Bio-Based Polymers with Potential for Biodegradability
Abstract
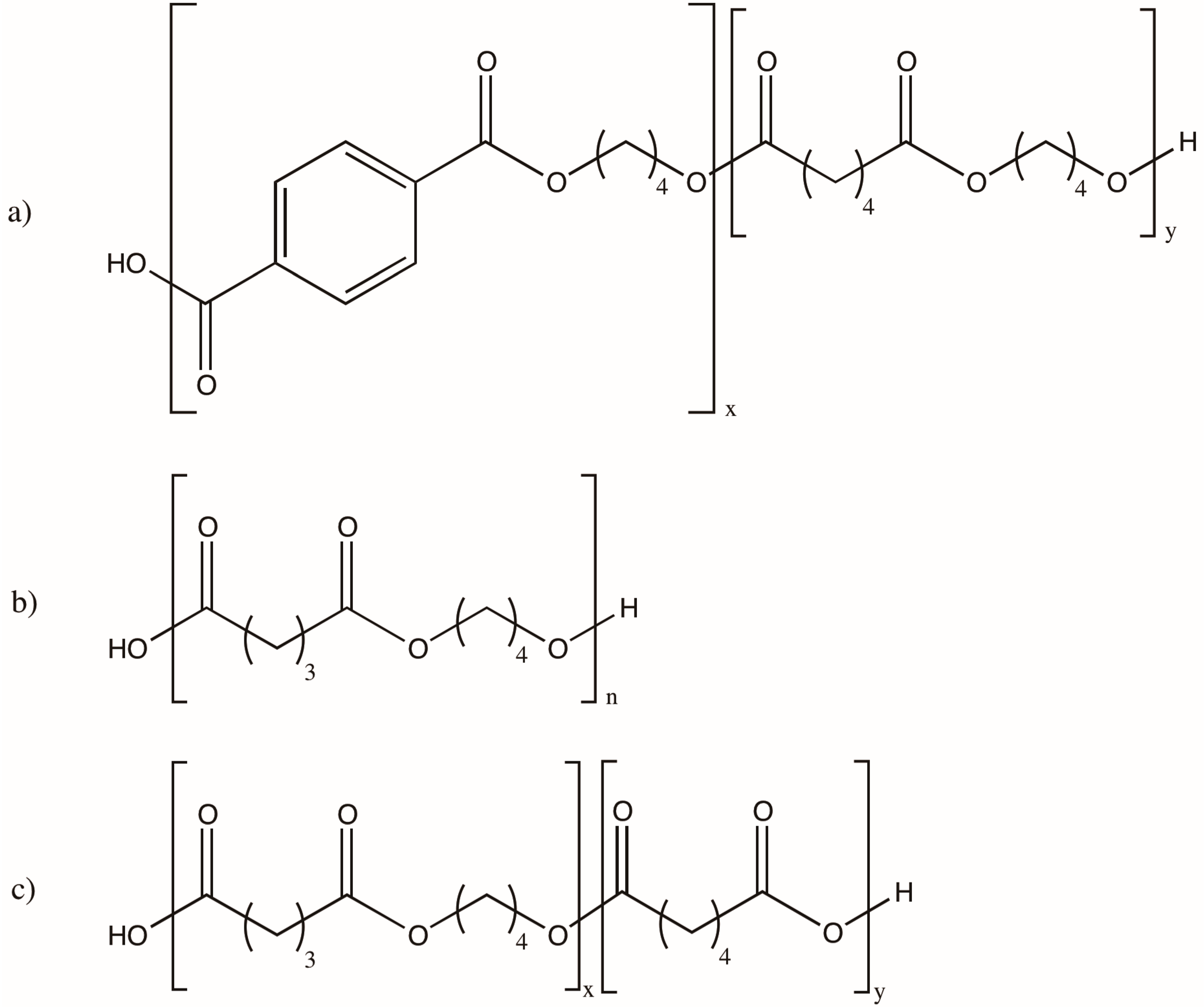
:1. Introduction
2. Bio-Based Polymers
2.1. Vegetable Oils
2.2. Cashew Nut Shell Liquid
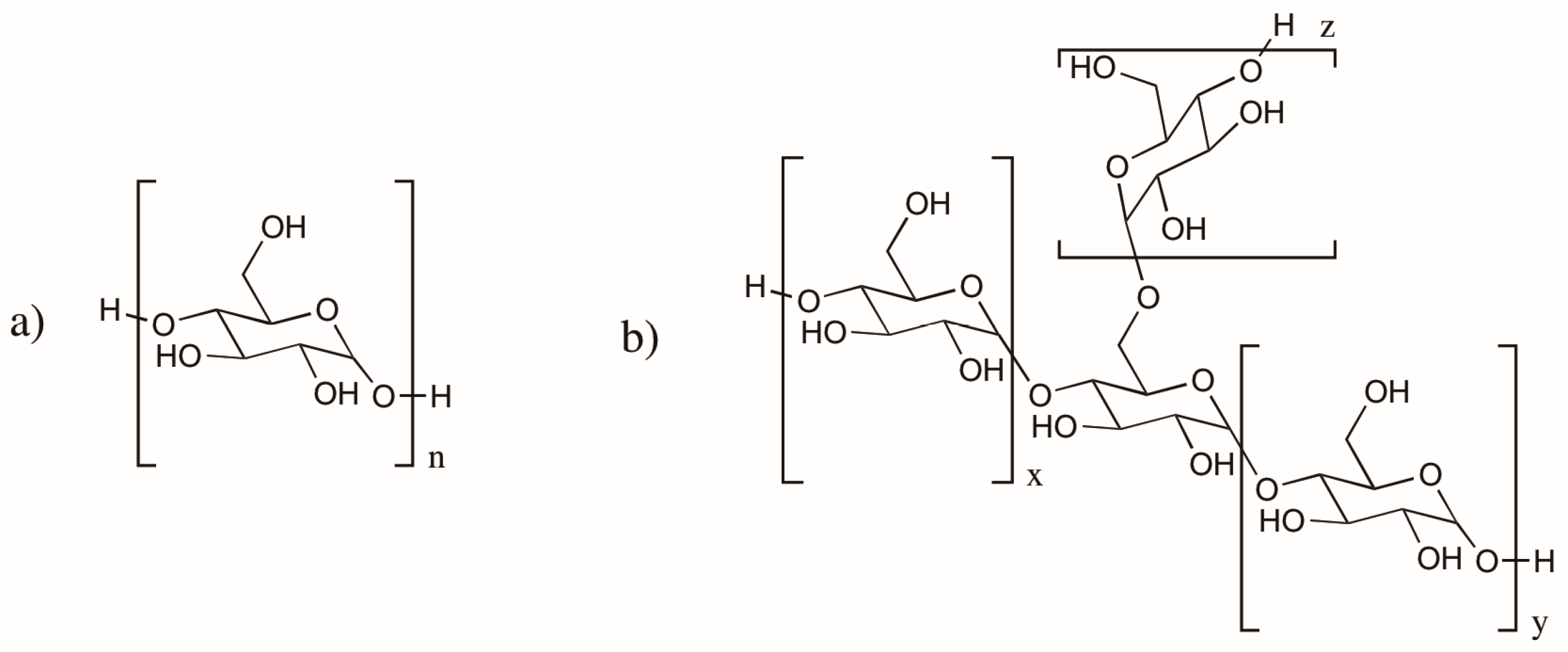
2.3. Cellulose and Chitosan
2.4. Polyhydroxyalkanoates (PHAs)
2.5. Proteins
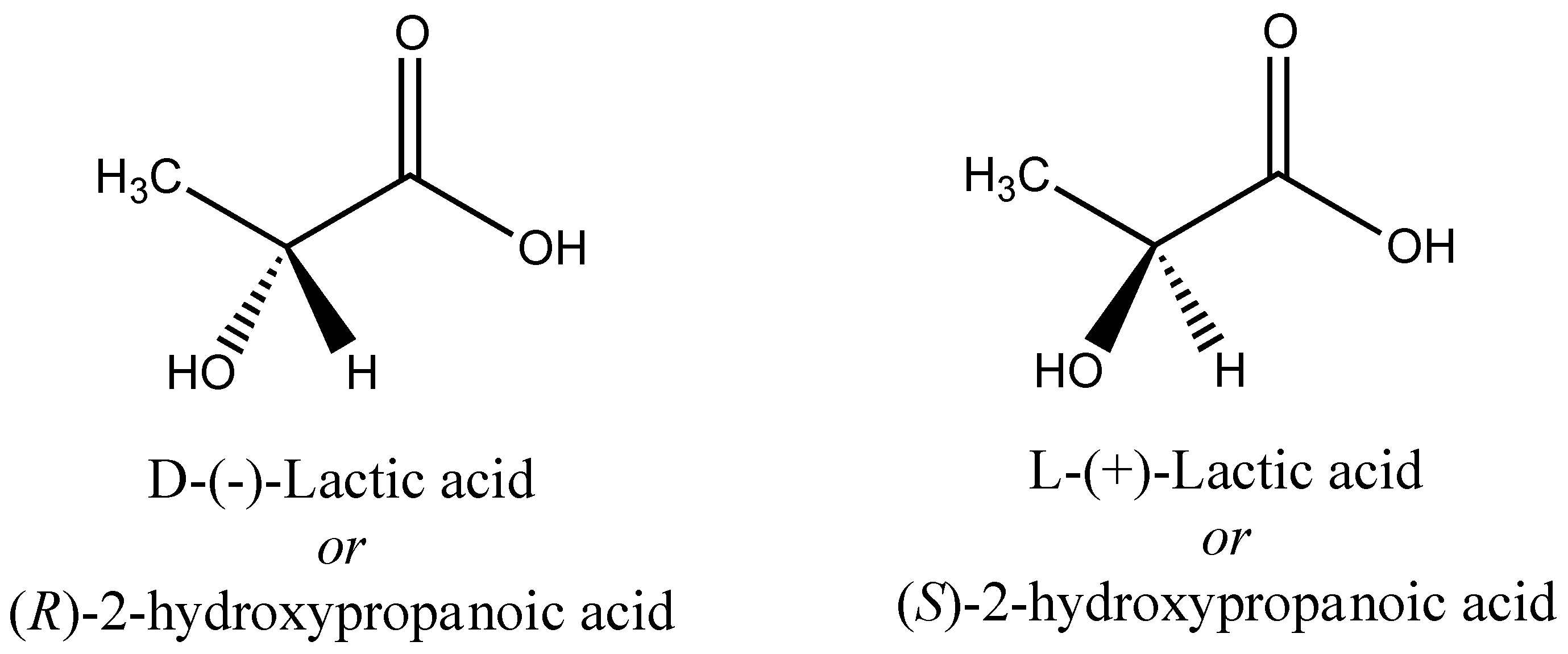
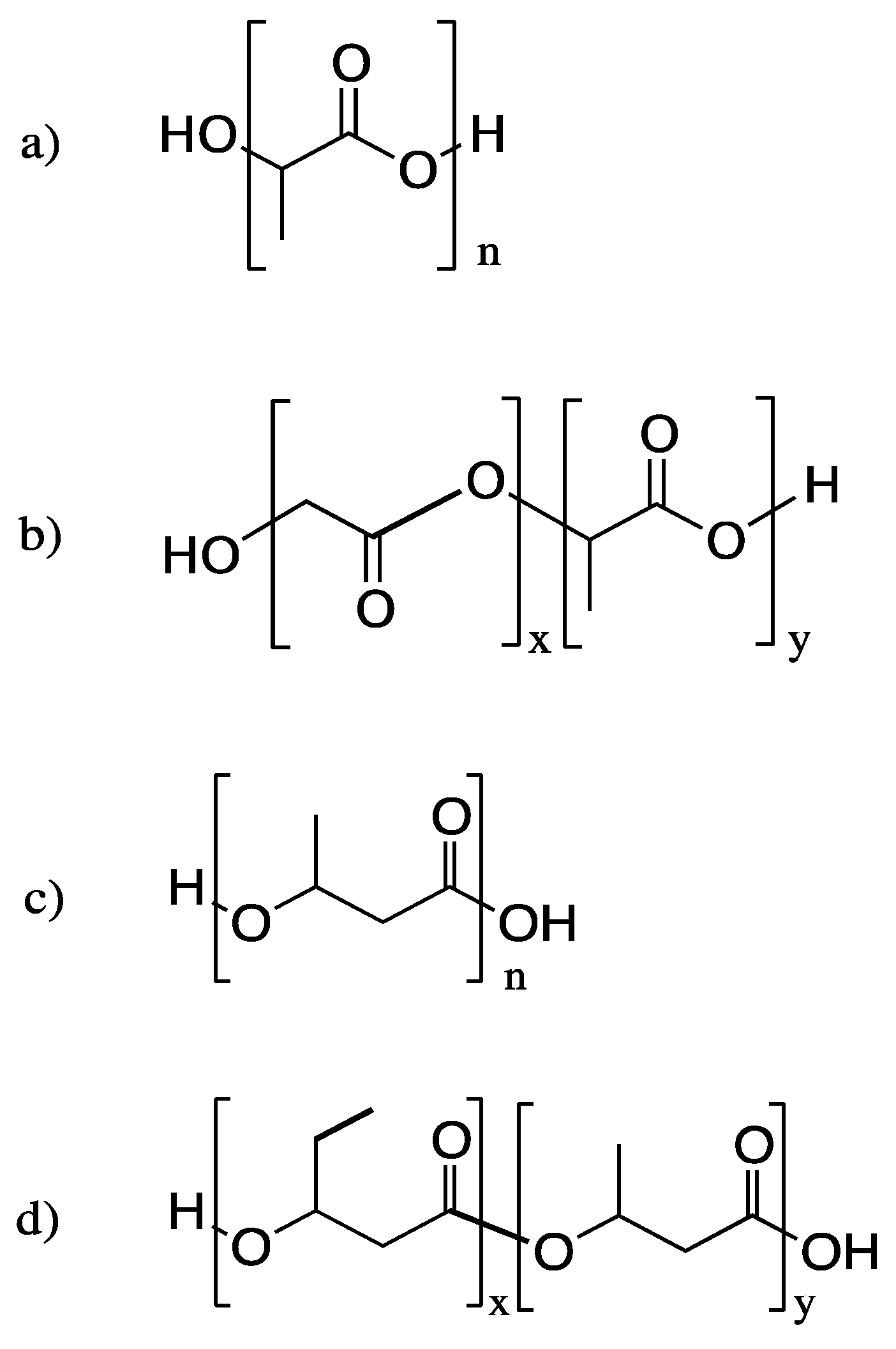
3. Poly(lactic acid) (PLA) and Related Polymers
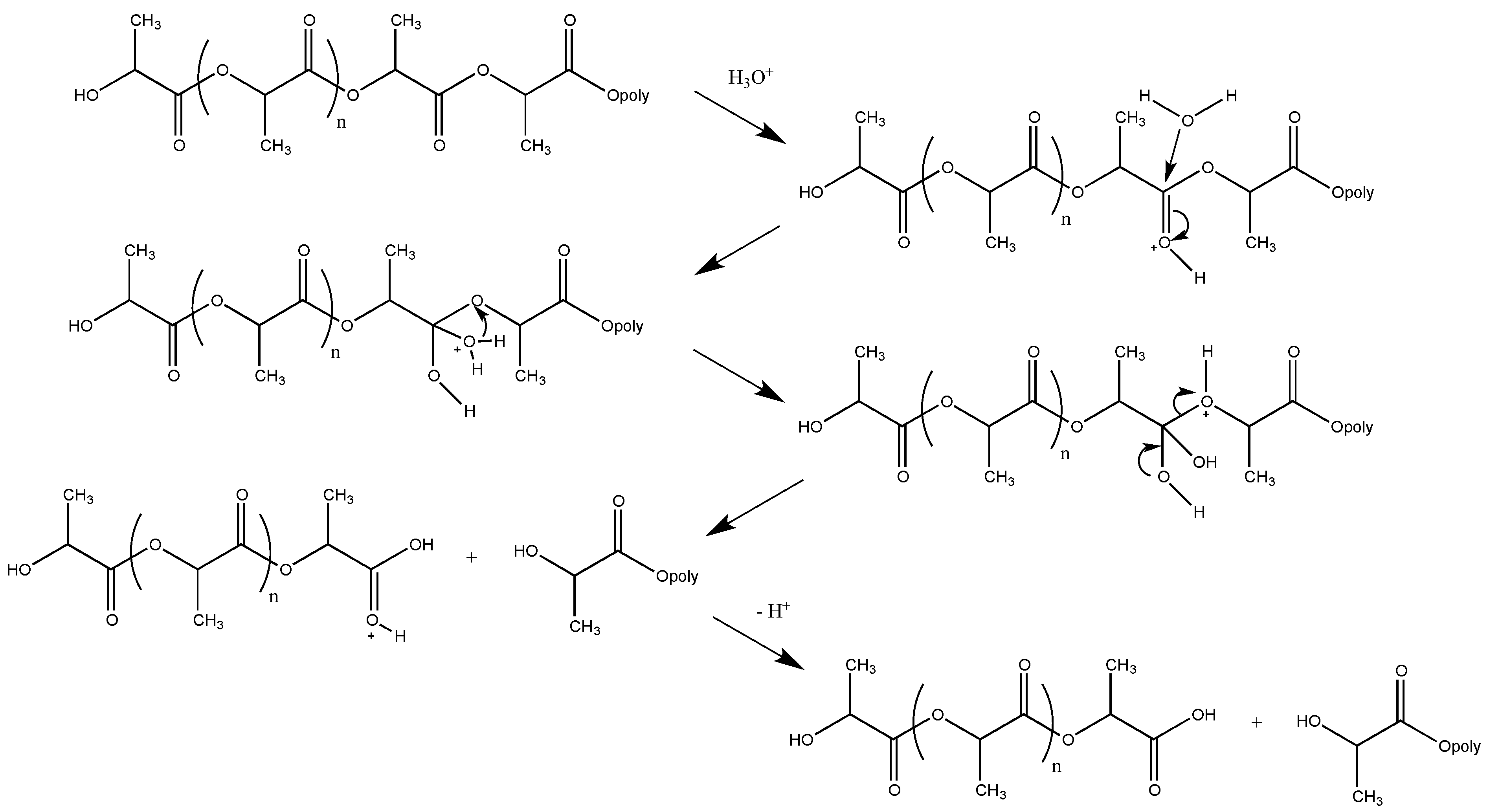
3.1. Biodegradation of PLA
3.2. Influence of UV Irradiation on Biodegradation
3.3. Degradation of PLA Composites and PLA Blends
3.4. PLA-Degrading Microorganisms
3.5. Methods of Monitoring Biodegradation
4. Other Bio-Based Polymers with Potential for Biodegradability
4.1. Medical Applications
4.2. Plastics
4.3. Adhesives and Elastomers
4.4. Compatibilizers, Biocomposites and Biofibers
4.5. Chemical Structure Influence on Biodegradation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goodman, S.H. Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; William Andrew Publishing: Westwood, MA, USA, 1999; p. 1. [Google Scholar]
- Williams, C.K.; Hillmyer, M.A. Polymers from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Lu, Y.S.; Larock, R.C. Novel polymeric materials from vegetable oils and vinyl monomers: Preparation, properties, and applications. ChemSusChem 2009, 2, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011, 13, 1061–1083. [Google Scholar] [CrossRef]
- Gandini, A. Polymers from renewable resources: A challenge for the future of macromolecular materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Gandini, A. Materials from Vegetable Oils: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources, 1st ed.; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 39–66. [Google Scholar]
- Van der Mark, M.R.; Sandefur, K. Vegetable Oils in Paint and Coatings. In Industrial Uses of Vegetable Oil; Erhan, S.Z., Ed.; AOCS Publishing: Peoria, IL, USA, 2009; pp. 143–162. [Google Scholar]
- Poth, U. Ullmann’s Encyclopedia of Industrial Chemistry; Bellussi, G., Bohnet, M., Bus, J., Drauz, K., Faulhammer, H., Greim, H., Jackel, K., Karst, U., Kleemann, A., Kutscher, B., et al., Eds.; John Wiley & Sons: New York, NY, USA, 2000; p. 11. [Google Scholar]
- Galià, M.; Espinosa, L.M.; Ronda, J.C.; Lligadas, G.; Cádiz, V. Vegetable oil-based thermosetting polymers. Eur. J. Lipid Sci. Technol. 2010, 112, 87–96. [Google Scholar]
- Cakmakli, B.; Hazer, B.; Tekin, I.O.; Comert, F.B. Synthesis and characterization of polymeric soybean oil-g-methyl methacrylate (and n-butyl methacrylate) graft copolymers: Biocompatibility and bacterial adhesion. Biomacromolecules 2005, 6, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, D.D.; Valverde, M.; Henna, P.; Li, F.K.; Larock, R.C. Novel thermosets prepared by cationic copolymerization of various vegetable oils—Synthesis and their structure-property relationships. Polymer 2005, 46, 9674–9685. [Google Scholar] [CrossRef]
- Lu, J.; Khot, S.; Wool, R.P. New sheet molding compound resins from soybean oil. I. Synthesis and characterization. Polymer 2005, 46, 71–80. [Google Scholar] [CrossRef]
- Tan, S.G.; Chow, W.S. Biobased epoxidized vegetable oils and its greener epoxy blends: A review. Polym. Plast. Technol. Eng. 2010, 49, 1581–1590. [Google Scholar] [CrossRef]
- Biermann, U.; Metzger, J.O.; Meier, M.A.R. Acyclic triene metathesis oligo- and polymerization of high oleic sun flower oil. Macromol. Chem. Phys. 2010, 211, 854–862. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Castor oil-based thermosets with varied crosslink densities prepared by ring-opening metathesis polymerization (ROMP). Polymer 2010, 51, 2508–2514. [Google Scholar] [CrossRef]
- Dutta, N.; Karak, N.; Dolui, S.K. Synthesis and characterization of polyester resins based on Nahar seed oil. Prog. Org. Coat. 2004, 49, 146–152. [Google Scholar] [CrossRef]
- Zlatanic, A.; Lava, C.; Zhang, W.; Petrovic, Z.S. Effect of structure on properties of polyols and polyurethanes based on different vegetable oils. J. Polym. Sci. B Polym. Phys. 2004, 42, 809–819. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Review biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Kalea, G.; Aurasa, R.; Singha, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Bozell, J.J. Feedstocks for the Future—Biorefinery production of chemicals from renewable carbon. Clean Soil Air Water 2008, 36, 641–647. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Grewell, D. Improvement of the mechanical properties of soy protein isolate based plastics through formulation and processing. Int. Polym. Proc. 2007, 489–496. [Google Scholar]
