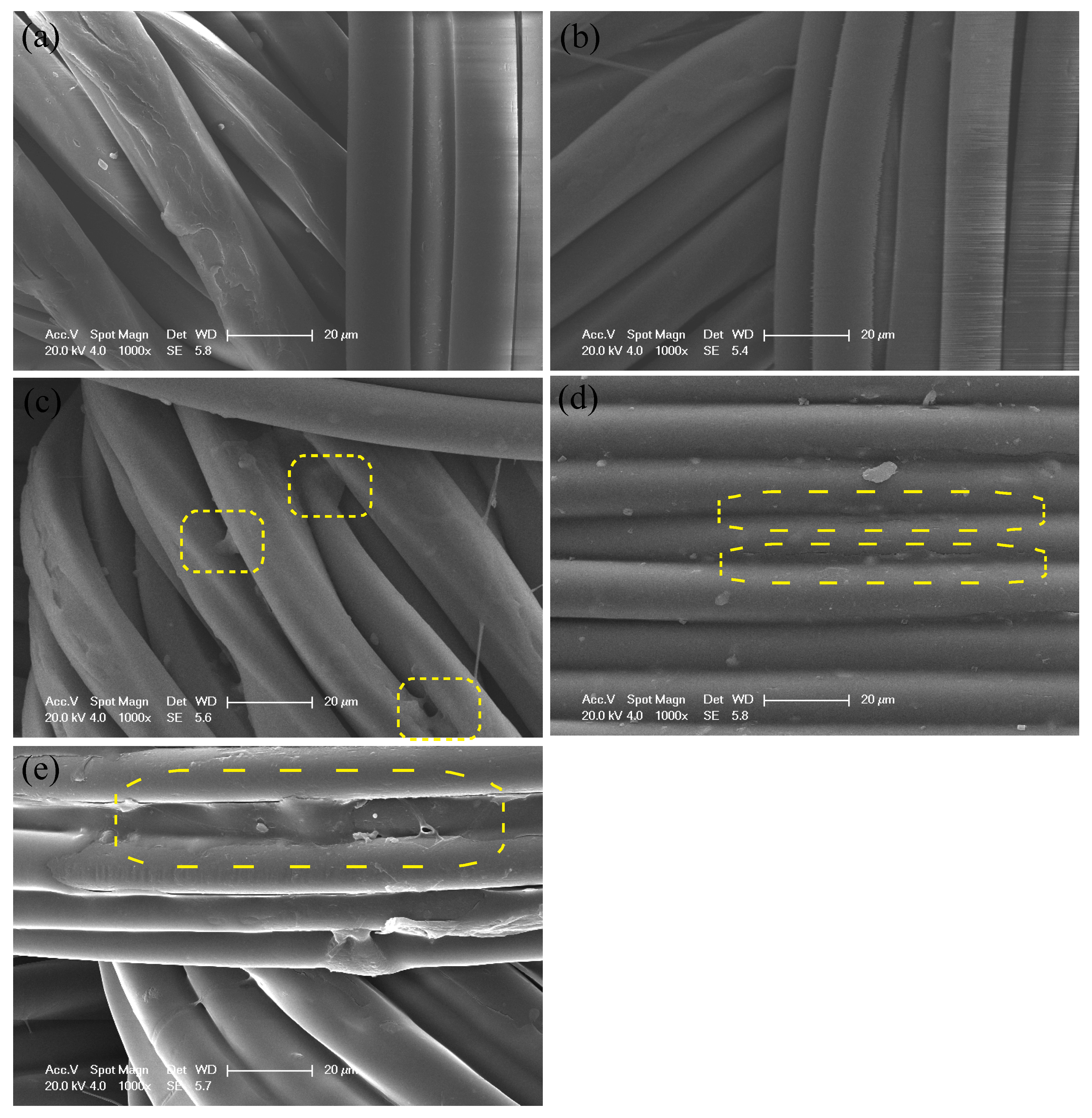
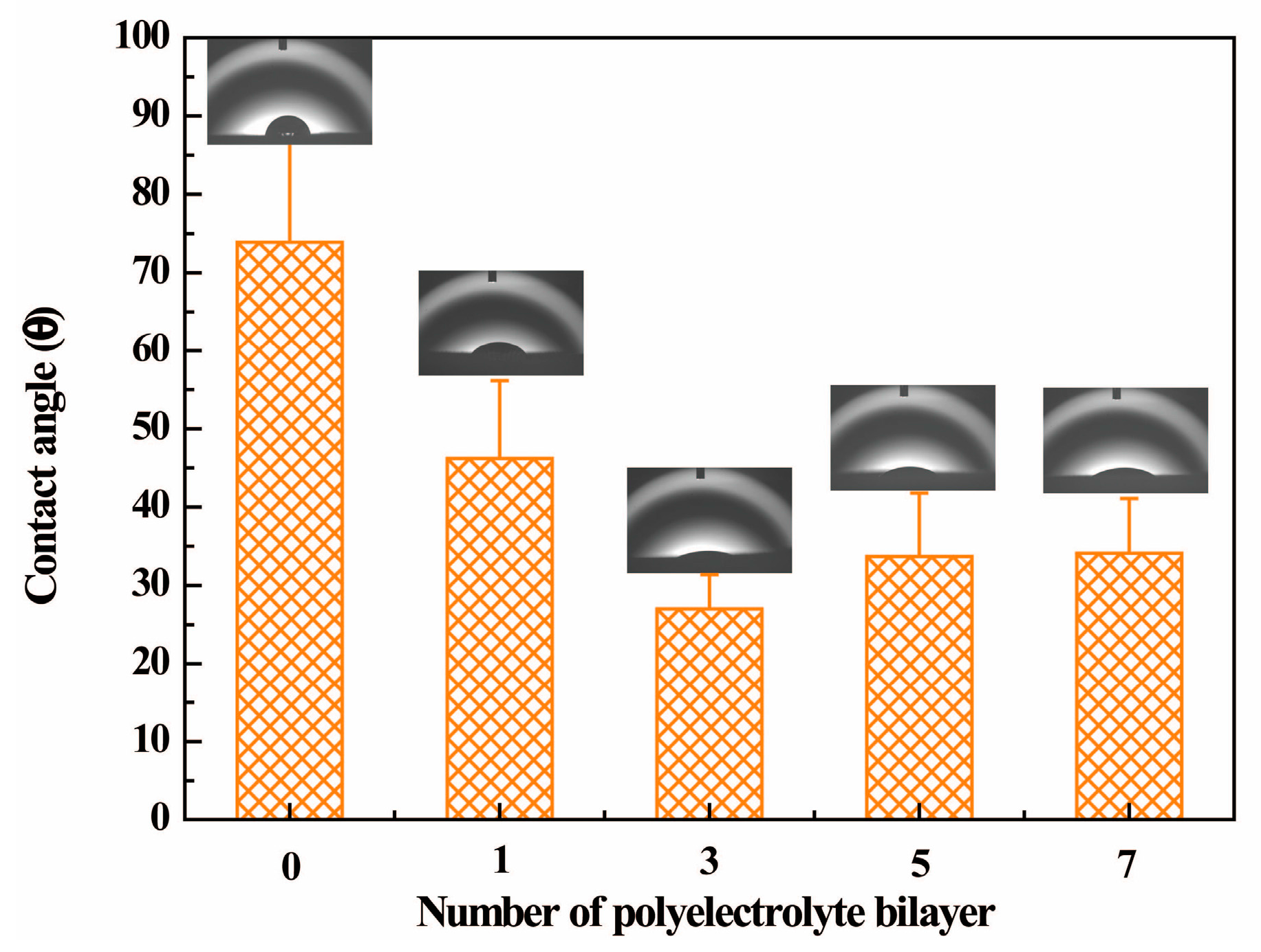
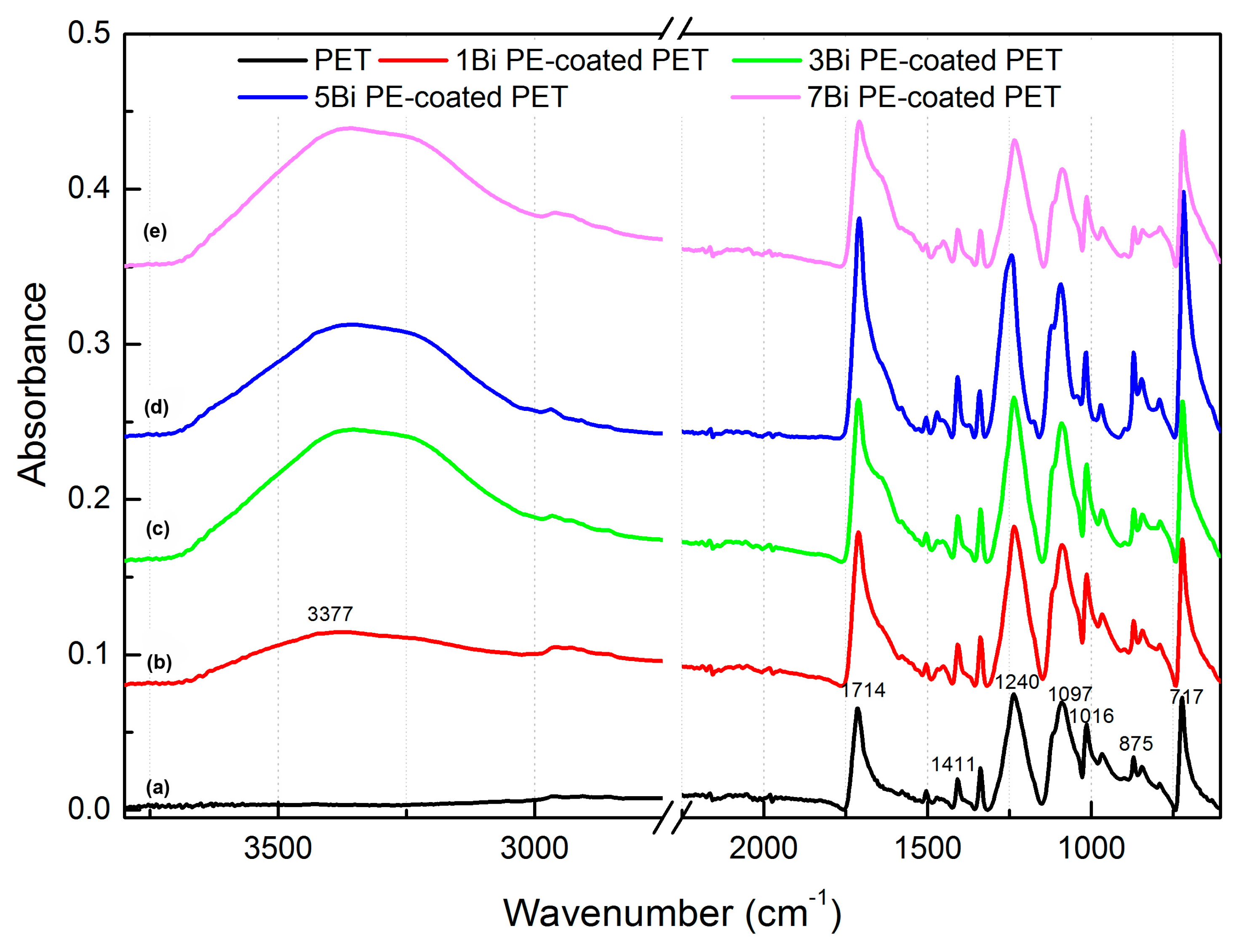
3.1. Preparation of Polyelectrolyte Multilayer-Deposited PET Fabric
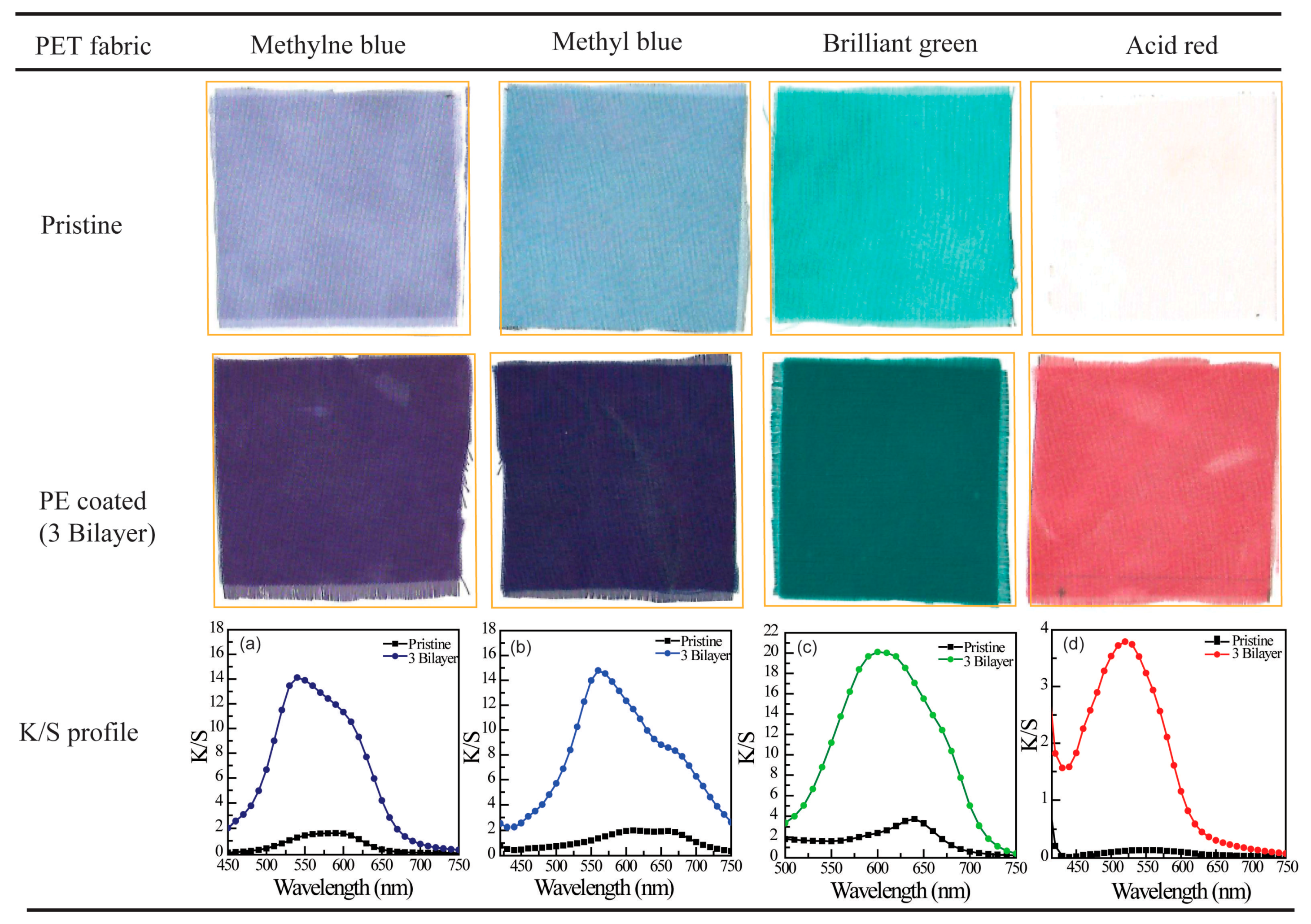
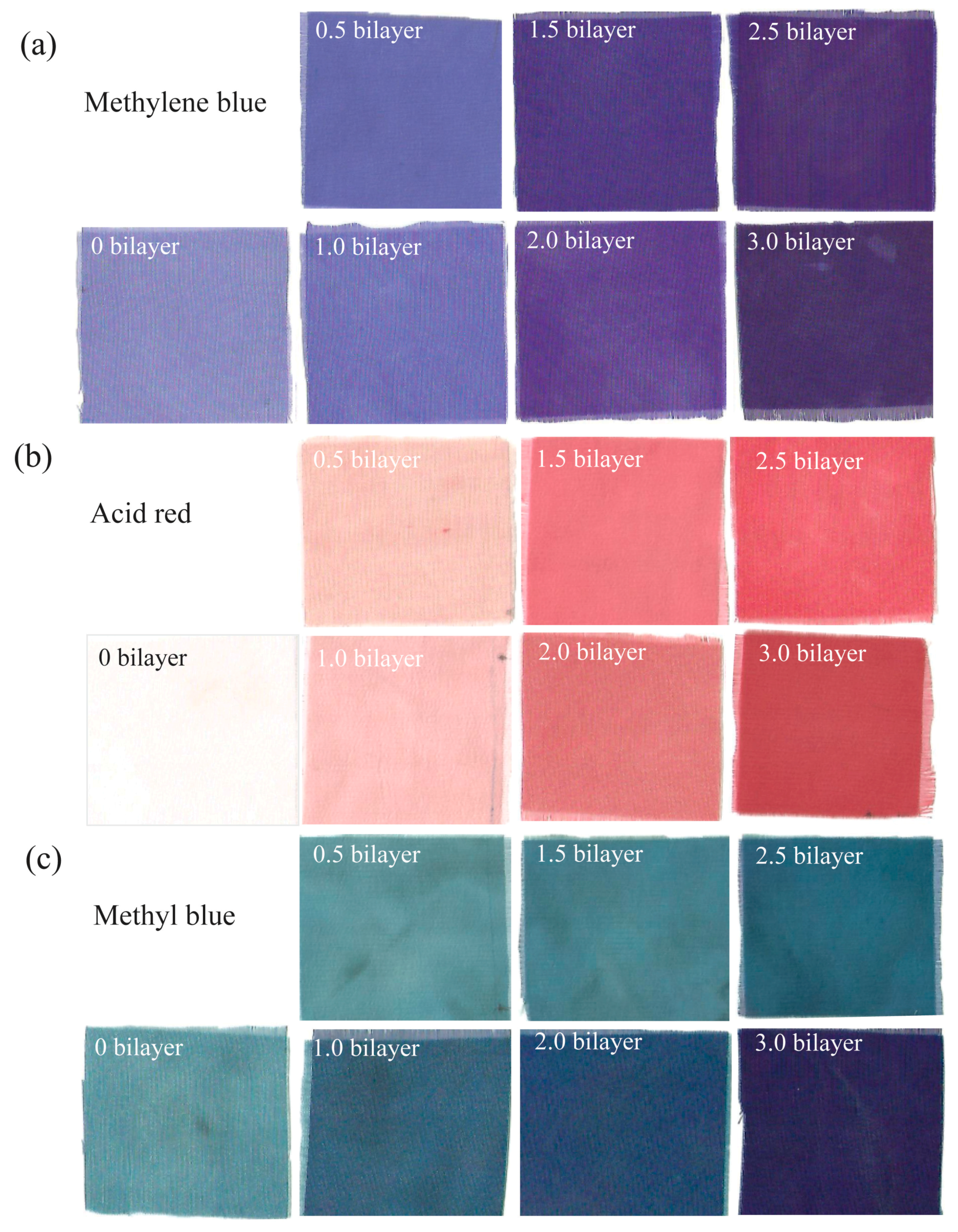
3.2. Coloration of PE Multilayer-Deposited PET Fabrics
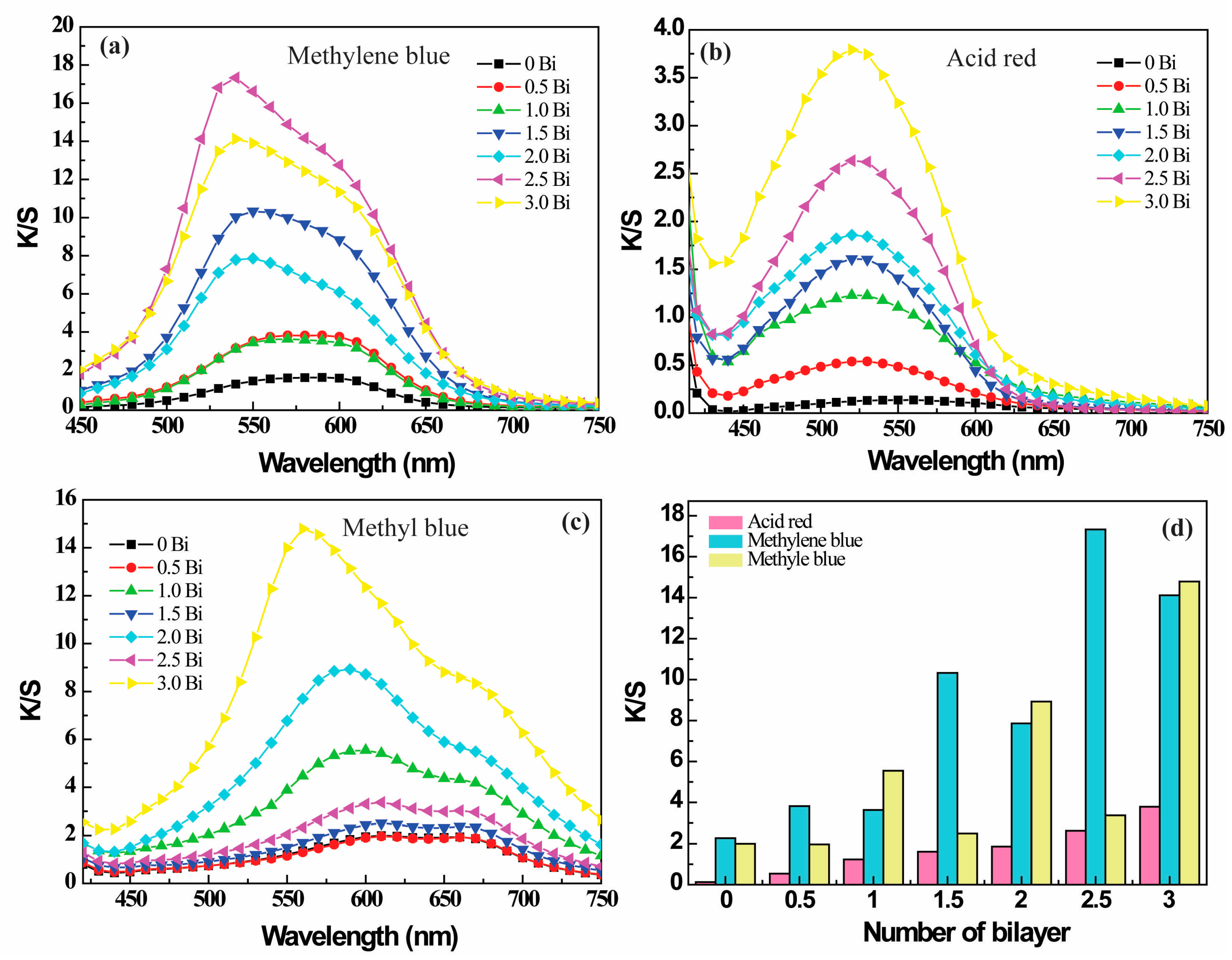
3.3. Effect of the Number of Bilayers on the Colorization of PE Multilayer-Deposited PET Fabrics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dodangeh, M.; Yousefi, N.; Mohammadian, M. Synthesis and functionalization of polyamidoamine dendrimers with thiazol derivatives to prepare novel disperse dyes and their application on polyethylene terephthalate (PET). Dyes Pigment. 2015, 116, 20–26. [Google Scholar] [CrossRef]
- Elabid, A.E.A.; Zhang, J.; Shi, J.; Guo, Y.; Ding, K.; Zhang, J. Improving the low temperature dyeability of polyethylene terephthalate fabric with dispersive dyes by atmospheric pressure plasma discharge. Appl. Surf. Sci. 2016, 375, 26–34. [Google Scholar] [CrossRef]
- Son, Y.A.; Hong, J.P.; Kim, T.K. An approach to the dyeing of polyester fiber using indigo and its extended wash fastness properties. Dyes Pigment. 2004, 61, 263–272. [Google Scholar] [CrossRef]
- Salem, T.; Uhlmann, S.; Nitschke, M.; Calvimontes, A.; Hund, R.D.; Simon, F. Modification of plasma pre-treated PET fabrics with poly-DADMAC and its surface activity towards acid dyes. Prog. Org. Coat. 2011, 72, 168–174. [Google Scholar] [CrossRef]
