Study on the Soy Protein-Based Wood Adhesive Modified by Hydroxymethyl Phenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydroxymethyl Phenol
2.3. Preparation of Soy-Based Adhesive
2.4. Preparation of HPF/AG
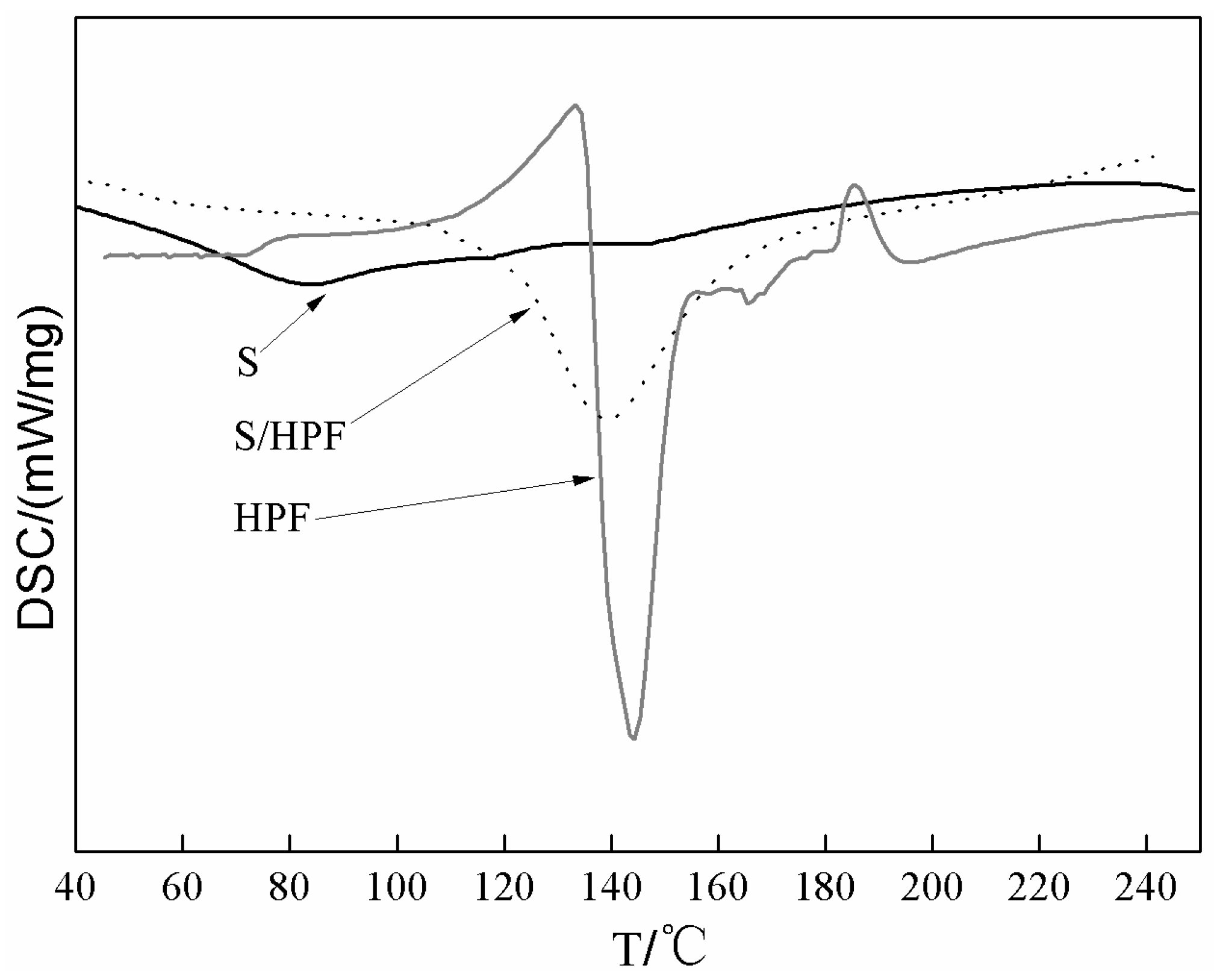
2.5. Differential Scanning Calorimetry (DSC)
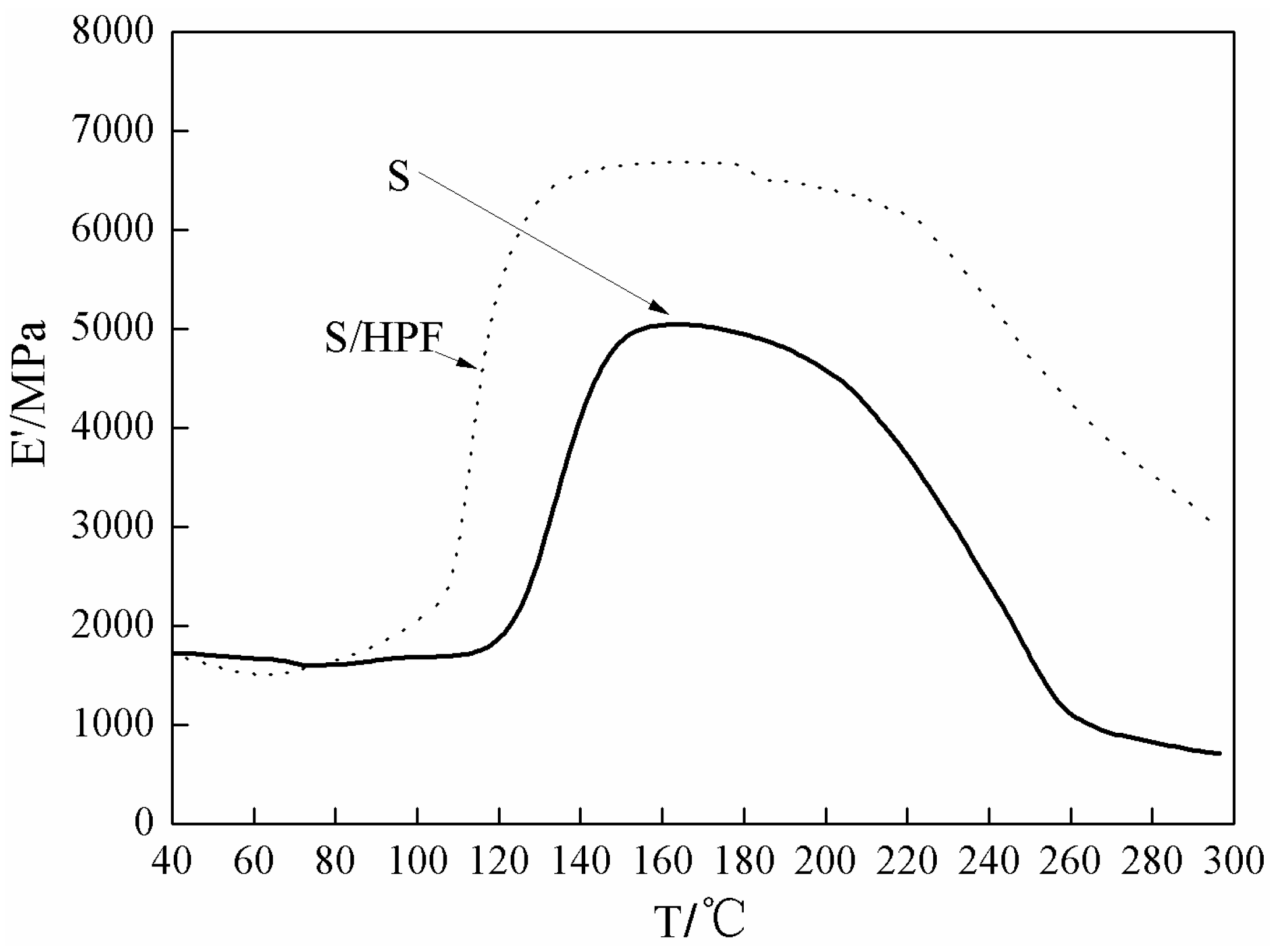
