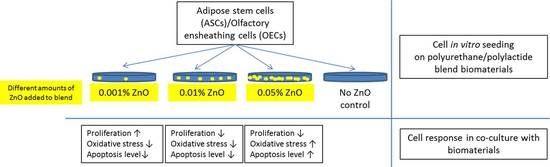
Polyurethane/Polylactide-Blend Films Doped with Zinc Ions for the Growth and Expansion of Human Olfactory Ensheathing Cells (OECs) and Adipose-Derived Mesenchymal Stromal Stem Cells (ASCs) for Regenerative Medicine Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
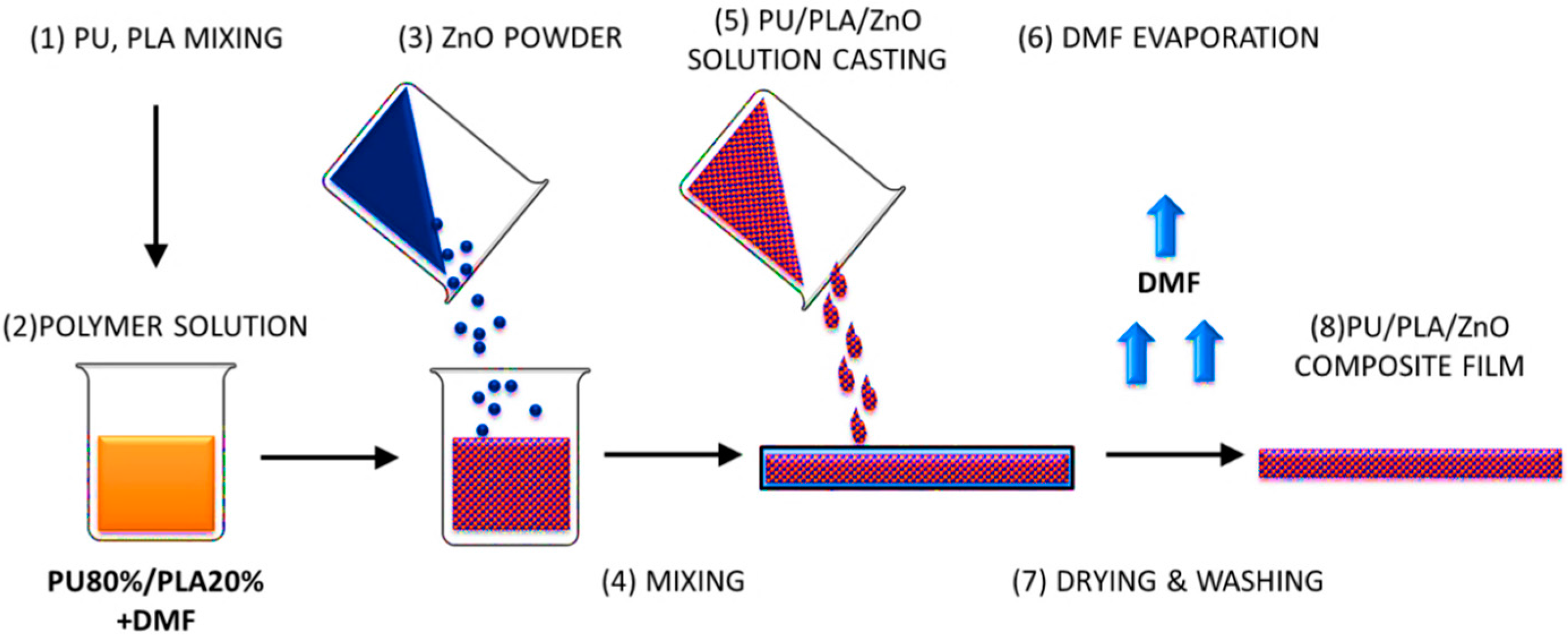
2.2. Preparation of Biomaterials
2.3. Morphology of the PU/PLDL/ZnO Films
2.4. Water Contact Angle Measurement
2.5. Atomic Force Microscopy
2.6. Cell Isolation
2.7. Cell Viability and Proliferation
2.8. OEC Phenotype
2.9. Scanning Electron Microscopy
2.10. ROS, SOD, and NO Assays
2.11. RT-PCR Analysis
3. Results
3.1. Morphology of Films
3.2. Wettability of Films
3.3. Atomic Force Microscopy
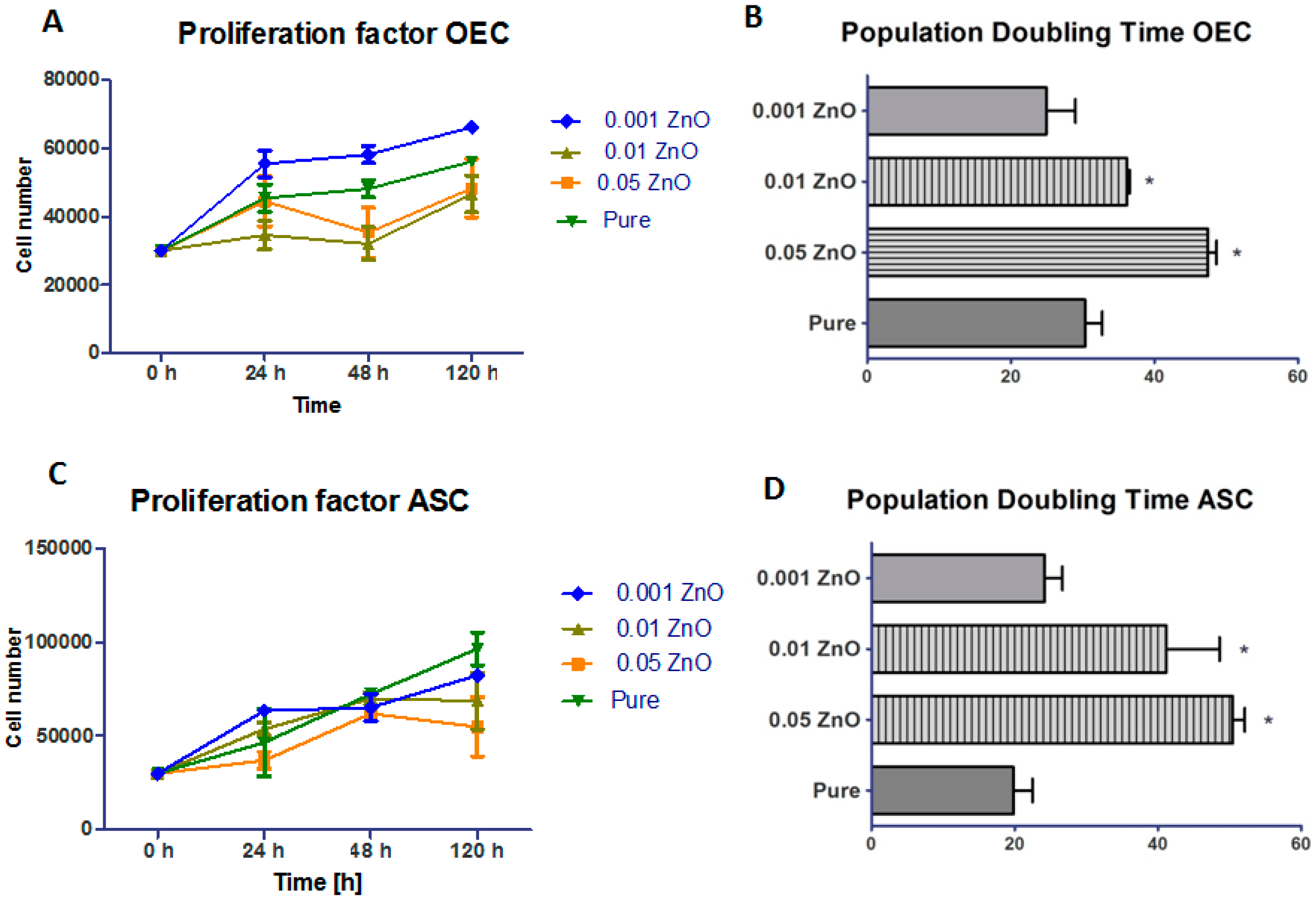
3.4. Proliferation Assay
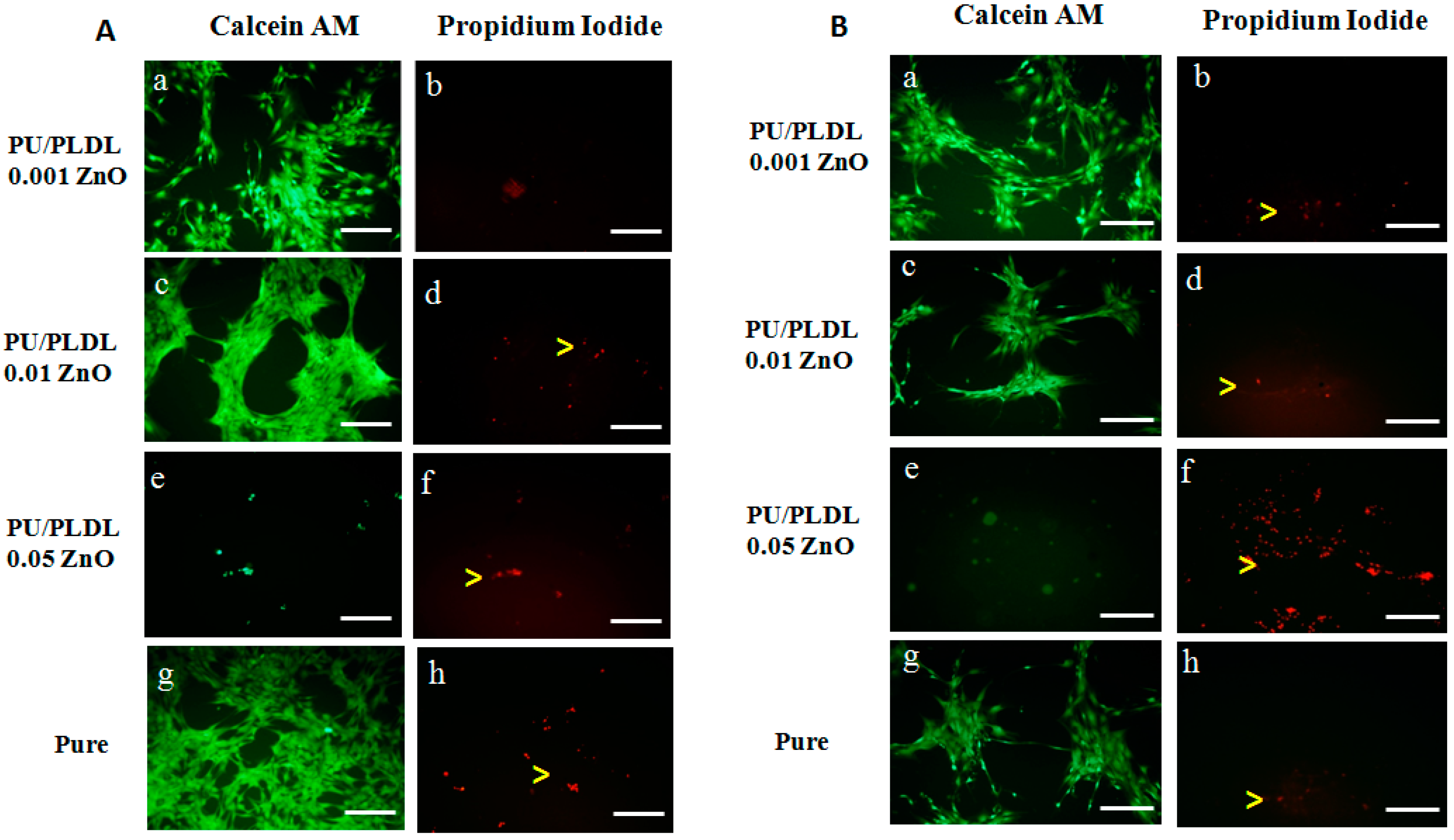
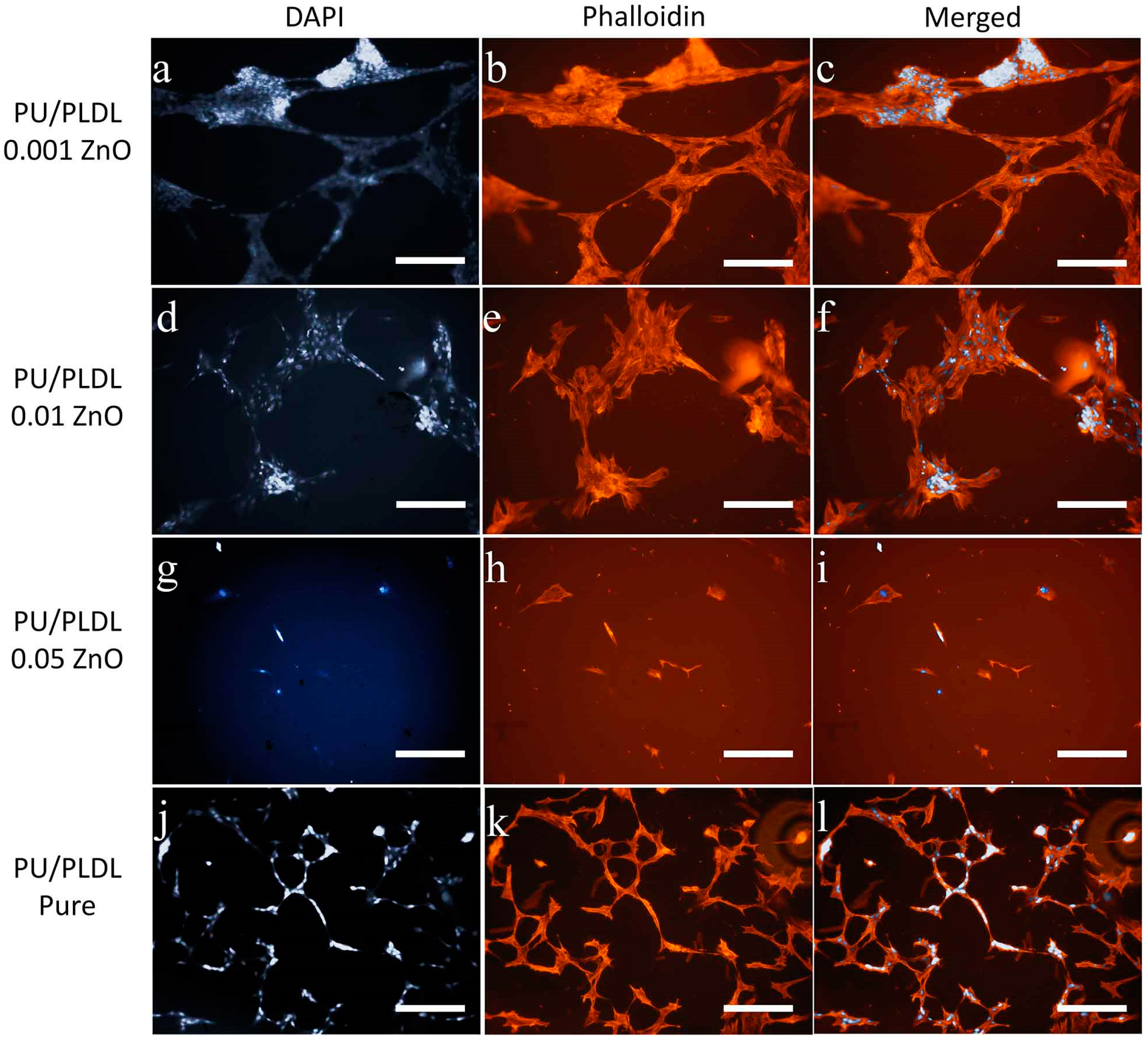
3.5. Cell Morphology
