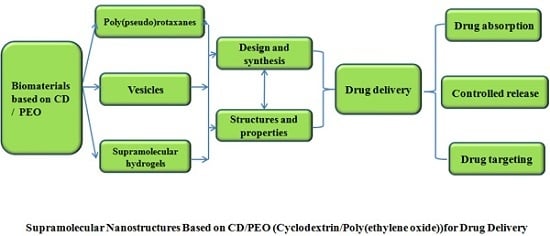
Supramolecular Nanostructures Based on Cyclodextrin and Poly(ethylene oxide): Syntheses, Structural Characterizations and Applications for Drug Delivery
Abstract
:1. Introduction
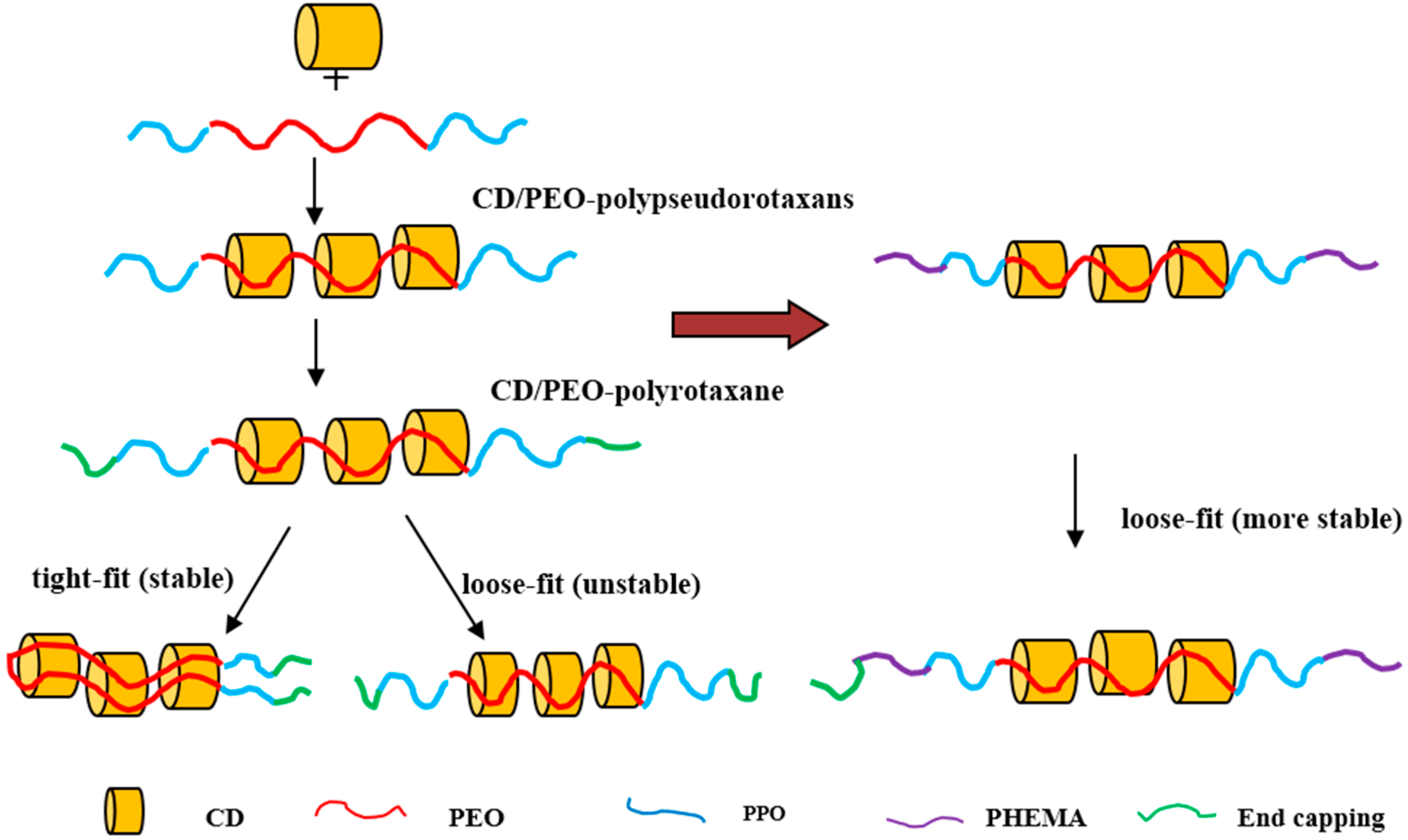
2. Supramolecular Structure and Properties of CD/PEO-Based Poly(pseudo)rotaxanes
2.1. CD/PEO-b-PPO-b-PEO
2.2. CD/PEO-b-PDEAEMA
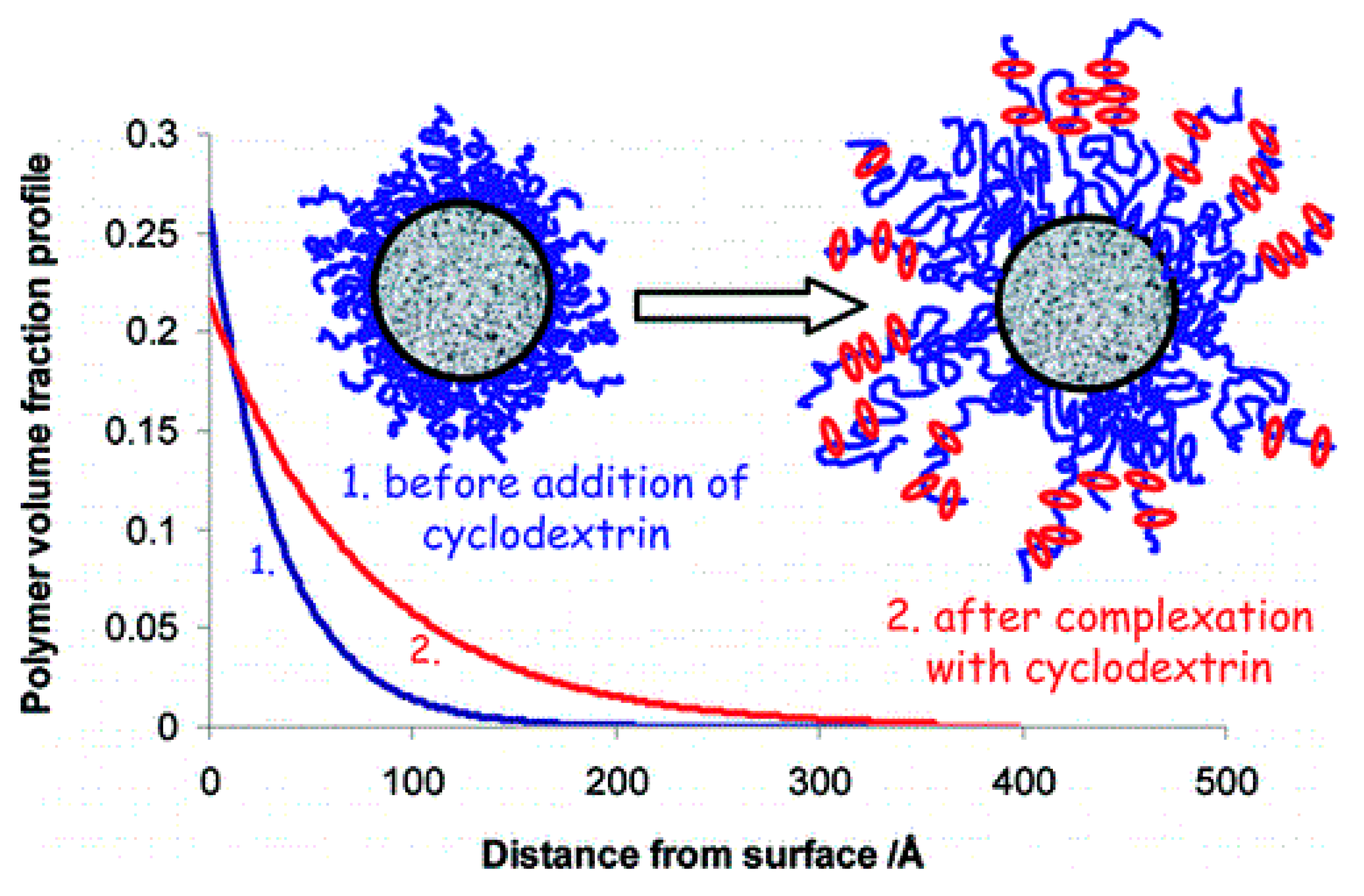
2.3. CD/PEO
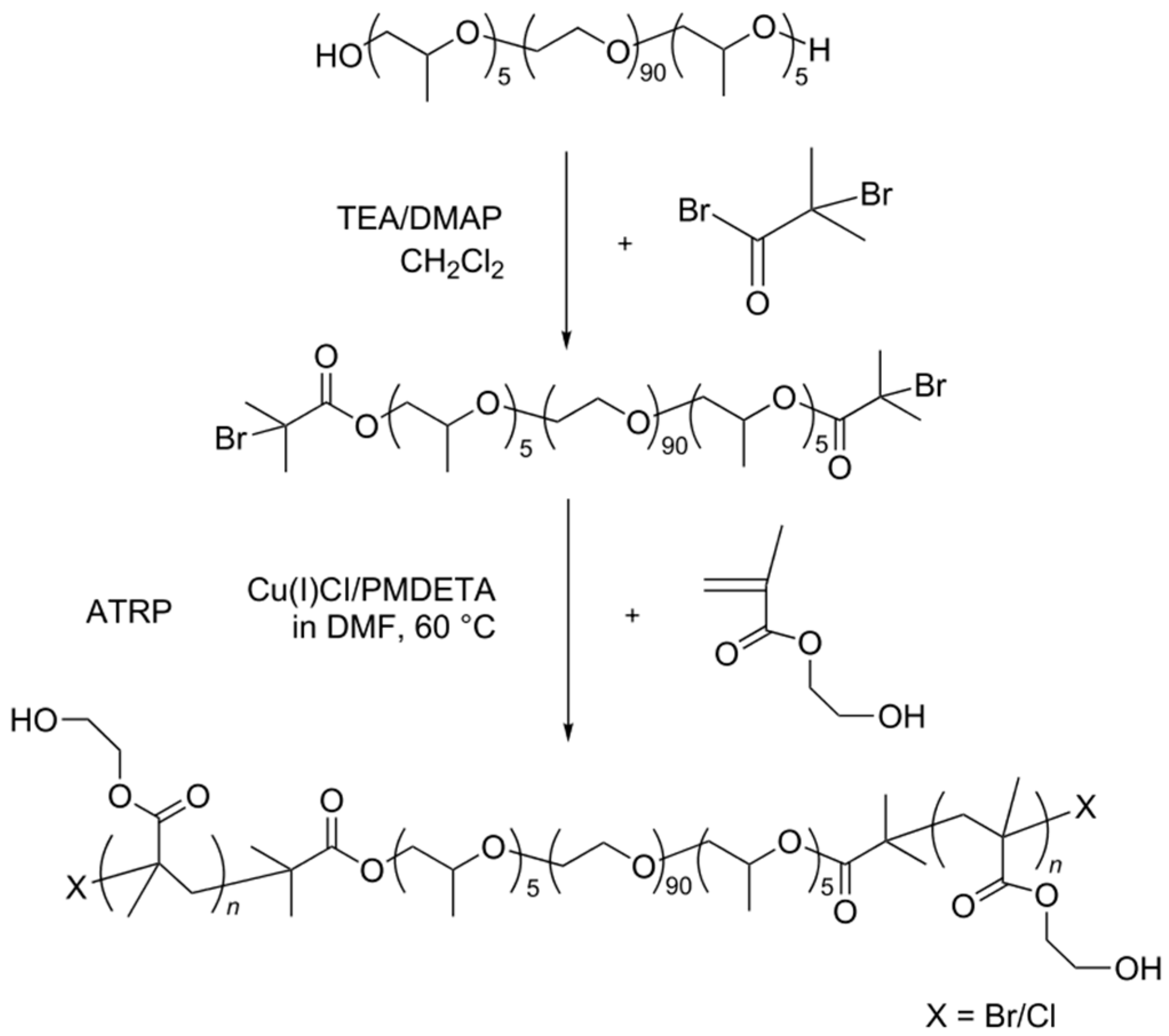
2.4. CD/PEO-b-POO-b-PEO
2.5. CD/PPO-b-PEO-b-PPO
3. Vesicles/Polymersomes of CD/PEO-Based Linear Block Copolymers for Drug Delivery
3.1. CD/PEO-b-PMPC
3.2. CD/PEO-b-PNIPAAm
3.3. CD/HBPO-Star-PEO
4. Supramolecular Hydrogels Based on CDs and PEO for Drug Delivery
4.1. CD/PPO-b-PEO-b-PPO
4.2. Poly-α-CD/PEO
4.3. CD/PEO-b-PVA/PBA
4.4. CD/PEO
4.5. CD/PEO-b-PPO
4.6. CD/PEO-b-PCL
4.7. CD/PEO-PPO Octablock Star Copolymers
5. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Augustynek, M.; Zemanova, A.; Kubicek, J.; Penhaker, M. Model of drugs penetration through biological membrane. Adv. Comput. Commun. Eng. Technol. 2015, 362, 481–492. [Google Scholar]
- Azad, T.D.; Pan, J.; Connolly, I.; Remington, A.; Wilson, C.; Grant, G.A. Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg. Focus 2015, 38, E9. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Agrahari, V. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ma, T.; Zhao, E.; Docter, D.; Yang, W.; Stauber, R.H.; Gao, M. Pharmacology & therapeutics. Small 2016, 12, 556–576. [Google Scholar] [PubMed]
- Deeken, J.F.; Loscher, W. The blood-brain barrier and cancer: Transporters, treatment, and Trojan horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sagare, A.P.; Ma, Q.; Halliday, M.R.; Kong, P.; Kisler, K.; Winkler, E.A.; Ramanathan, A.; Kanekiyo, T.; Bu, G.; et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 2015, 18, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Khumar, S.; Gupta, S.K.; Sharma, P.K. Recent developments in targeted drug delivery systems for crossing blood-brain barrier: A review. Int. J. Pharm. Pharm. Sci. 2012, 4, 36–41. [Google Scholar]
- Schneider, M.; Windbergs, M.; Daum, N.; Loretzd, B.; Collnot, E.-M.; Hansen, S.; Schaefer, U.F.; Lehr, C.-M. Crossing biological barriers for advanced drug delivery. Eur. J. Pharm. Biopharm. 2013, 84, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.T. Drug delivery: Get in there! Nat. Rev. Cancer 2015, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.L. Large cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 1–13. [Google Scholar] [CrossRef]
- Ueda, H. Physicochemical properties and complex formation abilities of large-ring cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 53–56. [Google Scholar] [CrossRef]
- Mondjinou, Y.A.; Hyun, S.-H.; Xiong, M.; Collins, C.J.; Thong, P.; Thompson, D.H. Impact of mixed β-cyclodextrin ratios on pluronicrotaxanation efficiency and product solubility. ACS Appl. Mater. Interfaces 2015, 7, 23831–23836. [Google Scholar] [CrossRef] [PubMed]
- Clementine, P.; Kevin, S.J.; Lisbeth, G.; Justin, J. Gelation kinetics and viscoelastic properties of Pluronic and α-cyclodextrin-based pseudopolyrotaxane hydrogels. Biomacromolecules 2013, 14, 3780–3792. [Google Scholar]
- Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P.H. Carbohydrates in supramolecular chemistry. Chem. Rev. 2016, 116, 1693–1752. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, Z.; Wang, Y.; Li, L.; Guo, X.; Pham, D.T.; Lincoln, S.F.; Prud’homme, R.K. Supramolecular polymer assembly in aqueous solution arising from cyclodextrin host-guest complexation. Beilstein J. Org. Chem. 2016, 12, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bellinger, A.M.; Glettig, D.L.; Barman, R.; Lee, Y.A.; Zhu, J.; Cleveland, C.; Montgomery, V.A.; Gu, L.; NashL, D.; et al. A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat. Mater. 2015, 14, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Woortman, A.J.J.; Rudolf, P.; Loos, K. Facile synthesis and structural characterization of amylose—Fatty acid inclusion complexes. Macromol. Biosci. 2015, 15, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Garcia-Rio, L. Interaction of bolaform surfactants withp-sulfonatocalix[4]arene: The role of two positive charges in the binding. Langmuir 2014, 30, 6748–6755. [Google Scholar] [CrossRef] [PubMed]
- Vecsernyѐs, M.; Fenyvesi, F.; Bacskay, I.; Deli, M.A.; Szente, L.; Fenyvesi, É. Cyclodextrins, bloode-brain barrier, and treatment of neurological diseases. Arch. Med. Res. 2014, 45, 711–729. [Google Scholar] [CrossRef] [PubMed]
- Shreyas, S.; Aniruddh, S.; Ki-Bum, L. Nanotechnology-based approaches for guiding neural regeneration. Acc. Chem. Res. 2016, 49, 17–26. [Google Scholar]
- Simões, S.M.N.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin-based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y. Biomedical applications of supramolecular systems based on host-guest interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present, and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Effects on drug permeation through biological membranes. J. Pharm. Pharmacol. 2011, 63, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.W.; Cheng, C.C.; Bastakoti, B.P.; Kuo, S.-W. Hierarchical mesoporous silicas templated by PE-b-PEO-b-PLA triblock copolymer for fluorescent drug delivery. RSC Adv. 2016, 6, 33811–33820. [Google Scholar] [CrossRef]
- Serizawa, T.; Fukuta, H.; Date, T.; Sawada, T. Affinity-based release of polymer-binding peptides from hydrogels with the target segments of peptides. Chem. Commun. 2016, 52, 2241–2244. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Ye, L. Effect of PEO on the network structure of PVA hydrogels prepared by freezing/thawing method. J. Appl. Polym. Sci. 2013, 128, 3325–3329. [Google Scholar] [CrossRef]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Zhang, L.; Eisenberg, A. multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.S.; Corbierre, M.K.; Eisenberg, A.C. 1998 E.W.R. Steacie Award Lecture Asymmetric amphiphilic block copolymers in solution: A morphological wonderland. J. Chem. 1999, 77, 1311–1326. [Google Scholar]
- Eisenberg, M.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar]
