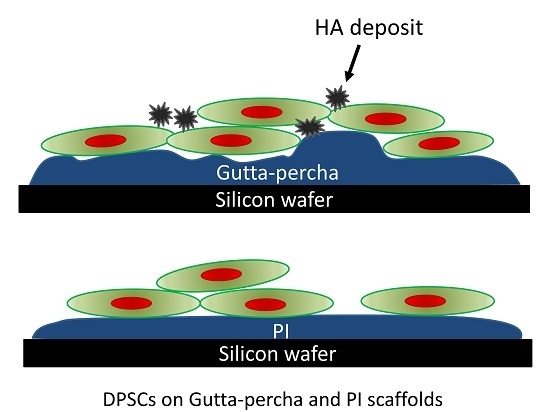
Differentiation of Dental Pulp Stem Cells on Gutta-Percha Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gutta-Percha Scaffolds
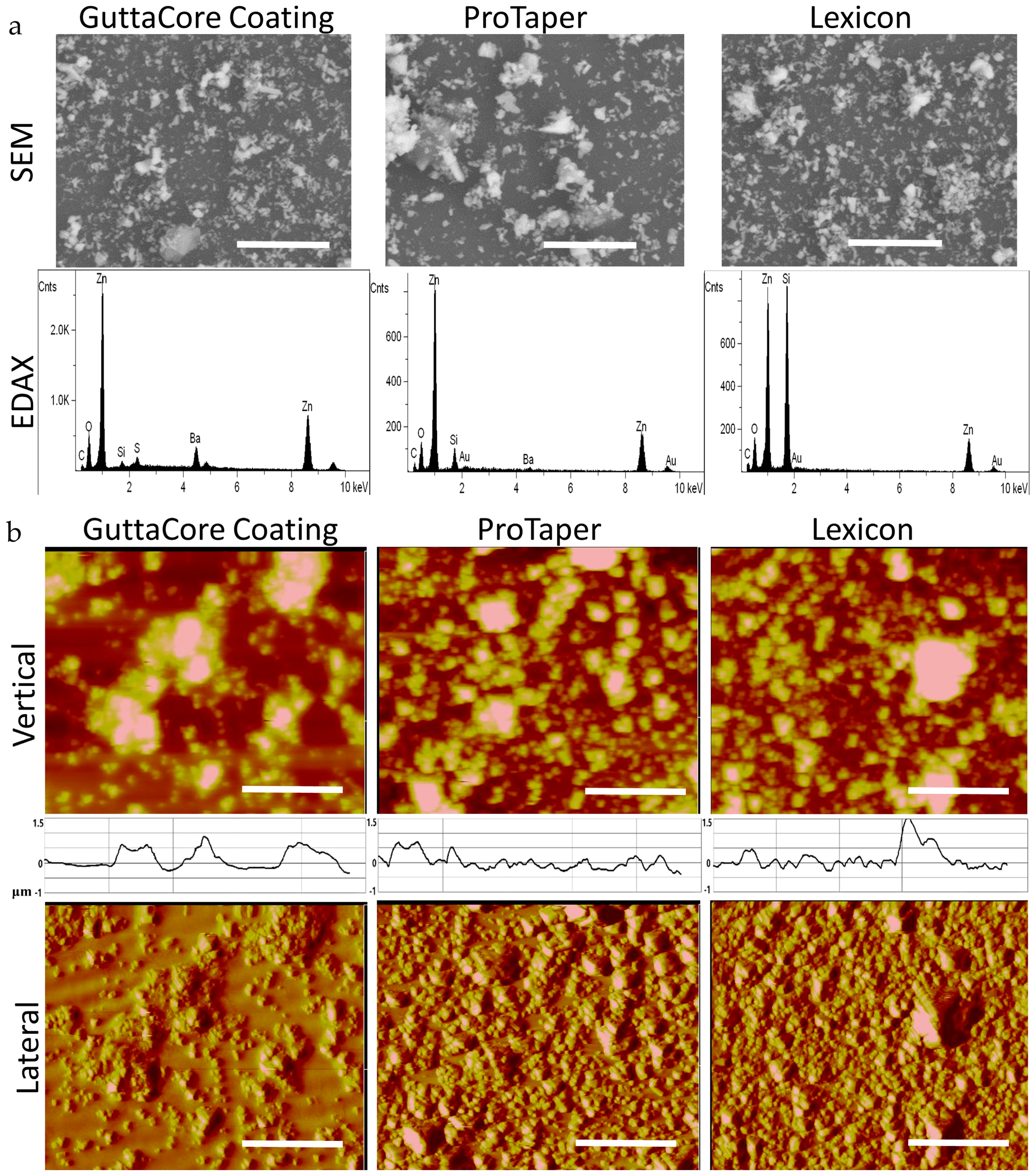
2.3. Scanning Electron Microscopy and Energy Dispersive Analysis X-ray (SEM-EDAX)
2.4. Scanning Probe Microscopy (SPM)
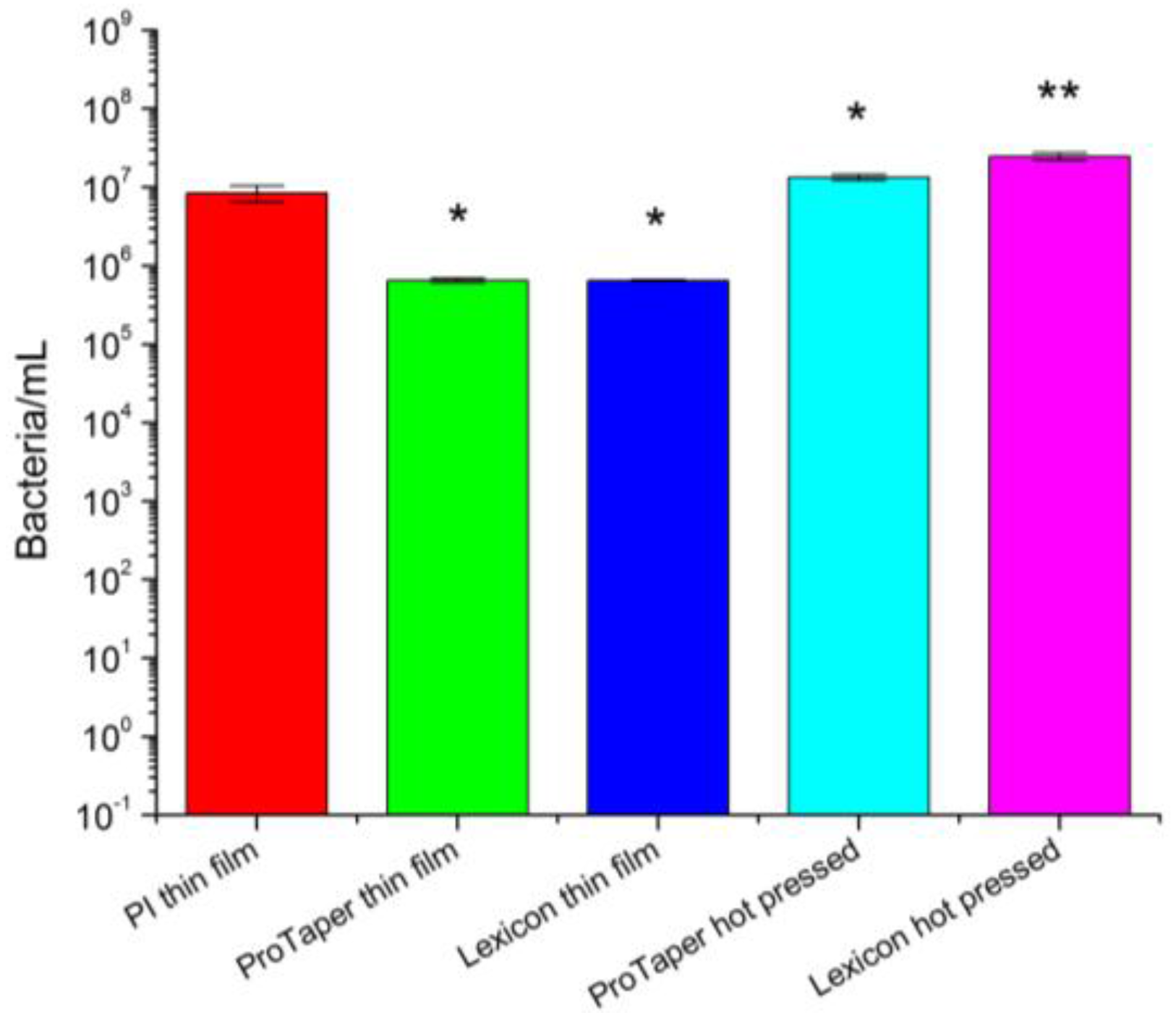
2.5. Antimicrobial Activity and Efficacy Test
2.6. Cell Isolation and Cell Plating
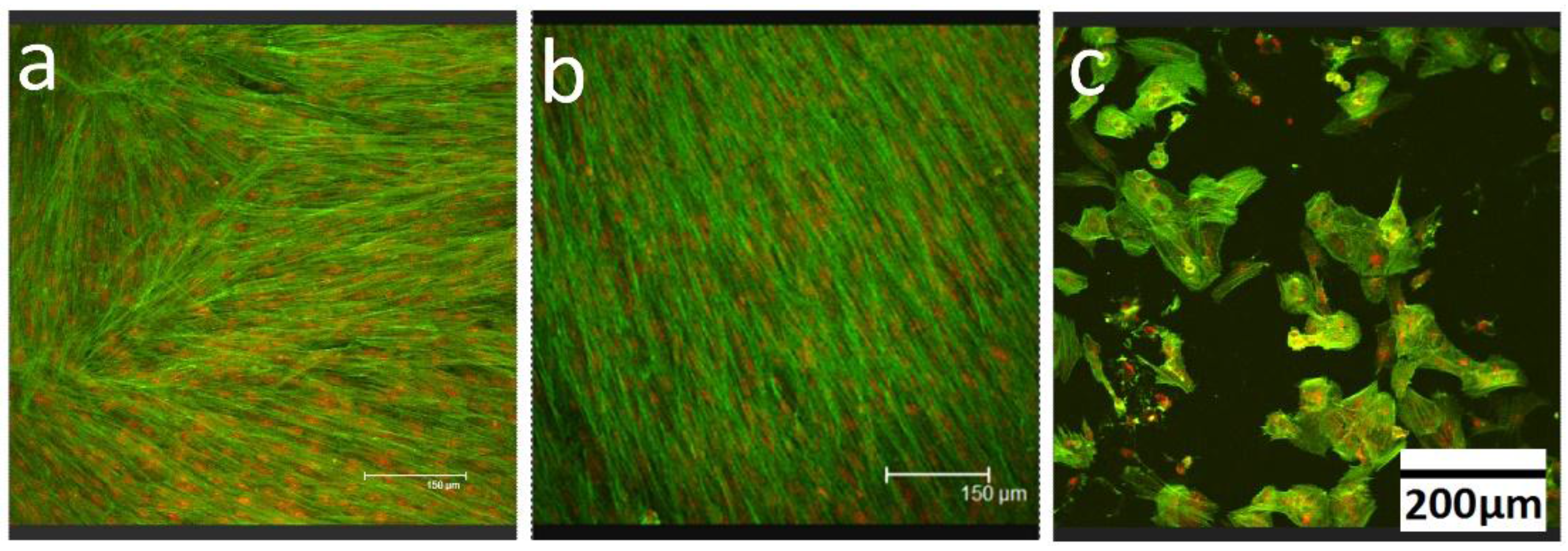
2.7. Confocal Laser Scanning Microscopy
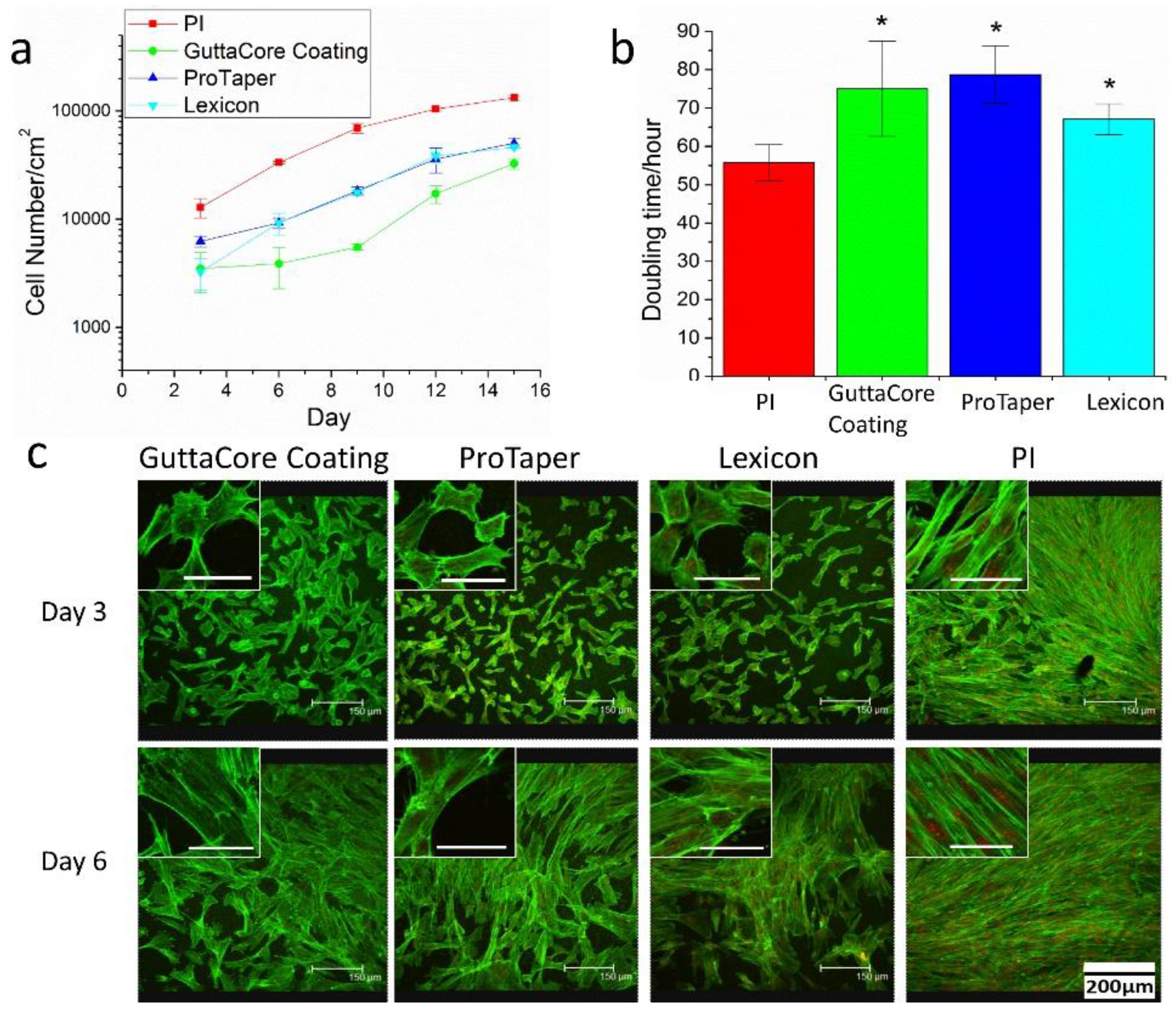
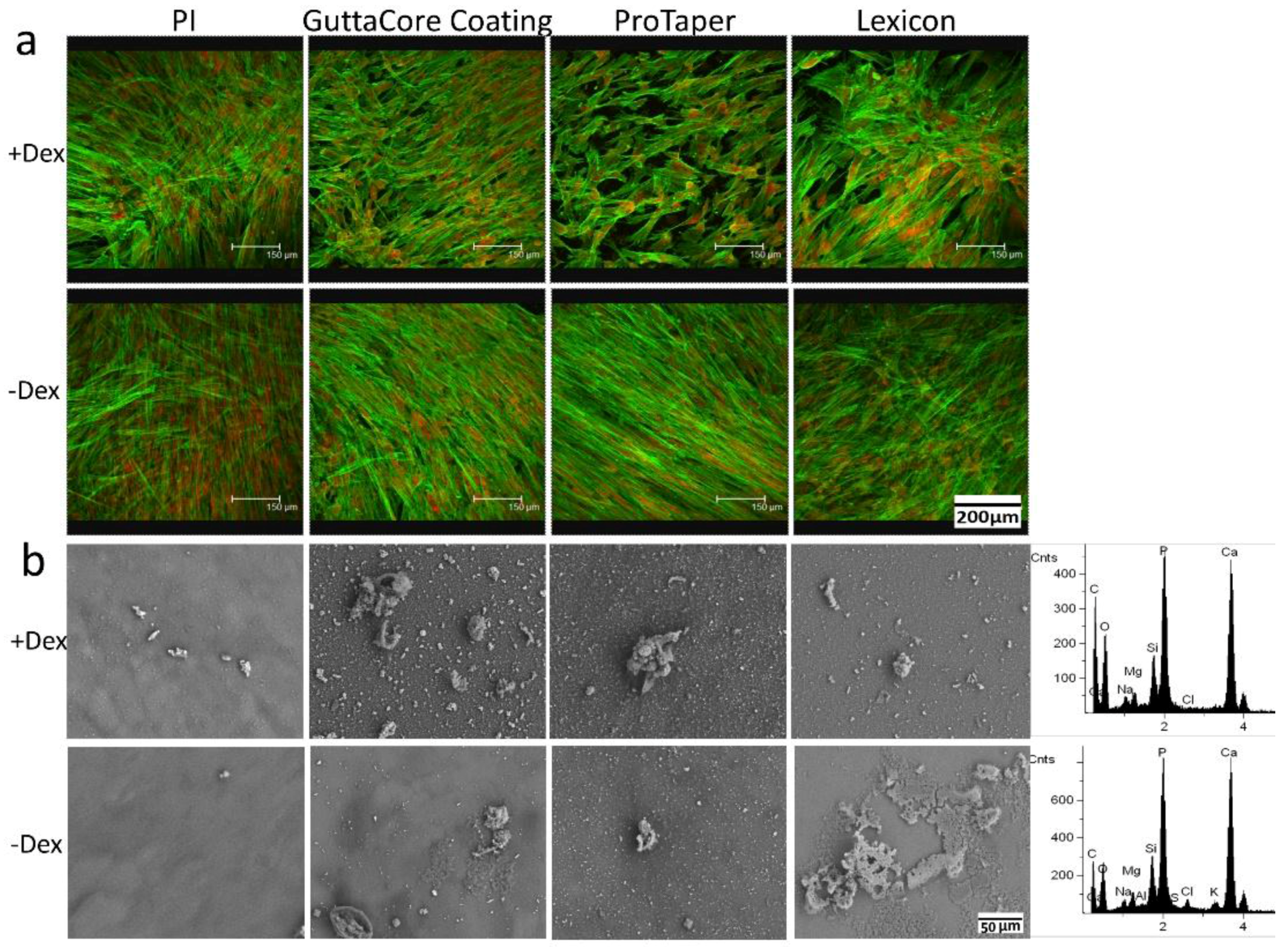
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cohen, S.; Hargreaves, K.M. Pathways of the Pulp, 9th ed.; Mosby Elsevier: St. Louis, MO, USA, 2006; pp. 499–565. [Google Scholar]
- Carrotte, P. Endodontics: Part 1. The modern concept of root canal treatment. Br. Dent. J. 2004, 197, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Barnes, J.J.; Manogue, M. The Principles of Endodontics, 2nd ed.; Oxford University Press: Oxford, UK, 2013; pp. 23–52. [Google Scholar]