2.6. Dynamic Mechanical Analysis (DMA)
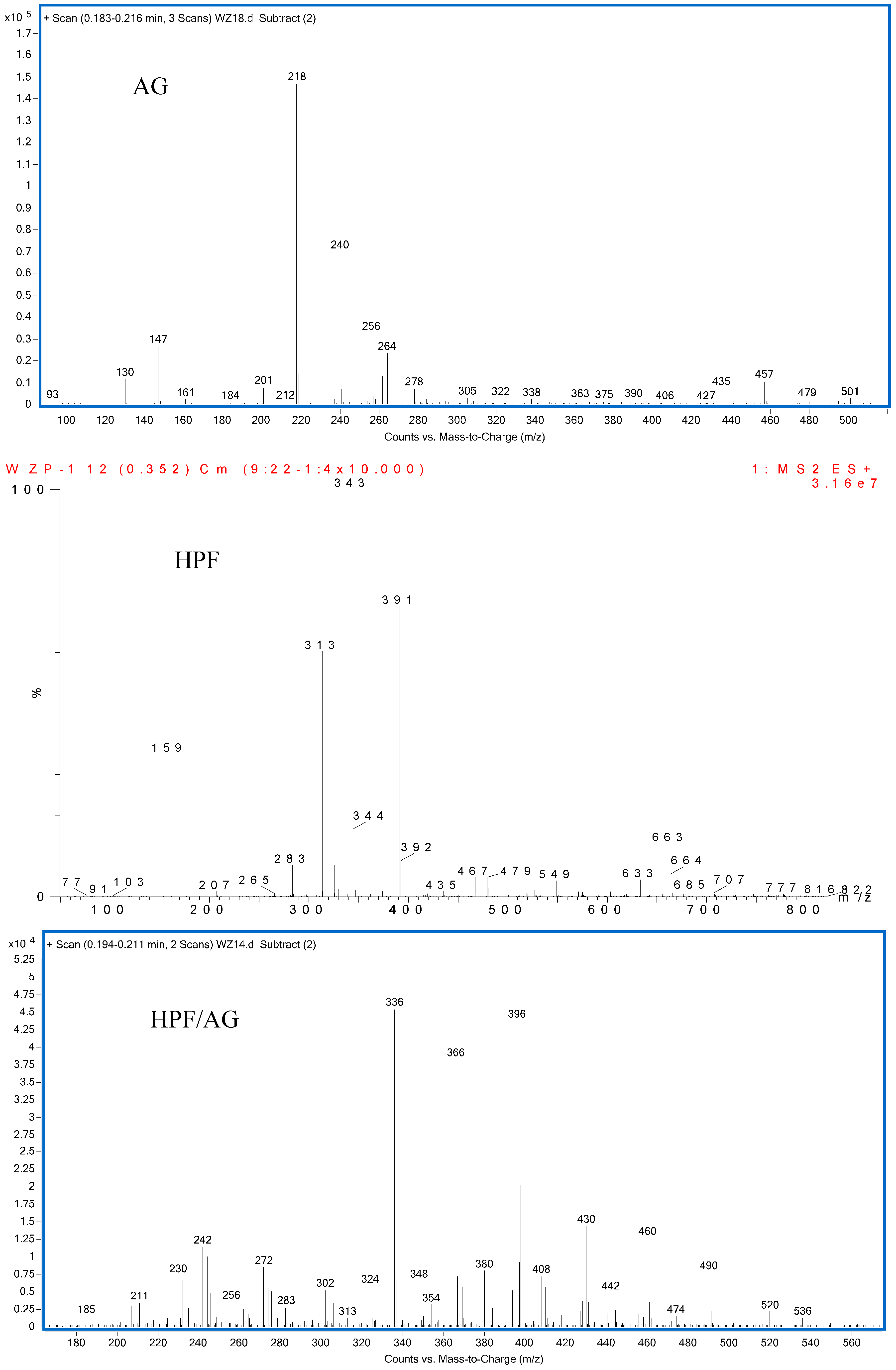
2.7. Electrospray Ionization Mass Spectrometry (ESI-MS)
2.8. FT-IR Analysis
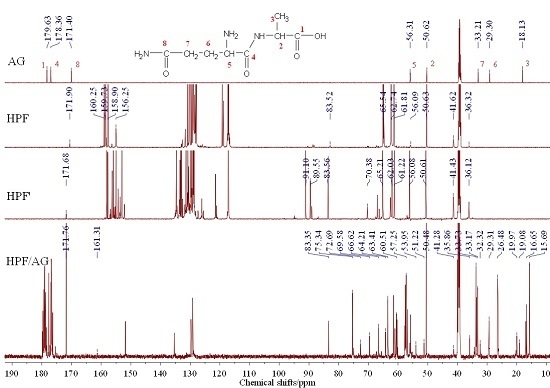
2.9. 13C-NMR
3. Results and Discussion
3.1. The DSC and DMA Analysis of Soy-Based Adhesive with HPF
3.2. The ESI-MS Analysis between HPF and AG