- Kim, T.K.; Son, Y.A.; Lim, Y.J. Thermodynamic parameters of disperse dyeing on several polyester fibers having different molecular structures. Dyes Pigment. 2005, 67, 229–234. [Google Scholar] [CrossRef]
- Choi, T.S.; Shimizu, Y.; Shriai, H.; Hamada, K. Disperse dyeing of polyester fiber using gemini surfactants containing ammonium cations as auxiliaries. Dyes Pigment. 2001, 50, 55–65. [Google Scholar] [CrossRef]
- Giorgi, M.R.D.; Cadoni, E.; Maricca, D.; Piras, A. Dyeing polyester fibers with disperse dyes in supercritical CO2. Dyes Pigment. 2000, 45, 75–79. [Google Scholar] [CrossRef]
- Pasquet, V.; Perwuelz, A.; Behary, N.; Isaad, J. Vanillin, a potential carrier for low temperature dyeing of polyester fabrics. J. Clean. Prod. 2013, 43, 20–26. [Google Scholar] [CrossRef]
- Nagata, A.; Mizuno, M.; Imahori, S.; Kimura, W. Studies on the dyeing assistants in high temperature dyeing of polyester fiber. J. Fiber Sci. Technol. 1962, 18, 361–370. (In Japanese) [Google Scholar] [CrossRef]
- Fakin, D.; Kleinschek, K.S.; Kurecic, M.; Ojstrsek, A. Effects of nano TiO2-SiO2 on the hydrophilicity/dyeability of polyester fabric and photostability of disperse dyes under UV irradiation. Surf. Coat. Technol. 2014, 253, 185–193. [Google Scholar] [CrossRef]
- Hori, T.; Kongdee, A. Dyeing of PET/co-PP composite fibers using supercritical carbon dioxide. Dyes Pigment. 2014, 105, 163–166. [Google Scholar] [CrossRef]