- Dietschi, D.; Duc, O.; Krejci, I.; Sadan, A. Biomechanical considerations for the restoration of endodontically treated teeth: A systematic review of the literature—Part 1. Composition and micro- and macrostructure alterations. Quintessence Int. 2007, 38, 733–743. [Google Scholar] [PubMed]
- Dietschi, D.; Duc, O.; Krejci, I.; Sadan, A. Biomechanical considerations for the restoration of endodontically treated teeth: A systematic review of the literature, Part II (Evaluation of fatigue behavior, interfaces, and in vivo studies). Quintessence Int. 2008, 39, 117–129. [Google Scholar] [PubMed]
- Alrahabi, M.K.; Ali, M.M. Root canal revascularization. The beginning of a new era in endodontics. Saudi Méd. J. 2014, 35, 429–434. [Google Scholar] [PubMed]
- Kitamura, C.; Nishihara, T.; Terashita, M.; Tabata, Y.; Jimi, E.; Washio, A.; Hirata, S. Regeneration approaches for dental pulp and periapical tissues with growth factors, biomaterials, and laser irradiation. Polymers 2011, 3, 1776–1793. [Google Scholar] [CrossRef]
- Oshima, M.; Tsuji, T. Whole tooth regeneration as a future dental treatment. Adv. Exp. Med. Biol. 2015, 881, 255–269. [Google Scholar] [PubMed]