- Harada, A.; Kamachi, M. Complex formation between cyclodextrin and poly(propylene glycol). J. Chem. Soc. Chem. Commun. 1990, 19, 1322–1323. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Preparation and properties of inclusion complexes of polyethylene glycol with alpha-cyclodextrin. Macromolecules 1993, 26, 5698–5703. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.; Zhou, J.; Xu, F.; Zrínyi, M.; Dussault, P.H.; Osadag, Y.; Chen, Y. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem. Soc. Rev. 2014, 43, 8114–8131. [Google Scholar] [CrossRef] [PubMed]
- Arranja, A.; Waton, G.; Schosseler, F.; Mendesb, E. Lack of a unique kinetic pathway in the growth and decay of Pluronic micelles. Soft Matter 2016, 12, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, M.; Hathout, R.M.; Geneidi, A.S.; Mansour, S. Exploring the use of nanocarrier systems to deliver the magical molecule; Curcumin and its derivatives. J. Control. Release 2016, 225, 1–30. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, R.; Siani, A.; Lallana, E.; Tirelli, N. Influence of primary structure on responsiveness. Oxidative, thermal, and thermo-oxidative responses in polysulfides. Macromolecules 2015, 48, 8108–8120. [Google Scholar] [CrossRef]
- Gao, N.; Lü, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Feng, C.; Liu, M. Injectable shell-crosslinked F127 micelle/hydrogel composites with pH and redox sensitivity for combined release of anticancer drugs. Chem. Eng. J. 2016, 287, 20–29. [Google Scholar] [CrossRef]
- Lisi, R.; Lazzara, G.; Milioto, S.; Muratore, N. Polystyrene nanoparticles in the presence of (ethylene oxide)13(propylene oxide)30(ethylene oxide)13, N,N-dimethyloctylamine-N-oxide and their mixtures. A calorimetric and dynamic light scattering study. Phys. Chem. Chem. Phys. 2008, 10, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, P.; Stocco, A.; Muñoz, J.; Miller, R. Interfacial rheology and conformations of triblock copolymers adsorbed onto the water-oil interface. J. Colloid Interface Sci. 2012, 378, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Lisi, R.; Lazzara, G.; Milioto, S.; Muratore, N. Volumes of aqueous block copolymers based on poly(propylene oxides) and poly(ethylene oxides) in a large temperature range: A quantitative description. J. Chem. Thermodyn. 2006, 38, 1344–1350. [Google Scholar] [CrossRef]
- Loh, W. Encyclopedia of Surface and Colloid Science; Somasundaran, P., Ed.; Taylor & Francis: New York, NY, USA, 2006; p. 1014. [Google Scholar]
- Kong, T.; Ye, L.; Zhang, A.Y. Loose-fit polypseudorotaxanes constructed from γ-CDs and PHEMA-PPG-PEG-PPG-PHEMA. Beilstein J. Org. Chem. 2014, 10, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ye, L.; Zhang, A.Y. Self-assembly of polyrotaxanes synthesized via click chemistry of azido-endcapped PNIPAAm-b-Pluronic F68-b-PNIPAAm/γ-CD with propargylamine-substituted β-CDs. Macromol. Chem. Phys. 2014, 215, 1022–1029. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Ye, L.; Zhang, A.; Feng, Z. Formation of a polypseudorotaxane via self-assembly of γ-cyclodextrin with poly(N-isopropylacrylamide). Macromol. Rapid Commun. 2012, 33, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, J.; Wang, P.J.; Ye, L.; Zhang, A.; Feng, Z. Loose-fit polypseudorotaxanes fabricated by γ-CDs threaded onto a single PNIPAAm-PEG-PNIPAAm chain in aqueous solution. Macromol. Chem. Phys. 2012, 213, 1532–1539. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. Double-stranded inclusion complexes of cyclodextrin threaded on poly(ethylene glycol). Nature 1994, 370, 126–128. [Google Scholar] [CrossRef]
