Effect of Small Reaction Locus in Free-Radical Polymerization: Conventional and Reversible-Deactivation Radical Polymerization †
Abstract
:1. Introduction
2. Conventional Free-Radical Polymerization
3. Reversible-Deactivation Radical Polymerization
3.1. Polymerization Rate Expression
3.2. SRMP and ATRP
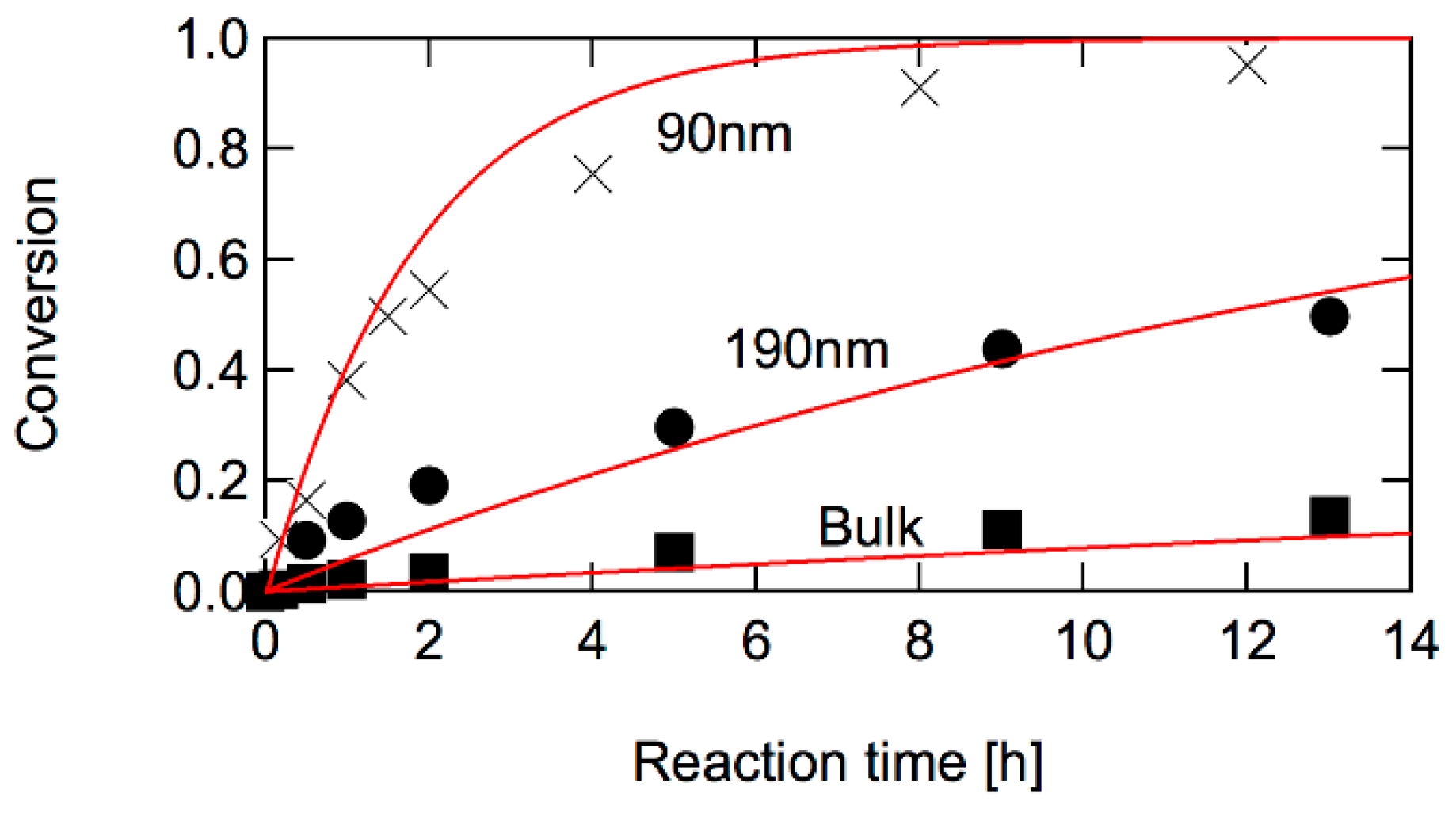
3.3. RAFT Polymerization
3.3.1. Two Conflicting RAFT Models
3.3.2. Application of Threshold Diameter to Discriminate RAFT Models
4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| AIBN | 2,2-azobisisobutyronitrile |
| ATRP | Atom-Transfer Radical Polymerization |
| IT model | Intermediate Termination model |
| RAFT | Reversible-Addition-Fragmentation chain-Transfer |
| RDRP | Reversible-Deactivation Radical Polymerization |
| RGS | Radical Generating Species |
| SF model | Slow Fragmentation model |
| SRMP | Stable-Radical-Mediated Polymerization |
References
- Tobita, H. Polymerization processes, 1. Fundamentals. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Gilbert, R.G. Emulsion Polymerization; Academic Press: London, UK, 1995. [Google Scholar]
- Tobita, H.; Takada, Y.; Nomura, M. Simulation model for the molecular weight distribution in emulsion polymerization. J. Polym. Sci. A 1995, 33, 441–453. [Google Scholar] [CrossRef]
- Tobita, H. Modeling controlled/living radical polymerization kinetics: Bulk and miniemulsion. Macromol. React. Eng. 2010, 4, 643–662. [Google Scholar] [CrossRef]
- Tobita, H. Threshold particle diameters in miniemulsion reversible-deactivation radical polymerization. Polymers 2011, 3, 1944–1971. [Google Scholar] [CrossRef]
- Save, M.; Guillaneuf, Y.; Gilbert, R.G. Controlled radical polymerization in aqueous dispersed media. Aust. J. Chem. 2006, 59, 693. [Google Scholar] [CrossRef]
- Oh, J.K. Recent advances in controlled/living radical polymerization in emulsion and dispersion. J. Polym. Sci. A Polym. Chem. 2008, 46, 6983–7001. [Google Scholar] [CrossRef]
- Cunningham, M.F. Controlled/living radical polymerization in aqueous dispersed systems. Prog. Polym. Sci. 2008, 33, 365–398. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Kagawa, Y.; Okubo, M. Controlled/living radical polymerization in dispersed systems. Chem. Rev. 2008, 108, 3747–3794. [Google Scholar] [CrossRef] [PubMed]
- Zetterlund, P.B. Controlled/living radical polymerization in nanoreactors: Compartmentalization effects. Polym. Chem. 2011, 2, 534–549. [Google Scholar] [CrossRef]
- Bechthold, N.; Landfester, K. Kinetics of miniemulsion polymerization as revealed by Calorimetry. Macromolecules 2000, 33, 4682–4689. [Google Scholar] [CrossRef]
- Tobita, H.; Takada, Y.; Nomura, M. Molecular weight distribution in emulsion polymerization. Macromolecules 1994, 27, 3804–3811. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Jones, R.G.; Moad, G. Terminology for reversible-deactivation radical polymerization previously called “controlled” radical or “living” radical polymerization (IUPAC Recommendations 2010). Pure Appl. Chem. 2010, 82, 483–491. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Okubo, M. Compartmentalization in TEMPO-mediated radical polymerization in dispersed systems: Effects of macroinitiator concentration. Macromol. Theory Simul. 2007, 16, 221–226. [Google Scholar] [CrossRef]