- Srinivasan, G.; Grewell, D. Investigation of Processability of Zein Based Plastics and Composites. In Proceedings of the 67th Society of Plastics Engineers ANTEC, Chicago, IL, USA, 22–24 June 2009; Society of Plastic Engineers: Brookfield, CT, USA, 2009. [Google Scholar]
- Erhan, S.Z. Industrial Uses of Vegetable Oils; AOCS Press: Champaign, IL, USA, 2005; p. 143. [Google Scholar]
- Lu, Y.; Larock, R.C. Aqueous cationic polyurethane dispersions from vegetable oils. ChemSusChem 2010, 3, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.; Ji, Y.; Shirley, W.M.; Petrovic, Z.S. Renewable and Sustainable Polymers; American Chemical Society: Washington, DC, USA, 2011; p. 73. [Google Scholar]
- Sharma, H.O.; Alam, M.; Riaz, U.; Ahmad, S.; Ashraf, S.M. Miscibility studies of polyesteramides of linseed oil and dehydrated castor oil with poly(vinyl alcohol). Int. J. Polym. Mater. 2007, 56, 437–451. [Google Scholar] [CrossRef]
- Zhan, M.; Wool, R.P. Biobased composite resins design for electronic materials. J. Appl. Polym. Sci. 2010, 118, 3274–3283. [Google Scholar] [CrossRef]
- Zhan, G.; Zhao, L; Hu, S.; Gan, W.; Yu, Y.; Tang, X. A novel biobased resin-epoxidized soybean oil modified cyanate ester. Polym. Eng. Sci. 2008, 48, 1322–1328. [Google Scholar] [CrossRef]
- Valverde, V.; Andjelkovic, D.; Kundu, P.P.; Larock, R.C. Conjugated low-saturation soybean oil thermosets: Free-radical copolymerization with dicyclopentadiene and divinylbenzene. J. Appl. Polym. Sci. 2008, 107, 423–430. [Google Scholar] [CrossRef]
- Andjelkovic, D.D.; Min, B.; Ahn, D.; Larock, R.C. Elucidation of structural isomers from the homogeneous rhodium-catalyzed isomerization of vegetable oils. J. Agric. Food Chem. 2006, 54, 9535–9543. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.; Meier, M.Ä.Ä. Acyclic diene metathesis with a monomer from renewable resources: Control of molecular weight and one-step preparation of block copolymers. ChemSusChem 2008, 1, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Panhuis, M.I.H.; Thielemans, W.; Minett, A.I.; Leahy, R.; Foulgoc, B.L.; Blau, W.J.; Wool, R.P. A composite from soy oil and carbon nanotubes. Int. J. Nanosci. 2003, 2, 185–194. [Google Scholar] [CrossRef]
- Thielemans, W.; Wool, R.P. Butyrated kraft lignin as compatibilizing agent for natural fiber reinforced thermoset composites. Compos. A Appl. Sci. Manuf. 2004, 35, 327–338. [Google Scholar] [CrossRef]
- Campanella, A.; Wool, R.P.; Bah, M.; Fita, S.; Abuobaid, A. Composites from northern red oak (Quercus robur) leaves and plant oil-based resins. J. Appl. Polym. Sci. 2013, 127, 18–26. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Corn oil-based composites reinforced with continuous glass fibers: Fabrication and properties. J. Appl. Polym. Sci. 2006, 102, 3345–3353. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Novel biobased nanocomposites from soybean oil and functionalized organoclay. Biomacromolecules 2006, 7, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Quirino, R.L.; Larock, R.C. Synthesis and properties of soy hull-reinforced biocomposites from conjugated soybean oil. J. Appl. Polym. Sci. 2009, 112, 2033–2043. [Google Scholar] [CrossRef]
- Quirino, R.L.; Larock, R.C. Rice hull biocomposites, part 2: Effect of the resin composition on the properties of the composite. J. Appl. Polym. Sci. 2011, 121, 2050–2054. [Google Scholar] [CrossRef]
- Quirino, R.L.; Woodford, J.; Larock, R.C. Soybean and linseed oil-based composites reinforced with wood flour and wood fibers. J. Appl. Polym. Sci. 2012, 124, 1520–1528. [Google Scholar] [CrossRef]
- Pfister, D.P.; Larock, R.C. Cationically cured natural oil-based green composites: Effect of the natural oil and the agricultural fiber. J. Appl. Polym. Sci. 2012, 123, 1392–1400. [Google Scholar] [CrossRef]
- Henna, P.H.; Kessler, M.R.; Larock, R.C. Fabrication and properties of vegetable-oil-based glass fiber composites by ring-opening metathesis polymerization. Macromol. Mater. Eng. 2008, 293, 979–990. [Google Scholar] [CrossRef]
- Deka, H.; Karak, N. Rheological study of vegetable oil based hyperbranched polyurethane/multi-walled carbon nanotube nanocomposites. Polym. Plast. Technol. Eng. 2011, 50, 797–803. [Google Scholar] [CrossRef]
- Garrison, T.F.; Kessler, M.R.; Larock, R.C. Effects of unsaturation and different ring-opening methods on the properties of vegetable oil-based polyurethane coatings. Polymer 2014, 55, 1004–1011. [Google Scholar] [CrossRef]
- Yeganeh, H.; Mehdizadeh, M.R. Synthesis and properties of isocyanate curable millable polyurethane elastomers based on castor oil as a renewable resource polyol. Eur. Polym. J. 2004, 40, 1233–1238. [Google Scholar] [CrossRef]