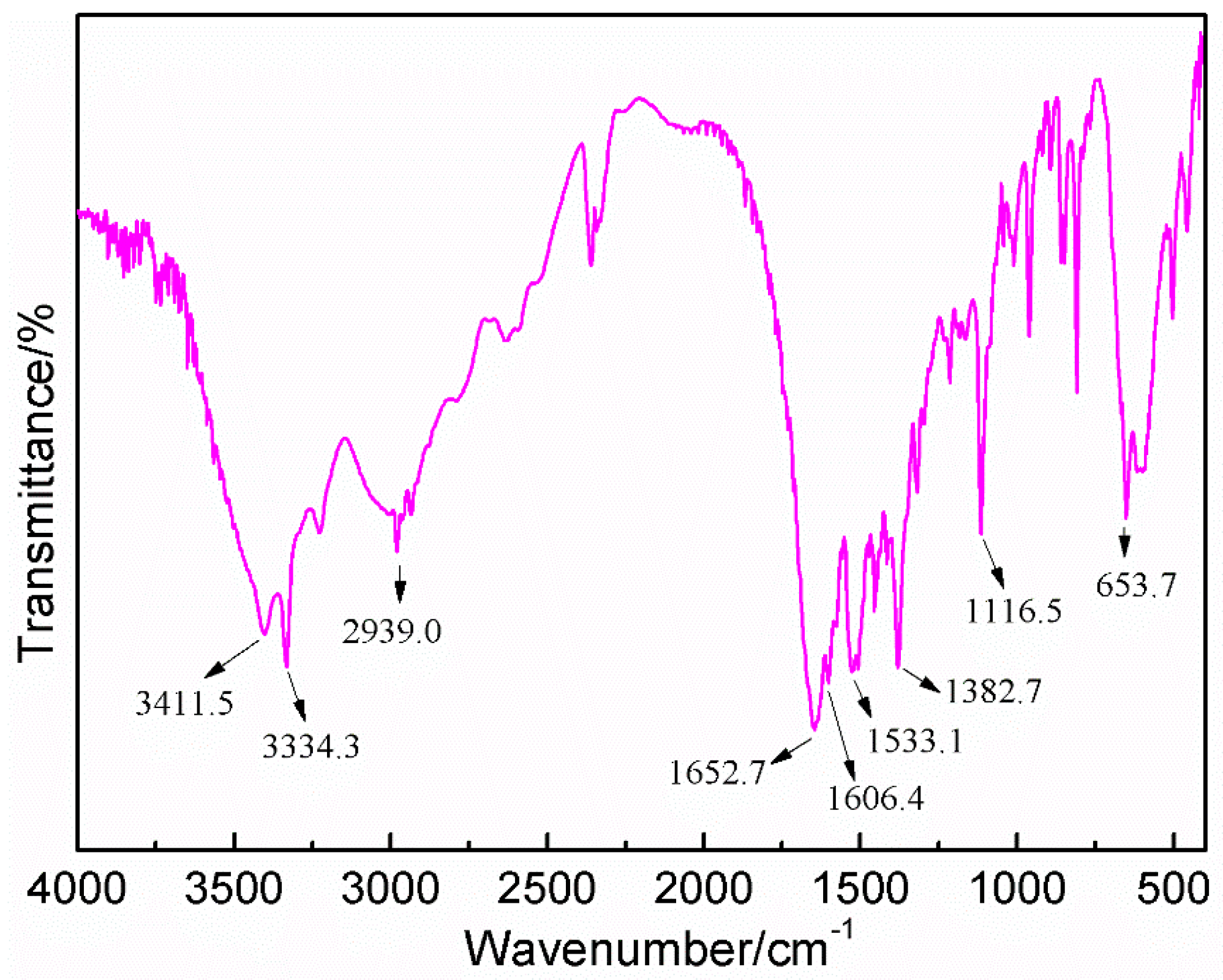
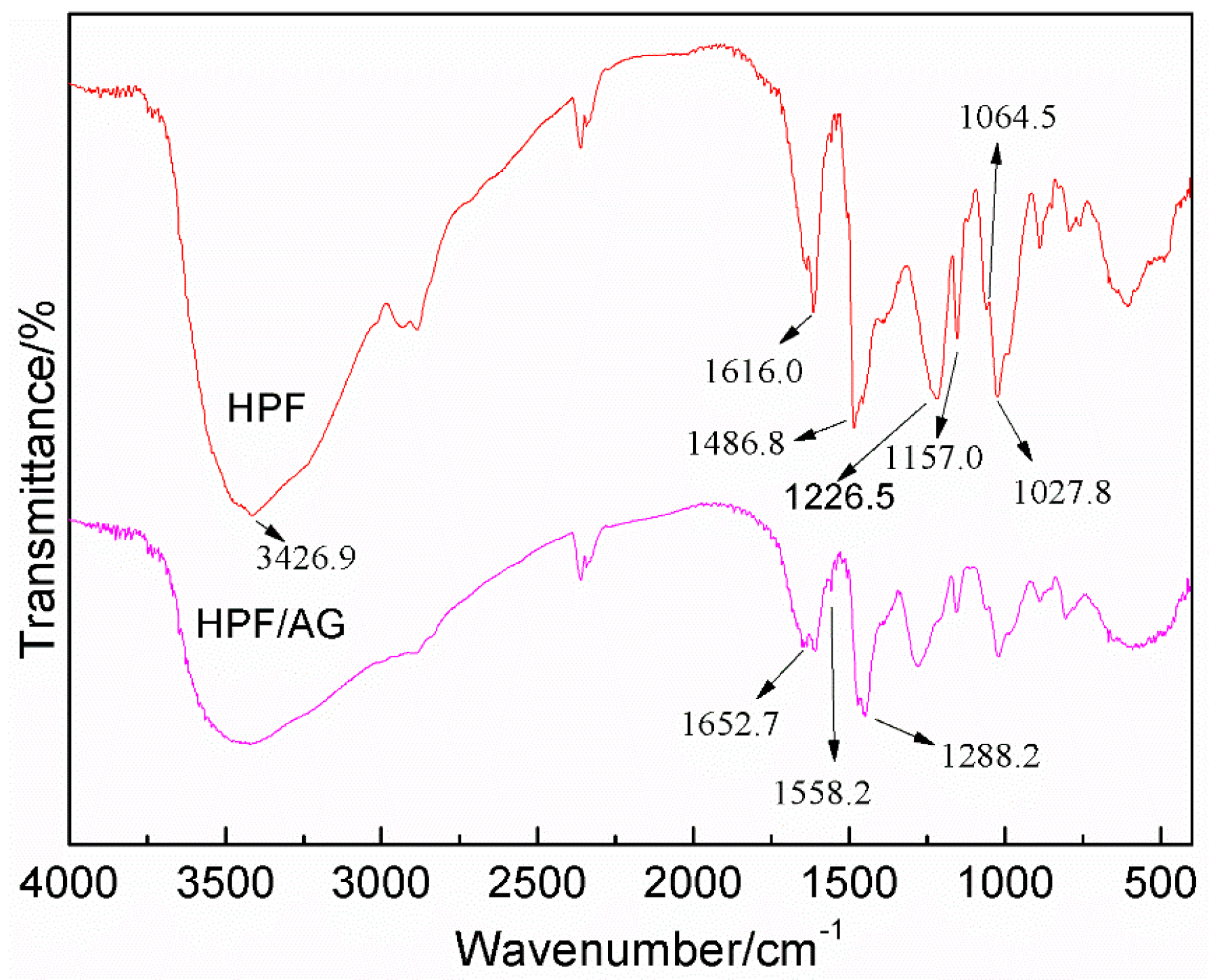
3.3. The FT-IR Analysis between HPF and AG
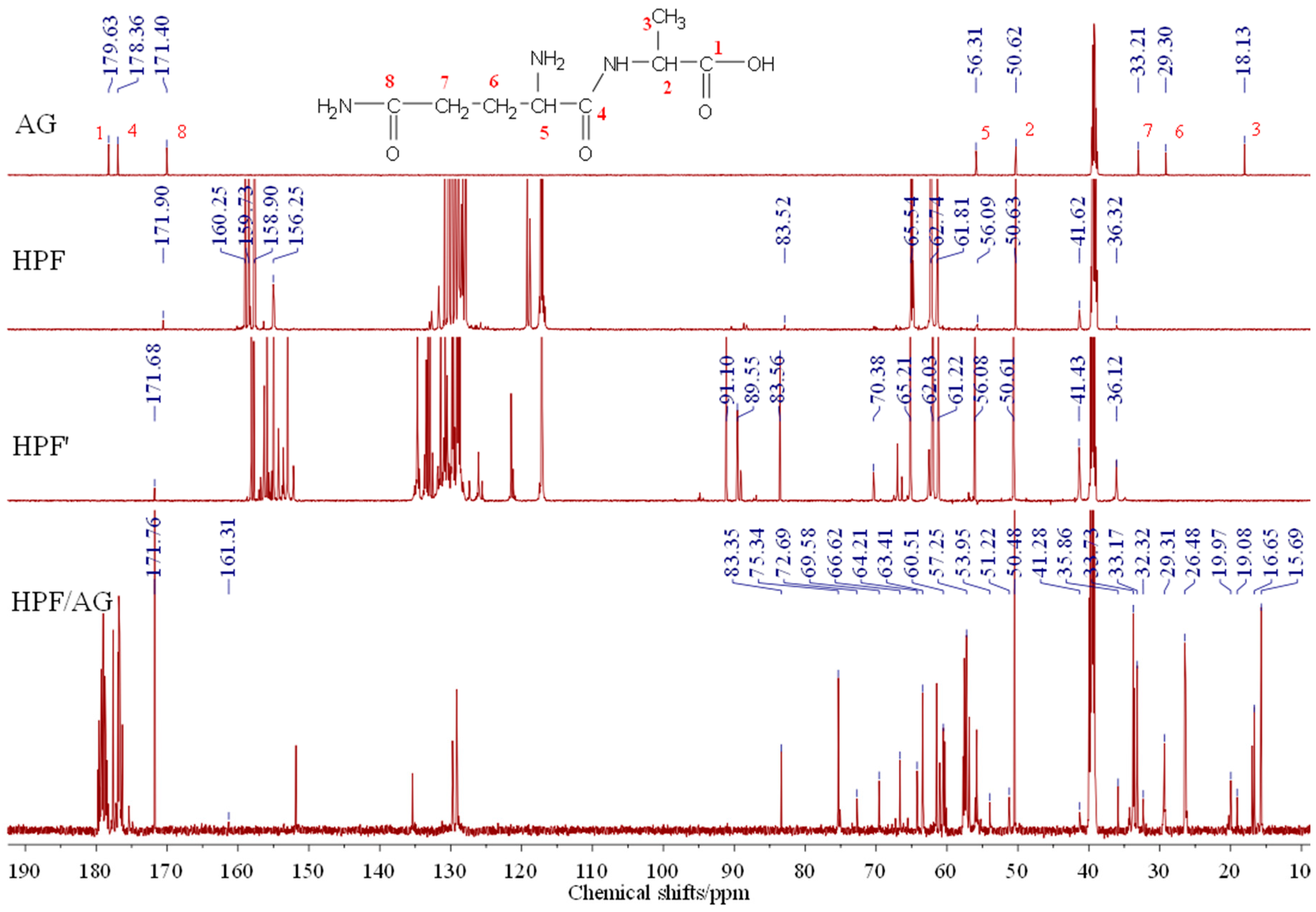
3.4. The 13C-NMR Analysis between HPF and AG
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, K.; Peshkova, S.; Geng, X. Investigation of soy