- Takahashi, A.; Katoono, R.; Yui, N. Loose-fit polyrotaxane composed of gamma-cyclodextrin and single poly(ethyelene glycol) chain: Making room in gamma-cd cavity for additional inclusion complexation. Macromolecules 2009, 42, 8587–8589. [Google Scholar] [CrossRef]
- Parmar, A.; Yerramilli, U.; Bahadur, P. Effect of hydrophobicity of PEO-PPO-PEO block copolymers on micellization and solubilization of a model drug nimesulide. J. Surfactants Deterg. 2012, 15, 367–375. [Google Scholar] [CrossRef]
- Dreiss, C.A.; Nwabunwanne, E.; Liu, R.; Brooks, N.J. Assembling and de-assembling micelles: Competitive interactions of cyclodextrins and drugs with Pluronics. Soft Matter 2009, 5, 1888–1896. [Google Scholar] [CrossRef]
- Wang, J.; Gao, P.; Ye, L.; Zhang, A.; Feng, Z. Solvent- and thermoresponsive polyrotaxanes with β-cyclodextrin dispersed/aggregated structures on a Pluronic F127 backbone. J. Phys. Chem. B 2010, 114, 5342–5349. [Google Scholar] [CrossRef] [PubMed]
- Lazzara, G.; Milioto, S. Copolymer-cyclodextrin inclusion complexes in water and in the solid state. A physico-chemical study. J. Phys. Chem. B 2008, 112, 11887–11895. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, K.; Chen, Y. Block copolymer micelles as carriers of transition metal ions Y(III) and Cu(II) and gelation thereof. Polymer 2014, 55, 6232–6238. [Google Scholar] [CrossRef]
- Wesley, R.D.; Cosgrove, T.; Thomson, L.; Armes, S.P.; Billingham, N.C.; Baines, F.L. Hydrodynamic layer thickness of a polybase brush in the presence of salt. Langmuir 2000, 16, 4467–4469. [Google Scholar] [CrossRef]
- Cosgrove, T.; Mears, S.J.; Obey, T.; Thompsonb, L.; Wesleya, R.D. Polymer, particle, surfactant interactions. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 149, 329–338. [Google Scholar] [CrossRef]
- Ceccato, M.; LoNostro, P.; Baglioni, P. α-Cyclodextrin/Polyethylene glycol polyrotaxane: A study of the threading process. Langmuir 1997, 13, 2436–2439. [Google Scholar] [CrossRef]
- Joseph, J.; Dreiss, C.A.; Cosgrove, T. Stretching a polymer brush by making in situ cyclodextrin inclusion complexes. Langmuir 2008, 24, 10005–10010. [Google Scholar] [CrossRef] [PubMed]
- Travelet, C.; Schlatter, G.; Hebraud, P.; Brochon, C.; Lapp, A.; Hadziioannou, G. Formation and self-organization kinetics of α-CD/PEO-based pseudo-polyrotaxanes in water. A specific behavior at 30 °C. Langmuir 2009, 25, 8723–8734. [Google Scholar] [CrossRef] [PubMed]
- Luis, N.; Eduardo, S.; José, L.; Francisco, J.O. Competitive displacement of drugs from cyclodextrin inclusion complex by polypseudorotaxane formation with poloxamer: Implications in drug solubilization and delivery. Eur. J. Pharm. Biopharm. 2012, 80, 585–595. [Google Scholar]
- Larrañeta, E.; Isasi, J.R. Self-assembled supramolecular gels of reverse poloxamers and cyclodextrins. Langmuir 2012, 28, 12457–12462. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ni, X.; Li, J. Synthesis of polyrotaxanes consisting of multiple a-cyclodextrin rings threaded on reverse Pluronic PPO-PEO-PPO triblock copolymers based on block-selected inclusion complexation. Eur. Polym. J. 2009, 45, 1570–1579. [Google Scholar] [CrossRef]