- Petrovic, Z.S.; Cvetkovic, I.; Hong, D.; Wan, X.; Zhang, W.; Abraham, T.; Malsam, J. Polyester polyols and polyurethanes from ricinoleic acid. J. Appl. Polym. Sci. 2008, 108, 1184–1190. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Soybean-oil-based waterborne polyurethane dispersions: Effects of polyol functionality and hard segment content on properties. Biomacromolecules 2008, 9, 3332–3340. [Google Scholar] [CrossRef] [PubMed]
- Garrison, T.F.; Zhang, Z.; Kim, H.J.; Mitra, D.; Xia, Y.; Pfister, D.P.; Brehm-Stecher, B.F.; Larock, R.C.; Kessler, M.R. Thermo-mechanical and antibacterial properties of soybean oil-based cationic polyurethane coatings: Effects of amine ratio and degree of crosslinking. Macromol. Mater. Eng. 2014, 299, 1042–1051. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. New hybrid latexes from a soybean oil-based waterborne polyurethane and acrylics via emulsion polymerization. Biomacromolecules 2007, 8, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Larock, R.C. Synthesis and properties of grafted latices from a soybean oil-based waterborne polyurethane and acrylics. J. Appl. Polym. Sci. 2011, 119, 3305–3314. [Google Scholar] [CrossRef]
- Lu, Y.; Xia, Y.; Larock, R.C. Surfactant-free core-shell hybrid latexes from soybean oil-based waterborne polyurethanes and poly(styrene-butyl acrylate). Prog. Org. Coat. 2011, 71, 336–342. [Google Scholar] [CrossRef]
- Guner, F.S.; Yagci, Y.; Erciyes, A.T. Polymers from triglyceride oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Bharathi, N.P.; Khan, N.U.; Shreaz, S.; Hashmi, A.A. Seed oil based zinc bioactive polymers: Synthesis, characterization and biological studies. J. Inorg. Organomet. Polym. Mater. 2009, 19, 558–565. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Unsaturated PA X,20 from renewable resources via metathesis and catalytic amidation. Macromol. Chem. Phys. 2009, 210, 1019–1025. [Google Scholar] [CrossRef]
- Santos, M.L.; Magalhães, G.C. Utilisation of cashew nut shell liquid from Anacardium occidentale as starting material for organic synthesis: A novel route to lasiodiplodin from cardols. J. Braz. Chem. Soc. 1999, 10, 13–20. [Google Scholar] [CrossRef]
- Gedam, P.H.; Sampathkumaran, P.S. Cashew nut shell liquid: Extraction, chemistry and applications. Prog. Org. Coat. 1986, 14, 115–157. [Google Scholar] [CrossRef]
- Mele, G.; Vasapollo, G. Fine chemicals and new hybrid materials from cardanol. Mini Rev. Org. Chem. 2008, 5, 243–253. [Google Scholar] [CrossRef]
- De Sousa Rios, M.A.; Nascimento, T.L.; Santiago, S.N.; Mazzetto, S.E. Cashew nut shell liquid: A versatile raw material utilized for syntheses of phosphorus compounds. Energy Fuels 2009, 23, 5432–5437. [Google Scholar] [CrossRef]
- Lubi, M.C.; Thachil, E.T. Cashew nut shell liquid (CNSL)—A versatile monomer for polymer synthesis. Des. Monomers Polym. 2000, 3, 123–153. [Google Scholar] [CrossRef]
- Vasapollo, G.; Mele, G.; Sole, R.D. Cardanol-based materials as natural precursors for olefin metathesis. Molecules 2011, 16, 6871–6882. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Kin-Tak, A.L.; Fedrigo, J. Novel phenolic resins with improved mechanical and toughness properties. J. Appl. Polym. Sci. 2012, 123, 2131–2139. [Google Scholar] [CrossRef]
- Nair, C.P.R.; Bindu, R.L.; Ninan, K.N. Recent advances in phenolic resins. Met. Mater. Process. 1997, 9, 179–200. [Google Scholar]
- Cardona, F.; Aravinthan, T.; Moscou, C. Modified PF resins for composite structures with improved mechanical properties. Polym. Polym. Compos. 2010, 18, 235–244. [Google Scholar]
- Mwaikambo, L.Y.; Ansell, M.P. Cure characteristics of alkali catalysed cashew nut shell liquid-formaldehyde resin. J. Mater. Sci. 2001, 36, 3693–3698. [Google Scholar] [CrossRef]
- Parameswaran, P.S.; Abraham, B.T.; Thachil, E.T. Cardanol-based resol phenolics—A comparative study. Prog. Rubber Plast. Recycl. Technol. 2010, 26, 31–50. [Google Scholar]
- Unnikrishnan, K.P.; Thachil, E.T. Studies on the modification of commercial epoxy resin using cardanol-based phenolic resins. J. Elastom. Plast. 2008, 40, 271–286. [Google Scholar] [CrossRef]
- Calo, E.; Maffezzoli, A.; Mele, G.; Martina, F.; Mazzetto, S.E.; Tarzia, A.; Stifani, C. Synthesis of a novel cardanol-based benzoxazine monomer and environmentally sustainable production of polymers and bio-composites. Green Chem. 2007, 9, 754–759. [Google Scholar] [CrossRef]
- Rao, B.S.; Palanisamy, A. Monofunctional benzoxazine from cardanol for bio-composite applications. React. Funct. Polym. 2011, 71, 148–154. [Google Scholar] [CrossRef]
- Chutayothin, P.; Ishida, H. Cationic ring-opening polymerization of 1,3-benzoxazines: Mechanistic study using model compounds. Macromolecules 2010, 43, 4562–4572. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastin, R.M.; Marquet, J.; Schnfeld, R. Mechanistic studies on ring-opening polymerization of benzoxazines: A mechanistically based catalyst design. Macromolecules 2011, 44, 4616–4622. [Google Scholar] [CrossRef]