protein-Kymene adhesive systems for wood composites. J. Am. Oil Chem. Soc. 2004, 81, 487–491. [Google Scholar] [CrossRef]
- Gao, Q.; Shi, Q.L.; Zhang, S. Soybean meal-based adhesive enhanced by MUF resin. J. Appl. Polym. Sci. 2008, 125, 3676–3681. [Google Scholar] [CrossRef]
- Fan, D.; Qin, T.; Chu, F. A soy flour-based adhesive reinforced by low addition of MUF resin. J. Adhes. Sci. Technol. 2011, 25, 323–333. [Google Scholar] [CrossRef]
- Jang, Y.; Huang, J.; Li, K. A new formaldehyde-free wood adhesive from renewable materials. Int. J. Adhes. Adhes. 2011, 31, 754–759. [Google Scholar] [CrossRef]
- Rogers, J.; Geng, X.; Li, K. Soy-based adhesives with 1,3-dichloro-2-propanol as a curing agent. Wood Fiber Sci. 2004, 36, 186–194. [Google Scholar]
- Lei, H.; Du, G.; Pizzi, A.; Zhou, X. Effects of glyoxal on chemical structure and properties of protein-based adhesives. J. Southwest For. Univ. China 2011, 31, 70–73. [Google Scholar]
- Kumar, R.; Choudhary, V.; Mishra, S.; Varma, I.K.; Mttiason, B. Adhesives and plastics based soy protein products. Ind. Crop. Prod. 2002, 16, 155–172. [Google Scholar] [CrossRef]
- Kreibich, R.E.; Steynberg, P.J.; Hemingway, R.W. End jointing green lumber with SoyBond. In Proceedings of the Wood Residues into Revenue, Residual Wood Conference, Richmond, BC, Canada, 4–5 November 1998.