- Garcia-Godoy, F.; Murray, P.E. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent. Traumatol. 2012, 28, 33–41. [Google Scholar] [CrossRef] [PubMed]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; de Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Martinez, E.; Mendoza-Nunez, V.M.; Santiago-Osorio, E. Mesenchymal stem cells derived from dental pulp: A review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cells Mater. 2009, 18, 75–83. [Google Scholar]
- Giuliani, A.; Manescu, A.; Langer, M.; Rustichelli, F.; Desiderio, V.; Paino, F.; de Rosa, A.; Laino, L.; d’Aquino, R.; Tirino, V.; et al. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: Biological and clinical implications. Stem Cells Transl. Med. 2013, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(l-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863. [Google Scholar] [CrossRef] [PubMed]
- Piva, E.; Silva, A.F.; Nor, J.E. Functionalized scaffolds to control dental pulp stem cell fate. J. Endod. 2014, 40, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Marrelli, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Odontogenic differentiation of human dental pulp stem cells on hydrogel scaffolds derived from decellularized bone extracellular matrix and collagen type I. PLoS ONE 2016, 11, e0148225. [Google Scholar] [CrossRef] [PubMed]
- Gennari, C. Differential effect of glucocorticoids on calcium absorption and bone mass. Br. J. Rheumatol. 1993, 32, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Bherwani, A.; Simon, M.; Rafailovich, M.; Jurukovski, V. Entangled polymer surface confinement, an alternative method to control stem cell differentiation in the absence of chemical mediators. Ann. Mater. Sci. Eng. 2014, 1, 1–7. [Google Scholar]
- Kemp, A.R.; Peters, H. Fractionation and molecular weight of rubber and Gutta-percha. Ind. Eng. Chem. 1941, 33, 1391–1398. [Google Scholar] [CrossRef]
- Friedman, C.M.; Sandrik, J.L.; Heuer, M.A.; Rapp, G.W. Composition and mechanical properties of Gutta-percha endodontic points. J. Dent. Res. 1975, 54, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Maniglia-Ferreira, C.; Gurgel-Filho, E.D.; de Araujo Silva, J.B., Jr.; de Paula, R.C.; de Andrade Feitosa, J.P.; de Sousa-Filho, F.J. Chemical composition and thermal behavior of five brands of thermoplasticized Gutta-percha. Eur. J. Dent. 2013, 7, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M.; Guggenheim, B. The mysterious appearance of enterococci in filled root