3.6. Immunophenotype of OECs
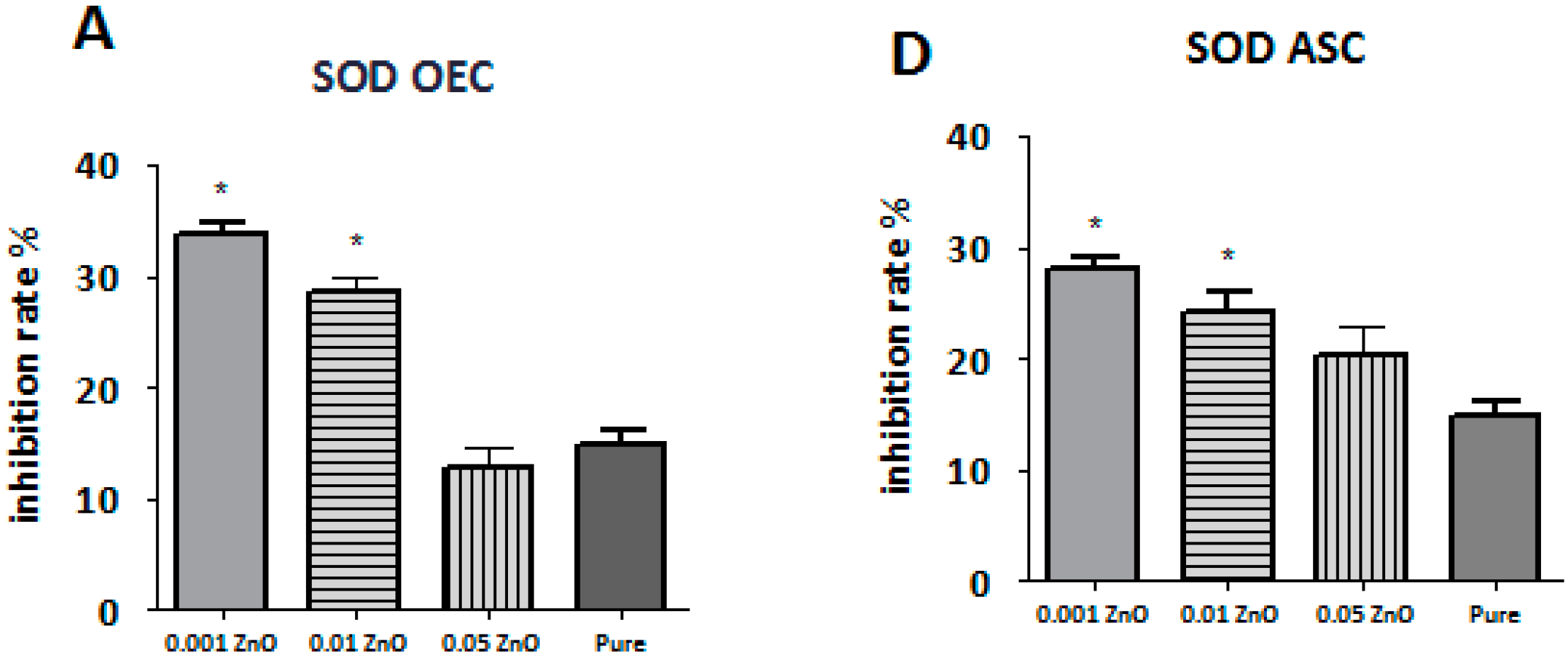
3.7. ROS, SOD, and NO Assays
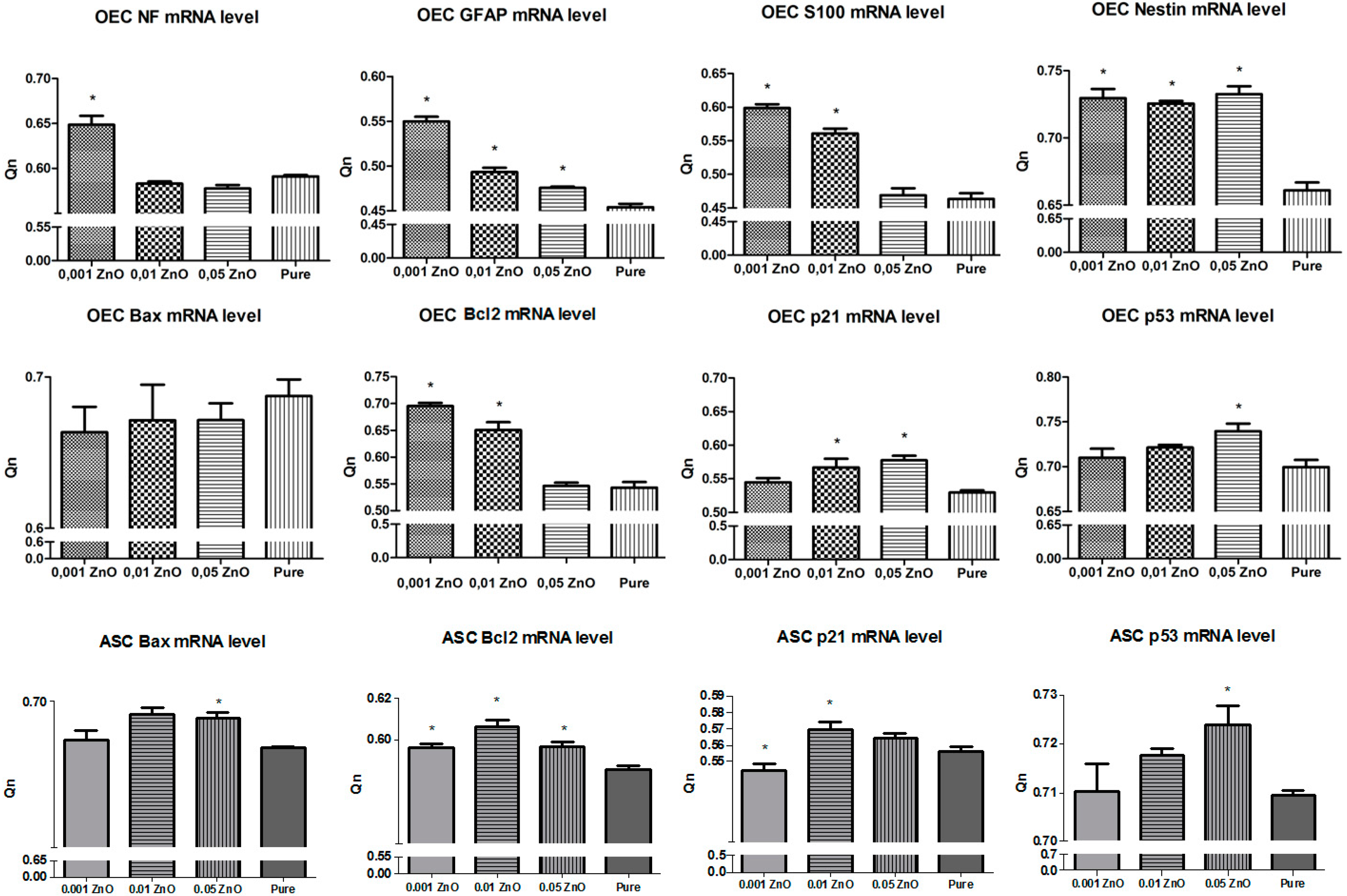
3.8. Real-Time RT-PCR Measurements
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| SCI | Spinal cord injury |
| PU | Polyurethane |
| PLDL | Poly(l-lactide-co-d,l-lactide) |
| OECs | Olfactory ensheathing cells |
| ASCs | Adipose stromal stem cells |
| ROS | Reactive oxygen species |
| NO | Nitric oxide |
| SOD | Superoxide dismutase |
| FBS | Fetal bovine serum |
| DMEM | Dulbecco’s Modified Eagle Medium |
| HBSS | Hank’s Balanced Salt Solution |
| GFAP | Glial fibrillary acidic protein |
References
- Schwab, M.E. Repairing the injured spinal cord. Science 2002, 295, 1029–1031. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Arinzeh, T.L. Electrospun nanofibrous materials for neural tissue engineering. Polymers 2011, 3, 413–426. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nanotoday 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, J.; Xu, Y.; Xiang, A.P.; Jiang, M.H.; Quan, D. The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats. Biomaterials 2013, 34, 7086–7096. [Google Scholar] [CrossRef] [PubMed]
- Moisenovich, M.M.; Pustovalova, O.; Shackelford, J.; Vasilijeva, T.V.; Druzhinina, T.V.; Kamenchuk, Y.A.; Guzeev, V.V.; Sokolova, O.S.; Bogush, V.G.; Debabov, V.G.; et al. Tissue regeneration in vivo within recombinant spidroin 1 scaffolds. Biomaterials 2012, 33, 3887–3898. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Kiasat-Dolatabadi, A.; Ebrahimi-Barough, S.; Ai, A.; Lotfibakhshaiesh, N.; Norouzi-Javidan, A.; Saberi, H.; Arjmand, B.; Aghayan, H.R. Polymeric scaffolds in neural tissue engineering: A review. Arch. Neurol. Sci. 2013, 1, 15–20. [Google Scholar] [CrossRef]
- Hausner, T.; Schmidhammer, R.; Zandieh, S.; Hopf, R.; Schultz, A.; Gogolewski, S.; Hertz, H.; Redl, H. Nerve regeneration using tubular scaffolds from biodegradable polyurethane. Acta Neurochir. Suppl. 2007, 100, 69–72. [Google Scholar] [PubMed]
- Giardino, R.; Fini, M.; Aldini, N.N.; Giavaresi, G.; Rocca, M. Polylactide bioabsorbable polymers for guided tissue regeneration. J. Trauma Injury Infect. Clin. Care 1999, 47, 303–308. [Google Scholar] [CrossRef]
- Grzesiak, J.; Marycz, K.; Szarek, D.; Bednarz, P.; Laska, J. Polyurethane/polylactide-based biomaterials combined with rat olfactory bulb-derived glial cells and adipose-derived mesenchymal stromal cells for neural regenerative medicine applications. Mater. Sci. Eng. C 2015, 52, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, J.; Lis, A.; Szarek, D.; Laska, J.; Marycz, K.; Fryczkowski, R. Characterization of olfactory ensheathing glial cells cultured on polyurethane/polylactide electrospun nonwovens. Int. J. Polym. Sci. 2015, 2015, 908328. [Google Scholar] [CrossRef]