- Iguchi, H.; Uchida, S.; Koyama, Y.; Takata, T. Polyester-containing α-cyclodextrin-based polyrotaxane: Synthesis by living ring-opening polymerization, polypseudorotaxanation, and end capping using nitrile N-oxide. ACS Macro Lett. 2013, 2, 527–530. [Google Scholar] [CrossRef]
- Stayton, P.S.; Shimoboji, T.; Long, C.; Chilkoti, A.; Ghen, G.; Harris, J.M.; Hoffman, A.S. Control of protein-ligand recognition using a stimuli-responsive polymer. Nature 1995, 378, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; Hoffman, A.S. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, J. Thermoresponsive behavior of cationic polyrotaxane composed of multiple pentaethylenehexamine-graftedα-cyclodextrins threaded on poly(propyleneoxide)-poly(ethylene oxide)-poly(propylene oxide) triblock copolymer. J. Phys. Chem. B 2009, 113, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Eisenberg, A. Polymer Vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhong, Z.; Feijen, J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules 2009, 10, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, Y.; Yana, D. Hyperbranched polymer vesicles: From self-assembly, characterization, mechanisms, and properties to applications. Chem. Soc. Rev. 2015, 44, 3874–3889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D. Real-time membrane fusion of giant polymer vesicles. J. Am. Chem. Soc. 2005, 127, 10468–10469. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Q.; Xiao, J. EpCAM-Antibody-labeled noncytotoxic polymer vesicles for cancer stem cells-targeted delivery of anticancer drug and siRNA. Biomacromolecules 2015, 16, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Li, Q.; Lu, H.; Fang, L.; Chen, Q.; Yang, Y.; Gao, H. Construction of stable polymeric vesicles based on azobenzene and beta-cyclodextrin grafted poly(glycerol methacrylate)s for potential applications in colon-specific drug delivery. Chem. Commun. 2015, 51, 4715–4718. [Google Scholar] [CrossRef] [PubMed]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Cara, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ni, Y.; Jiang, W.; Li, H.; Liu, Y.; Lin, S.; Zhou, Y.; Yan, D. Self-crosslinking and surface-engineered polymer vesicles. Small 2015, 11, 4485–4490. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Binder, W.H. Mixed hybrid lipid/polymer vesicles as a novel membrane platform. Macromol. Rapid Commun. 2015, 36, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, L.; Xiao, C.; Chen, L.; Zhuang, X.; Chen, X. Noncovalent interaction-assisted polymeric micelles for controlled drug delivery. Chem. Commun. 2014, 50, 11274–11290. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, Q. Multifunctional polymer vesicles for cancer stem cells-targeted drug/siRNA therapy. Cancer Cell Microenviron. 2016, 3, 1–5. [Google Scholar]
- Robbins, G.P.; Saunders, R.L.; Haun, J.B.; Rawson, J.; Therien, M.J.; Hammer, D.A. Tunable leuko-polymersomes that adhere specifically to inflammatory markers. Langmuir 2010, 26, 14089–14096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, J.; Chen, X.; Xiao, C.; He, C.; Zhuang, X.; Chen, L.; Chen, X. Intracellular pH-sensitive supramolecular amphiphiles based on host-guest recognition between benzimidazole and beta-cyclodextrin as potential drug delivery vehicles. Polym. Chem. 2013, 4, 3265–3271. [Google Scholar] [CrossRef]