- Yang, I.; Kuo, M.; Myers, D.J. Bond quality of soy-based phenolic adhesives in southern pine plywood. J. Am. Oil Chem. Soc. 2006, 83, 231–237. [Google Scholar] [CrossRef]
- Hse, C.Y.; Fu, F.; Bryant, B.S. Development of formaldehyde-based wood adhesives with co-reacted phenol/soybean flour. In Proceedings of the Wood adhesives 2000, Madison, WI, USA, 22–23 June 2000; Forest Products Society: Madison, WI, USA, 2000; pp. 13–19. [Google Scholar]
- Lei, H.; Wu, Z.; Du, G. Application of soy protein-based adhesives modified by cross-linker. China Wood Ind. 2013, 27, 8–11. [Google Scholar]
- Li, T.; Guo, X.; Liang, J.; Wang, H.; Xie, X.; Du, G. Competitive formation of the methylene and methylene ether bridges in the urea-formaldehyde reaction in alkaline solution: A combined experimental and theoretical study. Wood Sci. Technol. 2015, 49, 475–493. [Google Scholar] [CrossRef]
- Zuo, R. Preparation and Characterization of l-A lanyl-l-G lutam ine-Zn2+ Chelate. Ph.D. Thesis, College of Chemistry and Chemical Engineering, Chongqing University, Chongqing, China, 2007. [Google Scholar]
- Zhang, X. Multi-Aldehydes Modified Environmentally Friendly Phenolic Resin Adhesive. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2013. [Google Scholar]
- Sun, L.; Li, M.; Peng, B. Synthesis and characterization of a novel water soluble phenol-formaldehyde resin. Acta Pet. Sin. China 2008, 24, 63–68. [Google Scholar]
- Schmidt, K.; Grunwald, D.; Pasch, H. Preparation of phenol-urea-formaldehyde copolymer adhesives under heterogeneous catalysis. J. Appl. Polym. Sci. 2006, 102, 2946–2952. [Google Scholar] [CrossRef]
- He, G.; Yan, N. 13C–NMR study on structure, composition and curing behavior of phenol-urea-formaldehyde resole resins. Polymer 2004, 45, 6813–6822. [Google Scholar] [CrossRef]
- Higuchi, M.; Urakawa, T.; Morita, M. Condensation reaction of phenolic resin. 1. Kinetics and mechanisms of the base-catalyzed self-consensation of 2-hydroxymethylphenol. Polymer 2001, 42, 4563–4567. [Google Scholar] [CrossRef]
| Experimental (Da) | Chemical Species | |||
|---|---|---|---|---|
| Samples | M + H+ | M + Na+ | M + K+ | |
| AG | 218 | 240 | 256 |  |
| HPF | – | 159 | – | 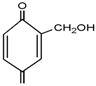 |
| – | 283 | – | 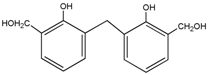 | |
| – | 313 | – |  | |
| – | 343 | – | 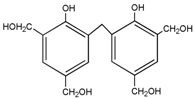 | |
| – | 391 | – | 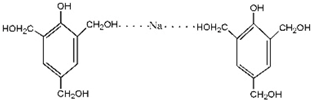 | |
| HPF/AG | 336 | – | – |  or or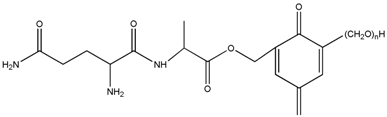 |
| 366 | – | – | ||
| 396 | – | – | ||
| 430 | – | – | 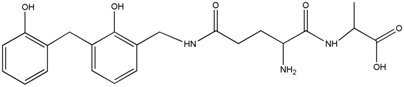 | |
| 460 | – | – | 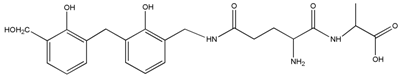 | |
| 490 | – | – | 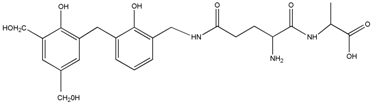 | |
| 520 | – | – | 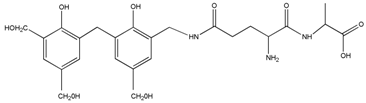 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Wu, Z.; Cao, M.; Du, G. Study on the Soy Protein-Based Wood Adhesive Modified by Hydroxymethyl Phenol. Polymers 2016, 8, 256. https://doi.org/10.3390/polym8070256
Lei H, Wu Z, Cao M, Du G. Study on the Soy Protein-Based Wood Adhesive Modified by Hydroxymethyl Phenol. Polymers. 2016; 8(7):256. https://doi.org/10.3390/polym8070256
Chicago/Turabian StyleLei, Hong, Zhigang Wu, Ming Cao, and Guanben Du. 2016. "Study on the Soy Protein-Based Wood Adhesive Modified by Hydroxymethyl Phenol" Polymers 8, no. 7: 256. https://doi.org/10.3390/polym8070256
APA StyleLei, H., Wu, Z., Cao, M., & Du, G. (2016). Study on the Soy Protein-Based Wood Adhesive Modified by Hydroxymethyl Phenol. Polymers, 8(7), 256. https://doi.org/10.3390/polym8070256