- Lochab, B.; Varma, I.K.; Bijwe, J. Blends of benzoxazine monomers: Effect of structure and composition on polymer properties. J. Therm. Anal. Calorim. 2013, 111, 1357–1364. [Google Scholar] [CrossRef]
- Swain, S.K.; Sahoo, S.; Mohapatra, D.K.; Mishra, B.K.; Lenka, S.; Nayak, P.L. Polymers from renewable resources. V. Synthesis and characterization of thermosetting resins derived from cashew nut shell liquid (CNSL)–furfural-substituted aromatic compounds. J. Appl. Polym. Sci. 1994, 54, 1413–1421. [Google Scholar] [CrossRef]
- Mishra, D.K.; Mishra, B.K.; Lenka, S.; Nayak, P.L. Polymers from renewable resources. VII: Thermal properties of the semi-interpenetrating polymer networks composed of castor oil polyurethanes and cardanol-furfural resin. Polym. Eng. Sci. 1996, 36, 1047–1051. [Google Scholar] [CrossRef]
- Kasemsiri, P.; Hiziroglu, S.; Rimdusit, S. Effect of cashew nut shell liquid on gelation, cure kinetics, and thermomechanical properties of benzoxazine resin. Thermochim. Acta 2011, 520, 84–92. [Google Scholar] [CrossRef]
- Kasemsiri, P.; Hiziroglu, S.; Rimdusit, S. Properties of wood polymer composites from eastern redcedar particles reinforced with benzoxazine resin/cashew nut shell liquid copolymer. Compos. A Appl. Sci. Manuf. 2011, 42, 1454–1462. [Google Scholar] [CrossRef]
- Lochab, B.; Varma, I.K.; Bijwe, J. Cardanol based bisbenzoxazines: Effect of structure on thermal behaviour. J. Therm. Anal. Calorim. 2012, 107, 661–668. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Puttipipatkhachorn, S. Xanthan–alginate composite gel beads: Molecular interaction and in vitro characterization. Int. J. Pharm. 2007, 331, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Wang, W.J.; Shao, Z.Q.; Zhu, H.D.; Li, Y.H.; Wang, F.J. The transparency and mechanical properties of cellulose acetate nanocomposites using cellulose nanowhiskers as fillers. Cellulose 2013, 20, 159–168. [Google Scholar] [CrossRef]
- Nishino, T.; Matsuda, I.; Hirao, K. All-cellulose composite. Macromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Gindl, W.; Schöberl, T.; Keckes, J. Structure and properties of a pulp fibre-reinforced composite with regenerated cellulose matrix. Appl. Phys. A 2006, 83, 19–22. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, B.; Li, H.; Li, Y.; Ou, S. Green composite films composed of nanocrystalline cellulose and a cellulose matrix regenerated from functionalized ionic liquid solution. Carbohydr. Polym. 2011, 84, 383–389. [Google Scholar] [CrossRef]
- Li, S.M.; Jia, N.; Ma, M.G.; Zhang, Z.; Liu, Q.H.; Sun, R.C. Cellulose–silver nanocomposites: Microwave-assisted synthesis, characterization, their thermal stability, and antimicrobial property. Carbohydr. Polym. 2011, 86, 441–447. [Google Scholar] [CrossRef]
- Luna-Martinez, J.F.; Reyes-Melo, E.; Gonzalez-Gonzalez, V.; Guerrero-Salazar, C.; Torres-Castro, A.; Sepulveda-Guzman, S. Synthesis and characterization of a magnetic hybrid material consisting of iron oxide in a carboxymethyl cellulose matrix. J. Appl. Polym. Sci. 2013, 127, 2325–2331. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Freire, C.S.R.; Fernandes, S.C.M.; Kallip, S.; Lisenkov, A.; Gandini, A.; Ferreira, M.G.S. Self-healing protective coatings with “green” chitosan based pre-layer reservoir of corrosion inhibitor. J. Mater. Chem. 2011, 21, 4805–4812. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Teong, L.C.; Hanafiah, M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Cocenza, D.S.; de Moraes, M.A.; Beppu, M.M.; Fraceto, L.F. Use of biopolymeric membranes for adsorption of paraquat herbicide from water. Water Air Soil Pollut. 2012, 223, 3093–3104. [Google Scholar] [CrossRef]
- Britto, D.; de Moura, M.R.; Aouada, F.A.; Mattoso, L.H.C.; Assis, O.B.G. N,N,N-Trimethyl chitosan nanoparticles as a vitamin carrier system. Food Hydrocoll. 2012, 27, 487–493. [Google Scholar] [CrossRef]
- Freitas, R.M.; Spin-Neto, R.; Spolidorio, L.C.; Campana, S.P.; Marcantonio, R.A.C.; Marcantonio, E. Different molecular weight chitosan-based membranes for tissue regeneration. Materials 2011, 4, 380–389. [Google Scholar] [CrossRef]
- Shelma, R.; Sharma, C.P. Development of lauroyl sulfated chitosan for enhancing hemocompatibility of chitosan. Colloid. Surf. B 2011, 84, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Martino, V.P.; Pollet, E.; Averous, L. Novative biomaterials based on chitosan and poly(ε-caprolactone): Elaboration of porous structures. J. Polym. Environ. 2011, 19, 819–826. [Google Scholar] [CrossRef]