- Koh, J. Alkali-clearable disperse dyeing of poly (ethyleneterephthalate) with azohydroxypyridone dyes containing a fluorosulfonyl group. Dyes Pigment. 2006, 69, 233–238. [Google Scholar] [CrossRef]
- Kanelli, M.; Vasilakos, S.; Nikolaivits, E.; Ladas, S.; Christakopoulos, P.; Topakas, E. Surface modification of poly(ethylene terephthalate) (PET) fibers by a cutinase from Fusarium oxysporum. Process Biochem. 2015, 50, 1885–1892. [Google Scholar] [CrossRef]
- Shahidi, S.; Ghoranneviss, M. Investigation on dyeability and antibacterial activity of nanolayer platinum coated polyester fabric using DC magnetron sputtering. Prog. Org. Coat. 2011, 70, 300–303. [Google Scholar] [CrossRef]
- Fávaro, S.L.; Rubira, A.F.; Muniz, E.C.; Radovanovic, E. Surface modification of HDPE, PP, and PET films with KMnO4/HCl solutions. Polym. Degrad. Stab. 2007, 92, 1219–1226. [Google Scholar] [CrossRef]
- Ng, R.; Zhang, X.; Liu, N.; Yang, S.T. Modifications of nonwoven polyethylene terephthalate fibrous matrices via NaOH hydrolysis: Effects on pore size, fiber diameter, cell seeding and proliferation. Process Biochem. 2009, 44, 992–998. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, D.; Zhang, F.; Zhang, G. Durable grafting of silkworm pupa protein onto the surface of polyethylene terephthalate fibers. Mater. Sci. Eng. C 2016, 69, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Manna, U.; Bharani, S.; Patil, S. Layer-by-layer self-assembly of modified hyaluronic acid/chitosan based on hydrogen bonding. Biomacromolecules 2009, 10, 2632–2639. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Yan, Y.; Chang, H.C.; Gao, H.; Philip, W.A. Mixed mosaic membranes prepared by layer-by-layer assembly for ionic separations. ACS Nano 2014, 8, 12338–12345. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Jiang, C.; Luo, C.; Zhang, Y.; Shi, F. Investigating zigzag film growth behaviors in layer-by-layer self-assembly of small molecules through a high-gravity technique. ACS Appl. Mater. Interfaces 2015, 7, 18824–18831. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Shen, M.; Mohwald, H. Polyelectrolyte multilayer nanoreactors toward the synthesis of diverse nanostructured materials. Prog. Polym. Sci. 2004, 29, 987–1019. [Google Scholar] [CrossRef]
- De Villiers, M.M.; Otto, D.P.; Strydom, S.J.; Lvov, Y.M. Introduction to nanocoatings produced by layer-by-layer (LbL) self-assembly. Adv. Drug Deliv. Rev. 2011, 63, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Poulos, N.G.; Hall, J.R.; Leopold, M.C. Functional layer-by-layer design of xerogel-based first-generation amperometric glucose biosensors. Langmuir 2015, 31, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Godman, N.P.; Deluca, J.L.; McCollum, S.R.; Schmidtke, D.W.; Glatzhofer, D.T. Electrochemical characterization of layer-by-layer assembled ferrocene-modified linear poly(ethylenimine)/enzyme bioanodes for glucose sensor and