- Ramon-Cueto, A.; Munoz-Quiles, C. Clinical application of adult olfactory bulb ensheathing glia for nervous system repair. Exp. Neurol. 2011, 229, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Raisman, G.; Barnett, S.C.; Ramón-Cueto, A. Repair of central nervous lesions by transplantation of olfactory ensheathing cells. Handb. Clin. Neurol. 2012, 109, 541–549. [Google Scholar] [PubMed]
- Sowa, Y.; Kishida, T.; Imura, T.; Numajiri, T.; Nishino, K.; Tabata, Y.; Mazda, O. Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into Schwann-like lineage. Plastic Reconstr. Surg. 2016, 137, 318e–330e. [Google Scholar] [CrossRef] [PubMed]
- Tabakow, P.; Jarmundowicz, W.; Czapiga, B.; Fortuna, W.; Międzybrodzki, R.; Czyż, M.; Huber, J.; Szarek, D.; Okurowski, S.; Szewczyk, P.; et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013, 22, 1591–1612. [Google Scholar] [CrossRef] [PubMed]
- Dasari, V.R.; Veeravalli, K.K.; Rao, J.S.; Fassett, D.; Dinh, D.H. Mesenchymal stem cell therapy for apoptosis after spinal cord injury. advanced understanding of neurodegenerative diseases. In Advanced Understanding of Neurodegenerative Diseases; Chang, R.C.-C., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Park, I.S.; Rhie, J.W.; Kim, S.H. A novel three-dimensional adipose-derived stem cell cluster for vascular regeneration in ischemic tissue. Cytotherapy 2014, 16, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Moncrieff, D.W. Zinc-containing neurons. Biol. Signals 1994, 3, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [PubMed]
- Frederickson, C.J.; Danscher, G. Zinc-containing neurons in hippocampus and related CNS structures. Prog. Brain Res. 1990, 83, 71–84. [Google Scholar] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem cells and the impact of ROS signalling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Marycz, K.; Tomaszewski, K.A.; Marędziak, M.; Śmieszek, A. The effect of age on osteogenic and adipogenic differentiation potential of human adipose derived stromal stem cells (hASCs) and the impact of stress factors in the course of the differentiation process. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Szarek, D.; Grzesiak, J.; Wrzeszcz, K. Influence of modified alginate hydrogels on mesenchymal stem cells and olfactory bulb-derived glial cells cultures. Biomed. Mater. Eng. 2014, 24, 1625–1637. [Google Scholar] [PubMed]
- Marędziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int. 2016, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Roth, V. Doubling Time Computing. 2006. Available online: accessed on www.doubling-time.com/compute.php (29 December 2015).