- Lee, Y.T.; Yu, B.Y.; Shao, H.J.; Chang, C.H.; Sun, Y.M.; Liu, H.C.; Hou, S.M.; Young, T.H. Effects of the surface characteristics of nano-crystalline and micro-particle calcium phosphate/chitosan composite films on the behavior of human mesenchymal stem cells in vitro. J. Biomater. Sci. Polym. E 2011, 22, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.G.S.; Santos, A.L.F.; Nunes, A.R.; Tavares, G.W.; Almeida, C.M. Designed formulation based on α-tocopherol anchored on chitosan microspheres for pH-controlled gastrointestinal controlled release. Colloid. Surf. B 2012, 96, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.P.; Hu, J.L.; Park, H.; Lee, M. Chitosan based nanoparticles as a sustained protein release carrier for tissue engineering applications. J. Biomed. Mater. Res. A 2012, 100, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Biodegradable and thermo-sensitive chitosan-g-poly (N-vinylcaprolactam) nanoparticles as a 5-fluorouracil carrier. Carbohydr. Polym. 2011, 83, 776–786. [Google Scholar] [CrossRef]
- Mesquita, J.P.; Donnici, C.L.; Teixeira, I.F.; Pereira, F.V. Bio-based nanocomposites obtained through covalent linkage between chitosan and cellulose nanocrystals. Carbohydr. Polym. 2012, 90, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Filho, G.C.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J. Food Sci. 2010, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Nakayama, A.; Aiba, S. Chitosan and gelatin based edible films: State diagrams, mechanical and permeation properties. Carbohydr. Polym. 1998, 37, 371–382. [Google Scholar] [CrossRef]
- Bangyekan, B.; Aht-Ong, D.; Srikulkit, K. Preparation and properties evaluation of chitosan-coated cassava starch films. Carbohydr. Polym. 2006, 63, 61–71. [Google Scholar] [CrossRef]
- Wu, T.F.; Pan, Y.Z.; Bao, H.Q.; Li, L. Preparation and properties of chitosan nanocomposite films reinforced by poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) treated carbon nanotubes. Mater. Chem. Phys. 2011, 129, 932–938. [Google Scholar] [CrossRef]
- Brondani, D.; Zapp, E.; Vieira, I.C.; Dupont, J.; Scheeren, C.W. Gold nanoparticles in an ionic liquid phase supported in a biopolymeric matrix applied in the development of a rosmarinic acid biosensor. Analyst 2011, 136, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Sanguanchaipaiwong, V.; Gabelish, C.L.; Hook, J.; Scholz, C.; Foster, L.J.R. Biosynthesis of natural-synthetic hybrid copolymers: Polyhydroxyoctanoate-diethylene glycol. Biomacromolecules 2004, 5, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Gogotov, I.N.; Gerasin, V.A.; Knyazev, Y.V.; Antipov, E.M.; Barazov, S.K. Composite biodegradable materials based on polyhydroxyalkanoate. Appl. Biochem. Microbiol. 2010, 46, 607–613. [Google Scholar] [CrossRef]
- Prochon, M.; Janowska, G.; Przepiorkowska, A.; Kucharska-Jastrzabek, A. Thermal properties and combustibility of elastomer–protein composites. J. Therm. Anal. Calorim. 2011, 109, 1563–1570. [Google Scholar] [CrossRef]
- Jong, L. Dynamic mechanical properties of soy protein filled elastomers. J. Polym. Environ. 2005, 13, 329–338. [Google Scholar] [CrossRef]
- Wu, M.; Xiong, Y.L.; Chen, J. Role of disulphide linkages between protein-coated lipid droplets and the protein matrix in the rheological properties of porcine myofibrillar protein-peanut oil emulsion composite gels. Meat Sci. 2011, 88, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.P.; Kumar, V. New emerging trends in synthetic biodegradable polymers—Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Dusselier, M.; van Wouwe, P.; Dewaele, A.; Makshina, E.; Sels, B.F. Lactic acid as a platform chemical in the biobased economy: The role of chemocatalysis. Energy Environ. Sci. 2013, 6, 1415–1442. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Balakrishnan, H.; Hassan, A.; Imran, M.; Wahit, M.U. Toughening of polylactic acid nanocomposites: A short review. Polym. Plast. Technol. Eng. 2012, 51, 175–192. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D.; Converti, A.; Oliveira, R.P.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Leejarkpai, T.; Suwanmanee, U.; Rudeekit, Y.; Mungcharoen, T. Biodegradable kinetics of plastics under controlled composting conditions. Waste Manag. 2011, 31, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Stloukal, P.; Pekařová, S.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Chitu, L.; Bodner, S.; Maier, G.; Slouf, M.; et al. Kinetics and mechanism of the biodegradation of PLA/clay nanocomposites during thermophilic phase of composting process. Waste Manag. 2015, 42, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Husárová, L.; Pekařová, S.; Stloukal, P.; Kucharzcyk, P.; Verney, V.; Commereuc, S.; Ramone, A.; Koutny, M. Identification of important abiotic and biotic factors in the biodegradation of poly(l-lactic acid). Int. J. Biol. Macromol. 2014, 71, 155–162. [Google Scholar]