biofuel cell applications. Langmuir 2016, 32, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wu, S.; Shen, M.; Guo, R.; Huang, Q.; Wang, S.; Shi, X. Polyelectrolyte multilayer-assisted immobilization of zero-valent iron nanoparticles onto polymer nanofibers for potential environmental applications. ACS Appl. Mater. Interfaces 2009, 1, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.R.; Wang, S.H.; Zhao, H.L.; Wu, S.B.; Xu, J.M.; Li, L.; Liu, X.Y. Layer-by-layer (LBL) assembly technology as promising strategy for tailoring pressure-driven desalination membranes. J. Membr. Sci. 2015, 493, 428–443. [Google Scholar] [CrossRef]
- Manna, U.; Patil, S. Glucose-triggered drug delivery from borate mediated layer-by-layer self-assembly. ACS Appl. Mater. Interfaces 2010, 2, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Choi, J.H.; Deng, Z.J.; Cho, N.J.; Hammond, P.T. Bimodal tumor-targeting from microenvironment responsive hyaluronan layer-by-layer (LbL) nanoparticles. ACS Nano 2014, 8, 8374–8382. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, D.; Jin, G.; Tan, B.H.; He, C. Facile layer-by-layer self-assembly toward enantiomeric Poly(lactide) stereocomplex coated magnetite nanocarrier for highly tunable drug deliveries. ACS Appl. Mater. Interfaces 2016, 8, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; McCarthy, T.J. Layer-by-layer deposition: A tool for polymer surface modification. Macromolecules 1997, 30, 78–86. [Google Scholar] [CrossRef]