- Jin, H.B.; Zhou, Y.F.; Huang, W.; Yan, D. Polymerization-like multilevel hierarchical self-assembly of polymer vesicles into macroscopic superstructures with controlled complexity. Langmuir 2010, 26, 14512–14519. [Google Scholar] [CrossRef] [PubMed]
- Smart, T.P.; Fernyhough, C.; Ryan, A.J.; Battaglia, G. Controlling fusion and aggregation in polymersome dispersions. Macromol. Rapid Commun. 2008, 29, 1855–1860. [Google Scholar] [CrossRef]
- Liu, G.; Jin, Q.; Liu, X.; Lv, L.; Chen, C.; Ji, J. Biocompatible vesicles based on PEO-b-PMPC/a-cyclodextrin inclusion complexes for drug delivery. Soft Matter 2011, 7, 662–669. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, G.; Ji, J. Supramolecular micelles and reverse micelles based on cyclodextrinpolyrotaxanes. Chin. J. Chem. 2014, 32, 73–77. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Y.; Zheng, Y.; Huang, W.; Zhou, Y.; Yan, D. Cytomimetic large-scale vesicle aggregation and fusion based on host-guest interaction. Langmuir 2012, 28, 2066–2072. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive Polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Ad. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Lin, X.; Wang, J.; Zheng, X.; Li, X.; Lin, Z.; Lin, G. Injectable micellar supramolecular hydrogel for delivery of hydrophobic anticancer drugs. J. Mater. Scie.Mater. Med. 2016, 27, 1–7. [Google Scholar] [CrossRef]
- De France, K.J.; Chan, K.J.W.; Cranston, E.D.; Hoare, T. Enhanced mechanical properties in cellulose nanocrystal–poly(oligoethylene glycol methacrylate) injectable nanocomposite hydrogels through control of physical and chemical cross-linking. Biomacromolecules 2016, 17, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(pro-pylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Yorgensen, Y.M.; Morasse, A.; Evans, J.T.; Burkhart, D.J. PEG modified liposomes containing CRX-601 adjuvant in combination with methylglycol chitosan enhance the murine sublingual immune response to influenza vaccination. J. Control. Release 2016, 223, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 5124–5132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zheng, M.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Ma, Y.; Cai, L. NIR-driven smart theranostic nanomedicine for on-demand drug release and synergistic antitumour therapy. Nat. Sci. Rep. 2015, 5, 14258–14272. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG tri-block copolymers. J. Control. Release 2000, 63, 155–163. [Google Scholar] [CrossRef]
- Song, G.; Zhang, L.; He, C.; Fang, D.; Whitten, P.G.; Wang, H. Facile fabrication of tough hydrogels physically cross-linked by strong cooperative hydrogen bonding. Macromolecules 2013, 46, 7423–7435. [Google Scholar] [CrossRef]