- Mueller, R.J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process. Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Ghorpade, V.M.; Gennadios, A.; Hanna, M.A. Laboratory composting of extruded poly(lactic acid) sheets. Bioresour. Technol. 2001, 76, 57–61. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Jarerat, A. Biodegradation of poly(l-lactide). Biotechnol. Lett. 2004, 26, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.P.; Schley, J.; Loy, B.; Lind, D.; Hobot, C.; Sparer, R.; Untereker, D. Kinetics and time−temperature equivalence of polymer degradation. Biomacromolecules 2007, 8, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Zhang, X.; Espiritu, M.; Bilyk, A.; Kurniawan, L. Morphological behaviour of poly(lactic acid) during hydrolytic degradation. Polym. Degrad. Stab. 2008, 93, 1964–1970. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Blends of crystalline and amorphous poly(lactide). III. Hydrolysis of solution-cast blend films. J. Appl. Polym. Sci. 1997, 63, 855–863. [Google Scholar] [CrossRef]
- Kucharczyk, P.; Hnatkova, E.; Dvorak, Z.; Sedlarik, V. Novel aspects of the degradation process of PLA based bulky samples under conditions of high partial pressure of water vapour. Polym. Degrad. Stab. 2013, 98, 150–157. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly(l-lactide). III. Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight poly(l-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Kruft, M.A. An atomic finite element model for biodegradable polymers. Part 2. A model for change in Young’s modulus due to polymer chain scission. J. Mech. Behav. Biomed. Mater. 2015, 51, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Rychlý, J.; Rychlá, L.; Stloukal, P.; Koutný, M.; Pekařová, S.; Verney, V.; Fiedlerová, A. UV initiated oxidation and chemiluminescence from aromatic–aliphatic co-polyesters and polylactic acid. Polym. Degrad. Stab. 2013, 98, 2556–2563. [Google Scholar] [CrossRef]
- Stloukal, P.; Verney, V.; Commereuc, S.; Rychly, J.; Matisova-Rychlá, L.; Pis, V.; Koutny, M. Assessment of the interrelation between photooxidation and biodegradation of selected polyesters after artificial weathering. Chemosphere 2012, 88, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Espi, E. Plastic films for agricultural applications. J. Plast. Film Sheeting 2006, 22, 85–102. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Ngouajio, M.; Fernandez, R.T. Assessment of aliphatic–aromatic copolyester biodegradable mulch films. Part I: Field study. Chemosphere 2008, 71, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Alvarado, E.; Camacho Montero, J.R.; Rosales, J.M. Atmospheric and soil degradation of aliphatic–aromatic polyester films. Polym. Degrad. Stab. 2010, 95, 99–107. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Ngouajio, M.; Fernandez, R.T. Assessment of aliphatic–aromatic copolyester biodegradable mulch films. Part II: Laboratory simulated conditions. Chemosphere 2008, 71, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Trinh Tan, F.; Cooper, D.G.; Marić, M.; Nicell, J.A. Biodegradation of a synthetic co-polyester by aerobic mesophilic microorganisms. Polym. Degrad. Stab. 2008, 93, 1479–1485. [Google Scholar] [CrossRef]
- Siotto, M.; Sezenna, E.; Saponaro, S.; Innocenti, F.D.; Tosin, M.; Bonomo, L.; Mezzanotte, V. Kinetics of monomer biodegradation in soil. J. Environ. Manag. 2012, 93, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Kim, M.N. Biodegradation of poly(l-lactide) (PLA) exposed to UV irradiation by a mesophilic bacterium. Int. Biodeterior. Biodegrad. 2013, 85, 289–293. [Google Scholar] [CrossRef]
- Van Cong, D.; Hoang, T.; Giang, N.V.; Ha, N.T.; Lam, T.D.; Sumita, M. A novel enzymatic biodegradable route for PLA/EVA blends under agricultural soil of Vietnam. Mater. Sci. Eng. C 2012, 32, 558–563. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Yamano, N.; Kawasaki, N.; Oshima, M.; Nakayama, A. Polyamide 4 with long-chain fatty acid groups—Suppressing the biodegradability of biodegradable polymers. Polym. Degrad. Stab. 2014, 108, 116–122. [Google Scholar] [CrossRef]
- Stloukal, P.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Koutny, M. The influence of a hydrolysis-inhibiting additive on the degradation and biodegradation of PLA and its nanocomposites. Polym. Test. 2015, 41, 124–132. [Google Scholar] [CrossRef]