- Ding, B.; Fujimoto, K.; Shiratori, S. Preparation and characterization of self-assembled polyelectrolyte multilayered films on electrospun nanofibers. Thin Solid Films 2005, 491, 23–28. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, G.; Zhang, F.; Zhang, Y. Facile preparation of super-hydrophilic poly(ethylene terephthalate) fabric using dilute sulfuric acid under microwave irradiation. Appl. Surf. Sci. 2015, 349, 437–444. [Google Scholar] [CrossRef]
- Tian, M.; Hu, X.; Qu, L.; Zhu, S.; Sun, Y.; Han, G. Versatile and ductile cotton fabric achieved via layer-by-layer self-assembly by consecutive adsorption of graphene doped PEDOT: PSS and chitosan. Carbon 2016, 96, 1166–1174. [Google Scholar] [CrossRef]
- Rieger, K.A.; Porter, M.; Schiffman, J.D. Polyelectrolyte-functionalized nanofiber mats control the collection and inactivation of Escherichia coli. Materials 2016, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Leng, C.; Yu, L.; He, K.; Brown, L.J.; Chen, Z.; Cho, J.; Wang, D. Ion-specific oil repellency of polyelectrolyte multilayers in water: Molecular insights into the hydrophilicity of charged surfaces. Angew. Chem. 2015, 127, 4933–4938. [Google Scholar] [CrossRef]
- Tamai, T.; Watanabe, M.; Kobayashi, Y.; Nakahara, Y.; Yajima, S. Surface modification of PEN and PET substrates by plasma treatment and layer-by-layer assembly of polyelectrolyte multilayer thin films and their application in electroless deposition. RSC Adv. 2017, 7, 33155–33161. [Google Scholar] [CrossRef]