- Szarek, D.; Laska, J.; Jarmundowicz, W.; Błażewicz, S.; Tabakow, P.; Marycz, K.; Woźniak, Z.; Mierzwa, J. Influence of alginates on tube nerve grafts of different elasticity-preliminary in vivo study. J. Biomater. Nanobiotechnol. 2012, 3, 20–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical applications of zinc oxide nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Gao, W. Surface wettability of nanostructured zinc oxide films. J. Electronic Mater. 2009, 38, 601–608. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Biazar, E.; Heidari, M.; Asefnezhad, A.; Montazeri, N. The relationship between cellular adhesion and surface roughness in polystyrene modified by microwave plasma radiation. Int. J. Nanomed. 2011, 6, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothia, P.C.; Chab, S.J.; Yanga, I.J.; Sreekanthc, T.V.M.; Kimb, K.J.; Shina, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B 2015, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, W.; Li, Y.; Wang, G.; Yang, L.; Jin, J.; Chen, Q.; Huang, M. Synthesis, characterization, antimicrobial activity and mechanism of a novel hydroxyapatite whisker/nano zinc oxide biomaterial. Biomed. Mater. 2014, 10, 015001. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Namvar, F.; Rahman, H.S.; Mohamad, R.; Azizi, S.; Tahir, P.M.; Chartrand, M.S.; Yeap, S.K. Cytotoxic effects of biosynthesized zinc oxide nanoparticles on murine cell lines. eCAM 2015, 2015, 593014. [Google Scholar] [CrossRef] [PubMed]
- Everetta, W.N.; Chernb, C.; Suna, D.; McMahonb, R.E.; Zhanga, X.; Chenc, W.J.A.; Hahnb, M.S.; Suea, H.J. Phosphate-enhanced cytotoxicity of zinc oxide nanoparticles and agglomerates. Toxicol. Lett. 2014, 225, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, S.; Mohanan, P.V. Investigation on cellular interactions of astrocytes with zinc oxide nanoparticles using rat C6 cell lines. Coll. Surf. B Biointerfaces 2015, 133, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Syama, S.; Sreekanhth, P.J.; Varma, H.K.; Mohanan, P.V. Zinc oxide nanoparticles induced oxidative stress in mouse bone marrow mesenchymal stem cells. Toxicol. Mech. Methods 2014, 24, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Czochara, M.; Grzywacz, A.; Gdula-Argasińska, J.; Librowski, T.; Wiliński, B.; Opoka, W. The role of zinc in the pathogenesis and treatment of central nervous system (CNS) diseases. Implications of zinc homeostasis for proper CNS function. Acta Pol. Pharm. 2014, 71, 369–377. [Google Scholar] [PubMed]





| Sample | Water contact angle (°) |
|---|---|
| PU/PLDL (control) | 81.3 ± 0.9 |
| PU/PLDL 0.05 ZnO | 95.7 ± 4.5 |
| PU/PLDL 0.01 ZnO | 101.3 ± 5.8 |
| PU/PLDL 0.001 ZnO | 115.7 ± 6.3 |
| Sample code | Rq (nm) | |
|---|---|---|
| Top side | Bottom side | |
| PU/PLDL (control) | 409 ± 65 | 198 ± 78 |
| PU/PLDL 0.001 ZnO | 575 ± 97 | 248 ± 56 |
| PU/PLDL 0.01 ZnO | 613 ± 85 | 267 ± 23 |
| PU/PLDL 0.05 ZnO | 747 ± 109 | 319 ± 92 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marycz, K.; Marędziak, M.; Grzesiak, J.; Szarek, D.; Lis, A.; Laska, J. Polyurethane/Polylactide-Blend Films Doped with Zinc Ions for the Growth and Expansion of Human Olfactory Ensheathing Cells (OECs) and Adipose-Derived Mesenchymal Stromal Stem Cells (ASCs) for Regenerative Medicine Applications. Polymers 2016, 8, 175. https://doi.org/10.3390/polym8050175
Marycz K, Marędziak M, Grzesiak J, Szarek D, Lis A, Laska J. Polyurethane/Polylactide-Blend Films Doped with Zinc Ions for the Growth and Expansion of Human Olfactory Ensheathing Cells (OECs) and Adipose-Derived Mesenchymal Stromal Stem Cells (ASCs) for Regenerative Medicine Applications. Polymers. 2016; 8(5):175. https://doi.org/10.3390/polym8050175
Chicago/Turabian StyleMarycz, Krzysztof, Monika Marędziak, Jakub Grzesiak, Dariusz Szarek, Anna Lis, and Jadwiga Laska. 2016. "Polyurethane/Polylactide-Blend Films Doped with Zinc Ions for the Growth and Expansion of Human Olfactory Ensheathing Cells (OECs) and Adipose-Derived Mesenchymal Stromal Stem Cells (ASCs) for Regenerative Medicine Applications" Polymers 8, no. 5: 175. https://doi.org/10.3390/polym8050175
APA StyleMarycz, K., Marędziak, M., Grzesiak, J., Szarek, D., Lis, A., & Laska, J. (2016). Polyurethane/Polylactide-Blend Films Doped with Zinc Ions for the Growth and Expansion of Human Olfactory Ensheathing Cells (OECs) and Adipose-Derived Mesenchymal Stromal Stem Cells (ASCs) for Regenerative Medicine Applications. Polymers, 8(5), 175. https://doi.org/10.3390/polym8050175