- Iovino, R.; Zullo, R.; Rao, M.A.; Cassar, L.; Gianfreda, L. Biodegradation of poly(lactic acid)/starch/coir biocomposites under controlled composting conditions. Polym. Degrad. Stab. 2008, 93, 147–157. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Ajji, A. Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef]
- Jašo, V.; Glenn, G.; Klamczynski, A.; Petrović, Z.S. Biodegradability study of polylactic acid/thermoplastic polyurethane blends. Polym. Test. 2015, 47, 1–3. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Kumar, M.; Mohanty, S.; Nayak, S.K.; Rahail Parvaiz, M. Effect of glycidyl methacrylate (GMA) on the thermal, mechanical and morphological property of biodegradable PLA/PBAT blend and its nanocomposites. Bioresour. Technol. 2010, 101, 8406–8415. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Arena, M.; Gennari, M.; Camino, G. Effect of clay type and loading on thermal, mechanical properties and biodegradation of poly(lactic acid) nanocomposites. React. Funct. Polym. 2013, 73, 540–549. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Dottori, M.; Armentano, I.; Kenny, J.M.; Camino, G. Effect of temperature and nanoparticle type on hydrolytic degradation of poly(lactic acid) nanocomposites. Polym. Degrad. Stab. 2011, 96, 2120–2129. [Google Scholar] [CrossRef]
- Suyama, T.; Tokiwa, Y.; Ouichanpagdee, P.; Kanagawa, T.; Kamagata, Y. Phylogenetic affiliation of soil bacteria that degrade aliphatic polyesters available commercially as biodegradable plastics. Appl. Environ. Microbiol. 1998, 64, 5008–5011. [Google Scholar] [PubMed]
- Pranamuda, H.; Tokiwa, Y.; Tanaka, H. Polylactide degradation by an amycolatopsis sp. Appl. Environ. Microbiol. 1997, 63, 1637–1640. [Google Scholar] [PubMed]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Jarerat, A.; Tokiwa, Y. Degradation of poly(l-lactide) by a fungus. Macromol. Biosci. 2001, 1, 136–140. [Google Scholar] [CrossRef]
- Kunioka, M.; Ninomiya, F.; Funabashi, M. Biodegradation of poly(lactic acid) powders proposed as the reference test materials for the international standard of biodegradation evaluation methods. Polym. Degrad. Stab. 2006, 91, 1919–1928. [Google Scholar] [CrossRef]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Anaerobic biodegradation tests of poly(lactic acid) and polycaprolactone using new evaluation system for methane fermentation in anaerobic sludge. Polym. Degrad. Stab. 2009, 94, 1397–1404. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer biodegradation: Mechanisms and estimation techniques—A review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, W.; Musiol, M.; Nowak, B.; Pajak, J.; Labuzek, S.; Kowalczuk, M.; Adamus, G. Degradability of polylactide and its blend with poly[(R,S)-3-hydroxybutyrate] in industrial composting and compost extract. Int. Biodeterior. Biodegrad. 2015, 101, 32–41. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Capellán-Pérez, I.; Mediavilla, M.; de Castro, C.; Carpintero, O.; Miguel, L.J. Fossil fuel depletion and socio-economic scenarios: An integrated approach. Energy 2014, 77, 641–666. [Google Scholar] [CrossRef]
- Quirino, R.L.; Garrison, T.F.; Kessler, M.R. Matrices from vegetable oils, cashew nut shell liquid, and other relevant systems for biocomposite applications. Green Chem. 2014, 16, 1700–1715. [Google Scholar] [CrossRef]
- Takashi, T.; Takeshi, T.; Hiroshi, U. Biodegradable shape memory polymeric material from epoxidized soybean oil and polycaprolactone. Polymers 2015, 7, 2165–2174. [Google Scholar]
- Thakur, V.K.; Kessler, M.R. Green Biorenewable Biocomposites: From Knowledge to Industrial Applications; Apple Academic-CRC Press: Boca Raton, FL, USA, 2015; pp. 1–568. [Google Scholar]
- Berezina, N.; Silvia, M.M. Bio-based Polymers and Materials. In Renewable Resources for Biorefineries; Berezina, N., Silvia, M.M., Eds.; The Royal Society of Chemistry: London, UK, 2014; pp. 1–28. [Google Scholar]
- Johns, A.; Morris, S.; Edwards, K.; Quirino, R.L. Asolectin from soybeans as a natural compatibilizer for cellulose-reinforced biocomposites from tung oil. J. Appl. Polym. Sci. 2015, 132, 41833–41842. [Google Scholar] [CrossRef]
- Biermann, U.; Butte, Q.; Holtgrefe, R.; Feder, W.; Metzger, J.O. Esters of calendula oil and tung oil as reactive diluents for alkyd resins. Eur. J. Lipid Sci. Technol. 2010, 112, 103–109. [Google Scholar] [CrossRef]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Sokolsky, M.; Kumar, N.; Domb, A. Fatty acid based biodegradable polymer. Polym. Rev. 2008, 48, 156–191. [Google Scholar]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A. Biodegradable polymers—An overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Casado, U.; Marcovich, N.E.; Aranguren, M.I. Polyurethanes from tung oil: Polymer characterization and composites. Polym. Eng. Sci. 2009, 49, 685–692. [Google Scholar] [CrossRef]