- Raffaele-Addamo, A.; Selli, E.; Barni, R.; Ricardi, C.; Orsini, F.; Poletti, G.; Meda, L.; Massafra, M.R.; Marcandalli, B. Cold plasma-induced modification of the dyeing properties of poly(ethylene terephthalate) fibers. Appl. Surf. Sci. 2006, 252, 2265–2275. [Google Scholar] [CrossRef]
- Dodangeh, M.; Gharanjig, K.; Arami, M.; Atashrouz, S. Surface alteration of polyamide fibers by polyamidoamine dendrimers and optimization of treatment process using neutral network towards improving their dyeing properties. Dyes Pigment. 2014, 111, 30–38. [Google Scholar] [CrossRef]
- Yu, X.; Wei, C.; Wu, H. Effect of molecular structure on the adsorption behavior of cationic dyes onto natural vermiculite. Sep. Purif. Technol. 2015, 156, 489–495. [Google Scholar] [CrossRef]
- Seghi, R.R.; Hewlett, E.R.; Kim, J. Visual and instrumental colorimetric assessments of small color differences on translucent dental porcelain. J. Dent. Res. 1989, 68, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
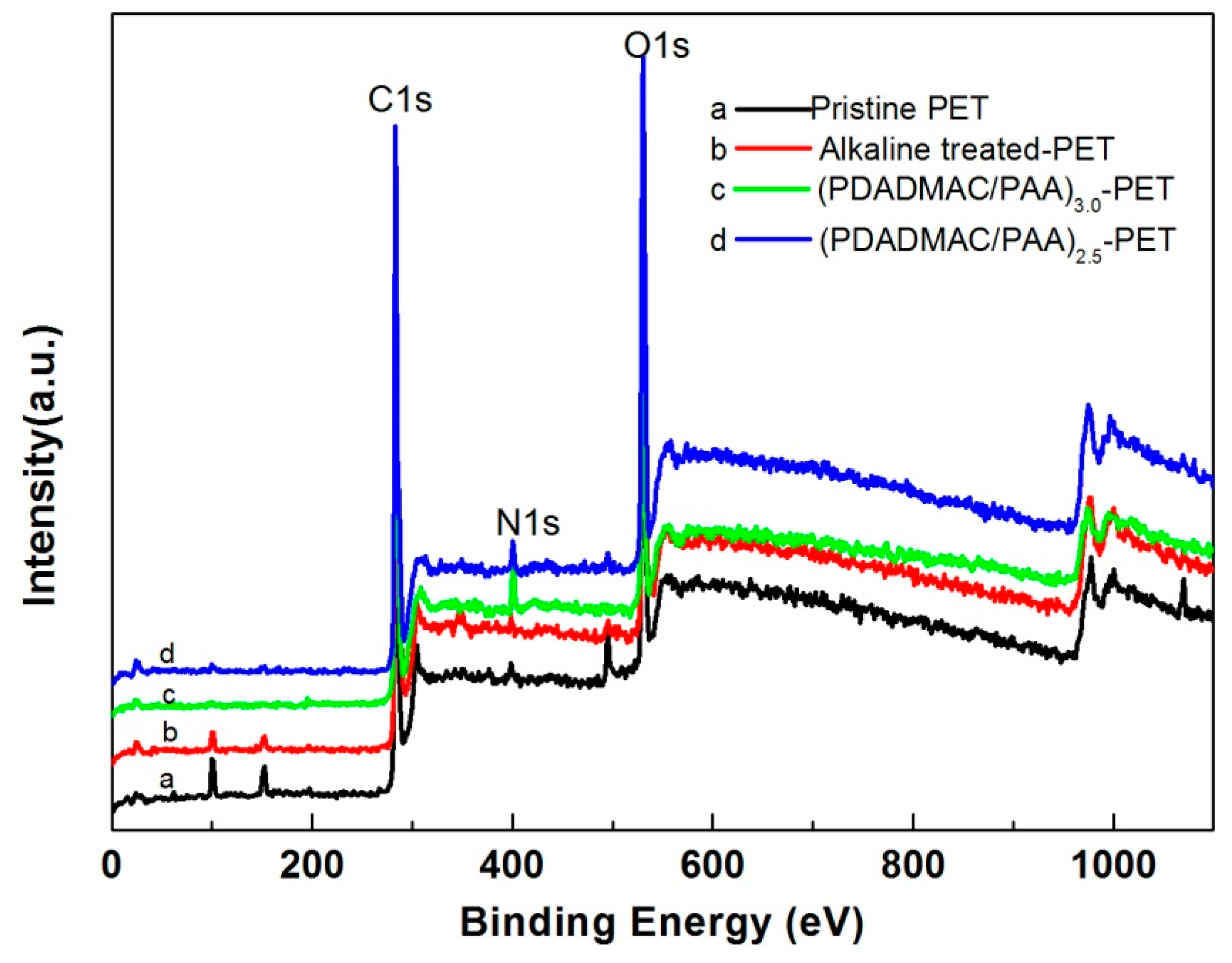
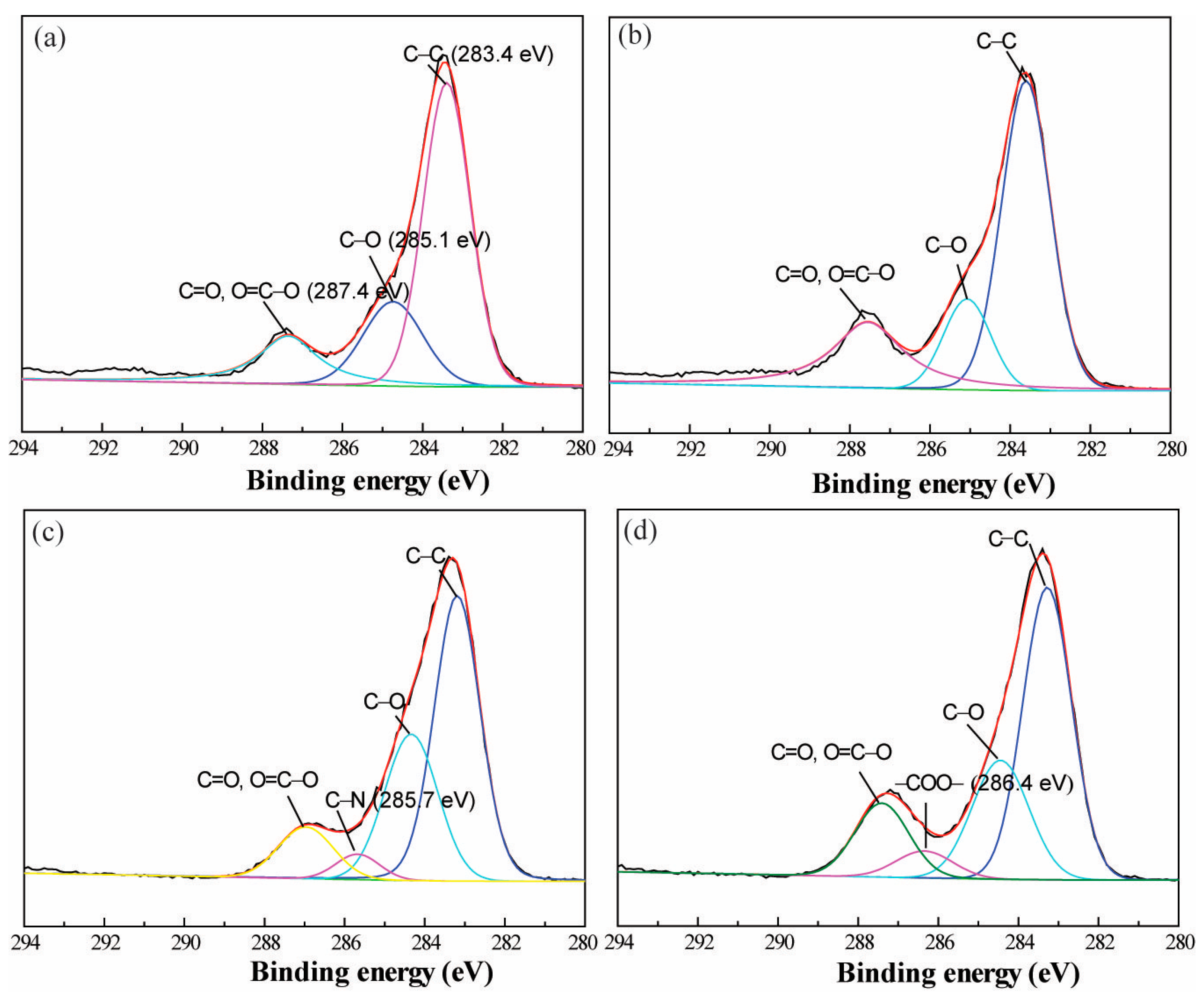
| Sample | Chemical Composition of Surface (%) | |||
|---|---|---|---|---|
| C1s | O1s | N1s | O1s/C1s | |
| Pristine PET | 73.5 | 25.7 | 0.8 | 34.9 |
| Alkaline-t-PET | 72.5 | 26.9 | 0.6 | 37.1 |
| (PDADMAC/PAA)3.0-PET | 74.7 | 21.6 | 3.7 | 28.9 |
| (PDADMAC/PAA)2.5-PET | 67.0 | 30.7 | 2.3 | 45.8 |