canals. Int. Endod. J. 2009, 42, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Moorer, W.R.; Genet, J.M. Antibacterial activity of Gutta-percha cones attributed to the zinc oxide component. Oral Surg. Oral Med. Oral Pathol. 1982, 53, 508–517. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Jeng, H.A.; Swanson, J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health Part A Toxic Hazard Subst. Environ. Eng. 2006, 41, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R.; Anand, T.; Khanum, F. Nanosized zinc oxide induces toxicity in human lung cells. ISRN Toxicol. 2013, 2013, 316075. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Juganson, K.; Bondarenko, O.; Mortimer, M.; Aruoja, V.; Kasemets, K.; Blinova, I.; Heinlaan, M.; Slaveykova, V.; Kahru, A. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: A comparative review. Nanotoxicology 2014, 8, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Pascon, E.A.; Spangberg, L.S. In vitro cytotoxicity of root canal filling materials: 1. Gutta-percha. J. Endod. 1990, 16, 429–433. [Google Scholar] [CrossRef]
- Gambarini, G.; Testarelli, L.; Al-Sudani, D.; Plotino, G.; Grande, N.M.; Lupi, A.; Giardina, B.; Nocca, G.; de Luca1, M. In vitro evaluation of the cytotoxicity of different root canal filling materials. Open Dent. J. 2011, 5, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Batouli, S.; Miura, M.; Brahim, J.; Tsutsui, T.W.; Fisher, L.W.; Gronthos, S.; Robey, PG.; Shi, S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J. Dent. Res. 2003, 82, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Bodrumlu, E.; Alacam, T. Evaluation of antimicrobial and antifungal effects of iodoform-integrating Gutta-percha. J. Can. Dent. Assoc. 2006, 72, 733. [Google Scholar] [PubMed]
- Jhamb, A.; Chaurasia, V.R.; Masamatti, V.K.; Agarwal, J.H.; Tiwari, S.; Nair, D. In vitro evaluation of antimicrobial activity of different Gutta-percha points and calcium hydroxide pastes. J. Int. Soc. Prev. Community Dent. 2014, 4, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Alliot-Licht, B.; Bluteau, G.; Magne, D.; Lopez-Cazaux, S.; Lieubeau, B.; Daculsi, G.; Guicheux, J. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005, 321, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Iaculli, F.; Di Filippo, E.S.; Piattelli, A.; Mancinelli, R.; Fulle, S. Dental pulp stem cells grown on dental implant titanium surfaces: An in vitro evaluation of differentiation and microRNAs expression. J. Biomed. Mater. Res. B 2016. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yu, Y.; Joubert, C.; Bruder, G.; Liu, Y.; Chang, C.-C.; Simon, M.; Walker, S.G.; Rafailovich, M. Differentiation of Dental Pulp Stem Cells on Gutta-Percha Scaffolds. Polymers 2016, 8, 193. https://doi.org/10.3390/polym8050193
Zhang L, Yu Y, Joubert C, Bruder G, Liu Y, Chang C-C, Simon M, Walker SG, Rafailovich M. Differentiation of Dental Pulp Stem Cells on Gutta-Percha Scaffolds. Polymers. 2016; 8(5):193. https://doi.org/10.3390/polym8050193
Chicago/Turabian StyleZhang, Liudi, Yingjie Yu, Christopher Joubert, George Bruder, Ying Liu, Chung-Chueh Chang, Marcia Simon, Stephen G. Walker, and Miriam Rafailovich. 2016. "Differentiation of Dental Pulp Stem Cells on Gutta-Percha Scaffolds" Polymers 8, no. 5: 193. https://doi.org/10.3390/polym8050193
APA StyleZhang, L., Yu, Y., Joubert, C., Bruder, G., Liu, Y., Chang, C.-C., Simon, M., Walker, S. G., & Rafailovich, M. (2016). Differentiation of Dental Pulp Stem Cells on Gutta-Percha Scaffolds. Polymers, 8(5), 193. https://doi.org/10.3390/polym8050193