- Lewinska, A.; Zebrowski, J.; Duda, M.; Gorka, A.; Wnuk, M. Fatty acid profile and biological activities of linseed and rapeseed oils. Molecules 2015, 20, 22872–22880. [Google Scholar] [CrossRef] [PubMed]
- Buahin, K.G.; Judy, K.D.; Hartke, C.; Domb, A.J.; Maniar, M.; Colvin, O.M.; Brem, H. Controlled release of 4-hydroperoxycyclophosphamide from the fatty acid dimer–sebacic acid copolymer. Polym. Adv. Technol. 1992, 3, 311–316. [Google Scholar] [CrossRef]
- Research and Markets: Tissue Engineering: Technologies and Therapeutic Areas—A Global Market Overview to 2022. Available online: http://www.businesswire.com/news/home/20150915005908/en/Research-Markets-Tissue-Engineering-Technologies-Therapeutic-Areas (accessed on 12 July 2016).
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 1–32. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of Eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Pascual, C. Review: Bio-based polymers, supercritical fluids and tissue engineering. Process. Biochem. 2015, 50, 826–838. [Google Scholar] [CrossRef]
- Scarfato, P.; di Maio, L.; Incarnato, L. Recent advances and migration issues in biodegradable polymers from renewable sources for food packaging. J. Appl. Polym. Sci. 2015, 132, 42597–42608. [Google Scholar] [CrossRef]
- Uyama, H. Current status & future perspective of Research & Development on bio-based polymers. Int. Polym. Sci. Technol. 2013, 40, 47–54. [Google Scholar]
- Abolibda, T.Y. Physical and Chemical Investigations of Starch Based Bio-Plastics. Ph.D. Dissertation, University of Leicester, Leicester, UK, 21 August 2015. [Google Scholar]
- Lai, S.; Sun, W.; Don, T. Preparation and characterization of biodegradable polymer blends from poly(3-hydroxybutyrate)/poly(vinyl acetate)-modified corn starch. Polym. Eng. Sci. 2015, 6, 1321–1329. [Google Scholar] [CrossRef]
- Tan, G.-Y.A.; Chen, C.-L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.-Y. Start a research on biopolymer polyhydroxyalkanoate (PHA): A review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Cohen, E.; Binshtok, O.; Dotan, A.; Dodiuk, H. Prospective materials for biodegradable and/or biobased pressure-sensitive adhesives: A review. J. Adhes. Sci. Technol. 2013, 27, 1998–2013. [Google Scholar] [CrossRef]
- Avérous, L.; Pollet, E. Chapter 2: Biodegradable Polymers. In Enivornmental Silicate Nano-Biocomposites, Green Energy and Technology Series; Avérous, L., Pollet, E., Eds.; Springer: London, UK, 2012; pp. 13–40. [Google Scholar]
- Pivsa-Art, W.; Chaiyasat, A.; Pivsa-Art, S.; Yamane, H.; Ohara, H. Preparation of polymer blends between poly(lactic acid) and poly(butylene adipate-co-terephthalate) and biodegradable polymers as compatibilizers. Energy Procedia 2013, 34, 549–554. [Google Scholar] [CrossRef]
- Ruellan, A.; Guinault, A.; Sollogoub, C.; Chollet, G.; Ait-Mada, A.; Ducruet, V.; Domenek, S. Industrial vegetable oil by-products increase the ductility of polylactide. Express Polym. Lett. 2015, 9, 1087–1103. [Google Scholar] [CrossRef]
- Zhang, D. Lightweight Materials from Biofibers and Biopolymers. In Lightweight Materials from Biopolymers and Biofibers, 1st ed.; Yang, Y., Xu, H., Yu, X., Eds.; ACS Symposium Series; ACS: Washington, DC, USA, 2014; pp. 1–20. [Google Scholar]
- Liqing, W.; McDonald, A. A review on grafting of biofibers for biocomposites. Materials 2016, 9, 1–23. [Google Scholar]
- Lu, Z.; Reif, R.; Gan, J. Isomer-specific biodegradation of nonylphenol in an activated sludge bioreactor and structure-biodegradability relationship. Water Res. 2015, 68, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Genovese, L.; Lotti, N.; Gazzano, M.; Finelli, L.; Munari, A. New eco-friendly random copolyesters based on poly(propylene cyclohexanedicarboxylate): Structure-properties relationships. Express Polym. Lett. 2015, 9, 972–983. [Google Scholar] [CrossRef]
- Beran, E.; Hull, S.; Steininger, M. The relationship between the chemical structure of poly(alkylene glycol)s and their aerobic biodegradability in an aqueous environment. J. Polym. Environ. 2013, 21, 172–180. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. https://doi.org/10.3390/polym8070262
Garrison TF, Murawski A, Quirino RL. Bio-Based Polymers with Potential for Biodegradability. Polymers. 2016; 8(7):262. https://doi.org/10.3390/polym8070262
Chicago/Turabian StyleGarrison, Thomas F., Amanda Murawski, and Rafael L. Quirino. 2016. "Bio-Based Polymers with Potential for Biodegradability" Polymers 8, no. 7: 262. https://doi.org/10.3390/polym8070262
APA StyleGarrison, T. F., Murawski, A., & Quirino, R. L. (2016). Bio-Based Polymers with Potential for Biodegradability. Polymers, 8(7), 262. https://doi.org/10.3390/polym8070262