| Sample | Relative Area Corresponding to Different Chemical Bonds (%) | ||||
|---|---|---|---|---|---|
| C–C | C–O | C=O, O–C=O | C–N | –COO– | |
| Pristine PET | 60.4 | 21.9 | 17.7 | / | / |
| Alkaline-t-PET | 58.1 | 16.0 | 25.9 | / | / |
| (PDADMAC/PAA)3.0-PET | 54.0 | 30.5 | 11.1 | 4.3 | / |
| (PDADMAC/PAA)2.5-PET | 53.8 | 25.3 | 15.1 | / | 5.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Xu, P.; Peng, Q.; Chen, J.; Huang, J.; Wang, F.; Noor, N. Layer-by-Layer Assembly of Polyelectrolyte Multilayer onto PET Fabric for Highly Tunable Dyeing with Water Soluble Dyestuffs. Polymers 2017, 9, 735. https://doi.org/10.3390/polym9120735
Xiao S, Xu P, Peng Q, Chen J, Huang J, Wang F, Noor N. Layer-by-Layer Assembly of Polyelectrolyte Multilayer onto PET Fabric for Highly Tunable Dyeing with Water Soluble Dyestuffs. Polymers. 2017; 9(12):735. https://doi.org/10.3390/polym9120735
Chicago/Turabian StyleXiao, Shili, Pengjun Xu, Qingyan Peng, Jiali Chen, Jiankang Huang, Faming Wang, and Nuruzzaman Noor. 2017. "Layer-by-Layer Assembly of Polyelectrolyte Multilayer onto PET Fabric for Highly Tunable Dyeing with Water Soluble Dyestuffs" Polymers 9, no. 12: 735. https://doi.org/10.3390/polym9120735
APA StyleXiao, S., Xu, P., Peng, Q., Chen, J., Huang, J., Wang, F., & Noor, N. (2017). Layer-by-Layer Assembly of Polyelectrolyte Multilayer onto PET Fabric for Highly Tunable Dyeing with Water Soluble Dyestuffs. Polymers, 9(12), 735. https://doi.org/10.3390/polym9120735