- De Jong, S.J.; De Smedt, S.C.; Demeester, J.; van Nostrum, C.F.; Kettenes-van den Bosch, J.J.; Hennink, W.E. Biodegradable hydrogels based on stereo complex formation between lactic acid oligomers grafted to dextran. J. Control. Release 2001, 72, 47–56. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nat. Commun. 2015, 6, 6295–6304. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Cheng, A.; Li, J. Supramolecular hydrogels based on self-assembly between PEO-PPO-PEO triblock copolymers and α-cyclodextrin. J. Biomed. Mater. Res. Part A 2009, 88, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, S.; Kang, B. Thermosensitive and mucoadhesive sol-gel composites of paclitaxel/dimethyl-β-cyclodextrin for buccal delivery. PLoS ONE 2014, 9, e109090. [Google Scholar] [CrossRef] [PubMed]
- Travelet, C.; Schlatter, G.; Hebraud, P.; Brochon, C.; Anokhin, D.V.; Ivanov, D.A.; Hadziioannou, G. Physical gels based on polyrotaxanes: Kinetics of the gelation, and relative contributions of α-cyclodextrin and poly(ethylene oxide) to the gel cohesion. Macromol. Symp. 2010, 291, 202–211. [Google Scholar] [CrossRef]
- Simões, S.; Veiga, F.; Ribeiro, A.C.F.; Figueiras, A.R.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular gels of poly-α-cyclodextrin and PEO-based copolymers for controlled drug release. Eur. J. Pharm. Biopharm. 2014, 87, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Danel, C.; Azaroual, N.; Chavaria, C. Comparativestudy of the complex forming ability and enantioselectivity of cyclodextrin polymers by CE and 1H NMR. Carbohydr. Polym. 2013, 92, 2282–2292. [Google Scholar] [CrossRef] [PubMed]
- Concheiro, A.; Alvarez-Lorenzo, C. Chemically cross-linked and grafted cyclodextrin hydrogels: From nanostructures to drug-eluting medical devices. Adv. Drug Deliv. Rev. 2013, 65, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
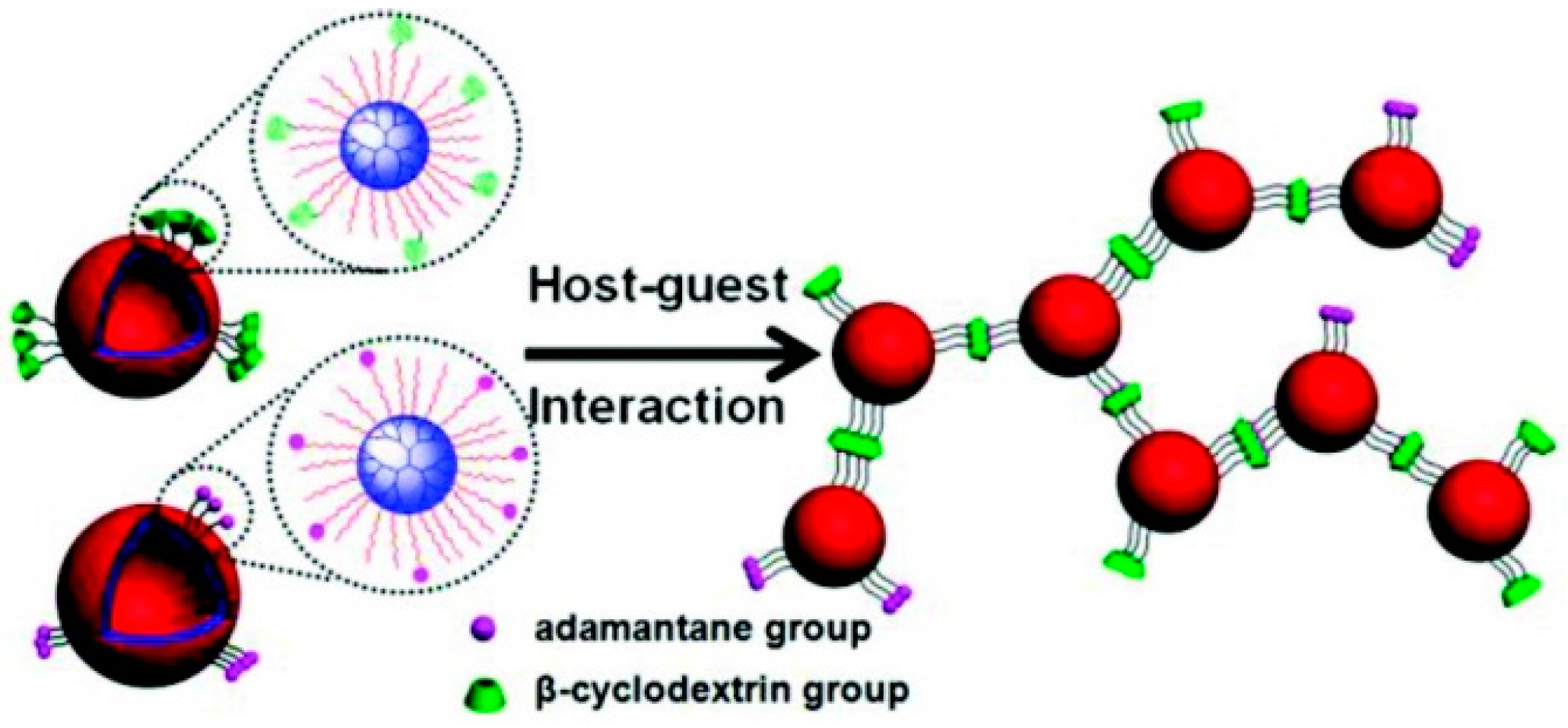
- Koopmans, C.; Ritter, H. Formation of physical hydrogels via host guest interactions of β-cyclodextrin polymers and copolymers bearing adamantly groups. Macromolecules 2008, 41, 7418–7422. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Steffensen, K.; Larsen, K.L. Self-assembling microparticles with controllable disruption properties based on cyclodextrin interactions. Colloids Surf. B Biointerfaces 2009, 73, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Amiel, C.; Molinard, K.; Daoud-Mahammed, S.; Sébille, B.; Gillet, B.; Beloeil, J.; Ringard, C.; Rosilio, V.; Poupaert, J.; et al. New self-assembled nanogels based on host-guest interactions: Characterization and drug loading. J. Control. Release 2006, 111, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Daoud-Mahammed, S.; Ringard-Lefebvre, C.; Razzouq, N. Spontaneous association of hydrophobized dextran and poly-β-cyclodextrin into nanoassemblies: Formation and interaction with a hydrophobic drug. J. Colloid Interface Sci. 2007, 307, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Ji, R.; Deng, X. Glucose-responsive hydrogels based on dynamic covalent chemistry and inclusion complexation. Soft Matter 2014, 10, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Chee, P.; Prasad, A.; Fang, X. Supramolecular cyclodextrin pseudorotaxane hydrogels: A candidate for sustained release? Mater. Sci. Eng. C 2014, 39, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Miro, A.; Rondinone, A.; Nappi, A. Modulation of release rate and barrier transport of Diclofenac incorporated in hydrophilic matrices: Role of cyclodextrins and implications in oral drug delivery. Eur. J. Pharm. Biopharm. 2009, 72, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Travelet, C.; Hebraud, P.; Perry, C. Temperature-dependent structure of γ-CD/PEO-based polyrotaxanes in concentrated solution in DMSO: Kinetics and multiblock copolymer behavior. Macromolecules 2010, 43, 1915–1921. [Google Scholar] [CrossRef]
- Larrañeta, E.; Arriz, C.; Velaz, I.; Zornoza, A.; Machín, R.; Isasi, J. In vitro release from reverse poloxamine/α-cyclodextrin matrices: Modelling and comparison of dissolution profiles. J. Pharm. Sci. 2014, 103, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J. Supramolecular hydrogels based on inclusion complexation between poly(ethylene oxide)-β-poly(ɛ-caprolactone) diblock copolymer and α-cyclodextrin and their controlled release property. J. Biomed. Mater. Res. Part A 2008, 86, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Isasi, J. Non-covalent hydrogels of cyclodextrins and poloxamines for the controlled release of proteins. Carbohydr. Polym. 2014, 102, 674–681. [Google Scholar] [CrossRef] [PubMed]
| Hydrogel (Cross-Linking Method) | Formation Mechanisms | System | Characteristics or Applications |
|---|---|---|---|
| Physical (reversible)gels | (1) Intermolecular forces hydrogen bonding (2) Hydrophobic injectable effect (3) Electrostatic ionic force or intermolecular assemblies (4) Stereo-complexation (5) Complementary binding | (a) CD/PEO-PPO–PEO | Injectable. Buccal-paclitaxel delivery system [98,99,100] |
| (b) Poly-α-CD/PEO | Injectable supramolecular gels [101] | ||
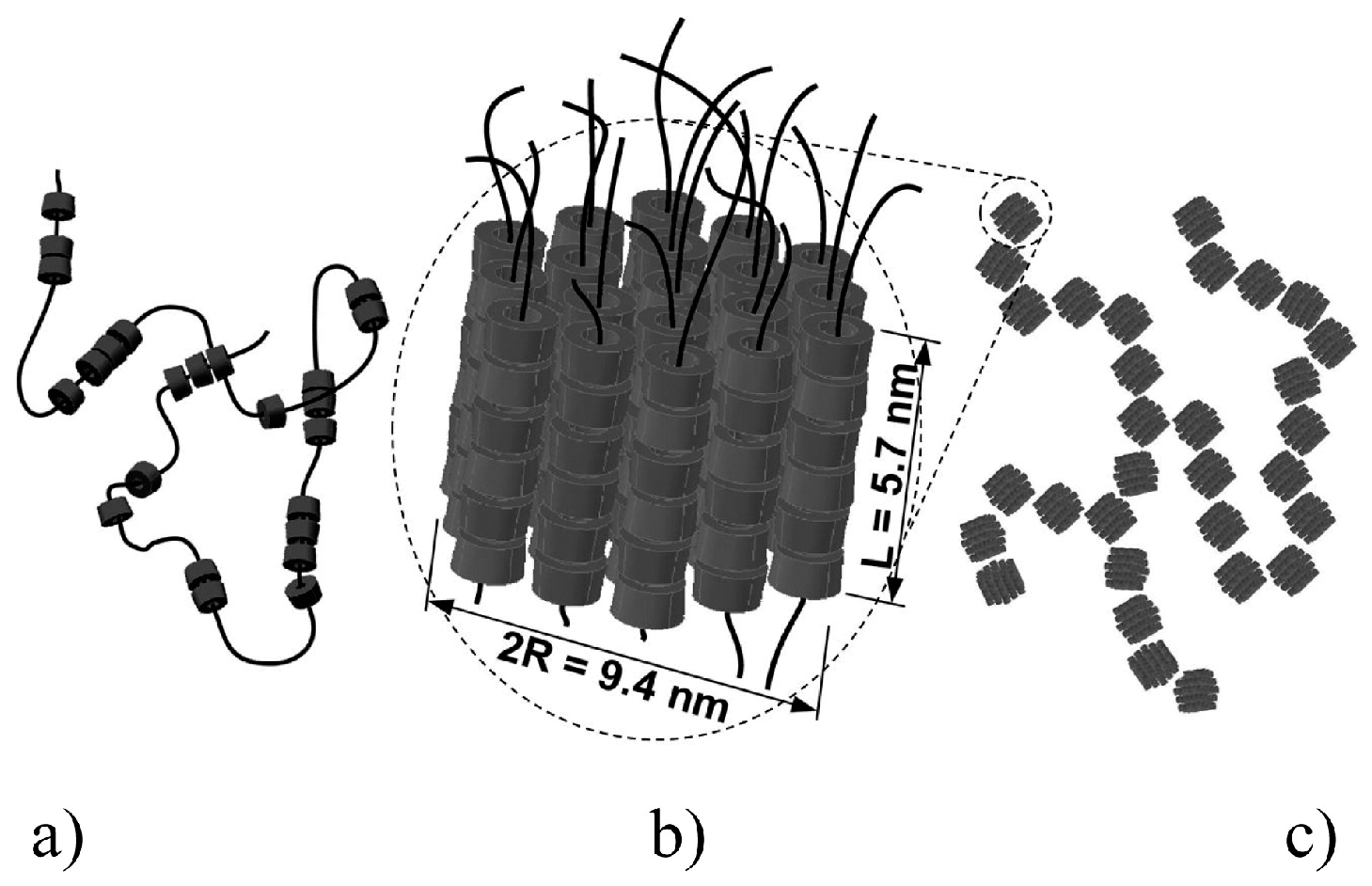
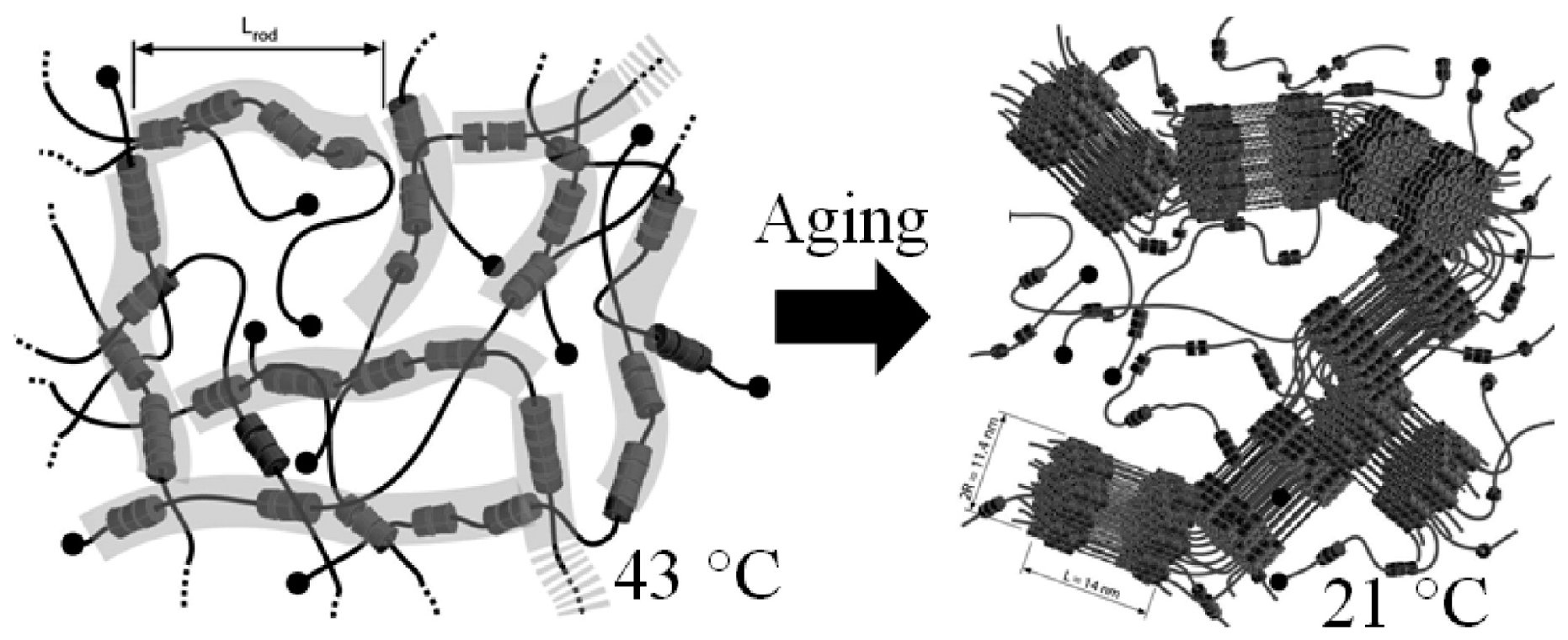
| (c) CD/PEO | CD/PRs with rigid block and flexible block type in DMSO; structure is regular bundles structure at 21 °C [109,110,111] | ||
| (d) CD/PEO-PPO | Sustained delivery [112] | ||
| (e) CD/PEO-PCL | Sustained release, lower molecular weight [113] | ||
| (f) CD/PEO-PPO octablock star copolymers | Biocompatible, sustained [114] | ||
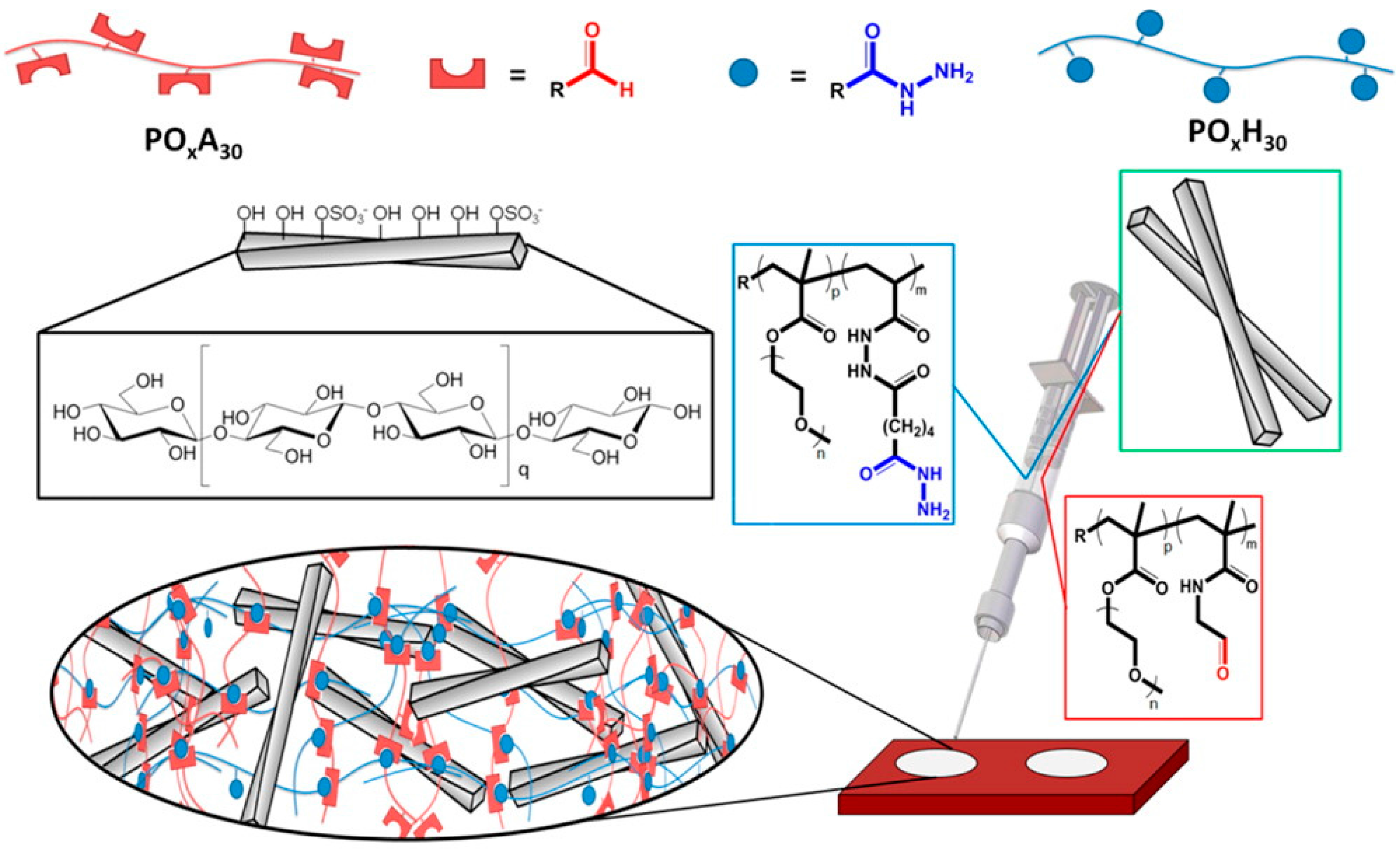
| Chemical (permanent)gels | (1) Redox reactions (2) Photo-polymerization (3) Click chemistry (4) Michael reactions (5) Schiff’s base reactions, enzymatic reactions(disulfide-forming reactions) | CD/PEO-PVA/PBA | Pharmaceutical fields (e.g., treatment of diabetes) [107] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wyman, I.W. Supramolecular Nanostructures Based on Cyclodextrin and Poly(ethylene oxide): Syntheses, Structural Characterizations and Applications for Drug Delivery. Polymers 2016, 8, 198. https://doi.org/10.3390/polym8050198
Zheng Y, Wyman IW. Supramolecular Nanostructures Based on Cyclodextrin and Poly(ethylene oxide): Syntheses, Structural Characterizations and Applications for Drug Delivery. Polymers. 2016; 8(5):198. https://doi.org/10.3390/polym8050198
Chicago/Turabian StyleZheng, Yue, and Ian W. Wyman. 2016. "Supramolecular Nanostructures Based on Cyclodextrin and Poly(ethylene oxide): Syntheses, Structural Characterizations and Applications for Drug Delivery" Polymers 8, no. 5: 198. https://doi.org/10.3390/polym8050198
APA StyleZheng, Y., & Wyman, I. W. (2016). Supramolecular Nanostructures Based on Cyclodextrin and Poly(ethylene oxide): Syntheses, Structural Characterizations and Applications for Drug Delivery. Polymers, 8(5), 198. https://doi.org/10.3390/polym8050198