- Tobita, H. Kinetics of stable free radical mediated polymerization inside submicron particles. Macromol. Theory Simul. 2007, 16, 810–823. [Google Scholar] [CrossRef]
- Tobita, H. Effects of fluctuation and segregation in the rate acceleration of ARTP miniemulsion polymerization. Macromol. Theory Simul. 2011, 20, 179–190. [Google Scholar] [CrossRef]
- Maehata, H.; Buragina, C.; Cunningham, M.; Keoshkerian, B. Compartmentalization in TEMPO-mediated styrene miniemulsion polymerization. Macromolecules 2007, 40, 7126–7131. [Google Scholar] [CrossRef]
- Monteiro, M.J.; Brouwer, H. Intermediate radical termination as the mechanism for retardation in reversible addition-fragmentation chain transfer polymerization. Macromolecules 2001, 34, 349–352. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Quinn, J.F.; Uyen Nguyen, T.L.; Heuts, J.P.A.; Davis, T.P. Kinetic investigations of reversible addition fragmentation chain transfer polymerizations: Cumyl phenyldithioacetate mediated homopolymerizations of styrene and methyl methacrylate. Macromolecules 2001, 34, 7849–7857. [Google Scholar] [CrossRef]
- Wang, A.R.; Zhu, S.; Kwak, Y.; Goto, A.; Fukuda, T.; Monteiro, M.S. A difference of six orders of magnitude: A reply to “the magnitude of the fragmentation rate coefficient”. J. Polym. Sci. A 2003, 41, 2833–2839. [Google Scholar] [CrossRef]
- Tobita, H.; Yanase, F. Monte carlo simulation of controlled/living radical polymerization in emulsified systems. Macromol. Theory Simul. 2007, 16, 476–488. [Google Scholar] [CrossRef]
- Tobita, H. RAFT miniemulsion polymerization kinetics, 1. Polymerization rate. Macromol. Theory Simul. 2009, 18, 108–119. [Google Scholar] [CrossRef]
- Tobita, H. On the discrimination of RAFT models using miniemulsion polymerization. Macromol. Theory Simul. 2013, 22, 399–409. [Google Scholar] [CrossRef]
- Suzuki, K.; Nishimura, Y.; Kanematsu, Y.; Masuda, Y.; Satoh, S.; Tobita, H. Experimental validation of intermediate tertmination in RAFT polymerization with dithiobenzoate via comparison of miniemulsion and bulk polymerization rate. Macromol. React. Eng. 2012, 6, 17–23. [Google Scholar] [CrossRef]
- Suzuki, K.; Kanematsu, Y.; Miura, T.; Minami, M.; Satoh, S.; Tobita, H. Experimental method to discriminate RAFT models between intermediate termination and slow fragmentation via comparison of rates of miniemulsion and bulk polymerization. Macromol. Theory Simul. 2014, 23, 136–146. [Google Scholar] [CrossRef]
- Meiser, W.; Buback, M.; Ries, O.; Ducho, C.; Sidoruk, A. EPR study into cross-termination and fragmentation with the phenylethyl-phenylethyl dithiobenzoate RAFT Model System. Macromol. Chem. Phys. 2013, 214, 924–933. [Google Scholar] [CrossRef]
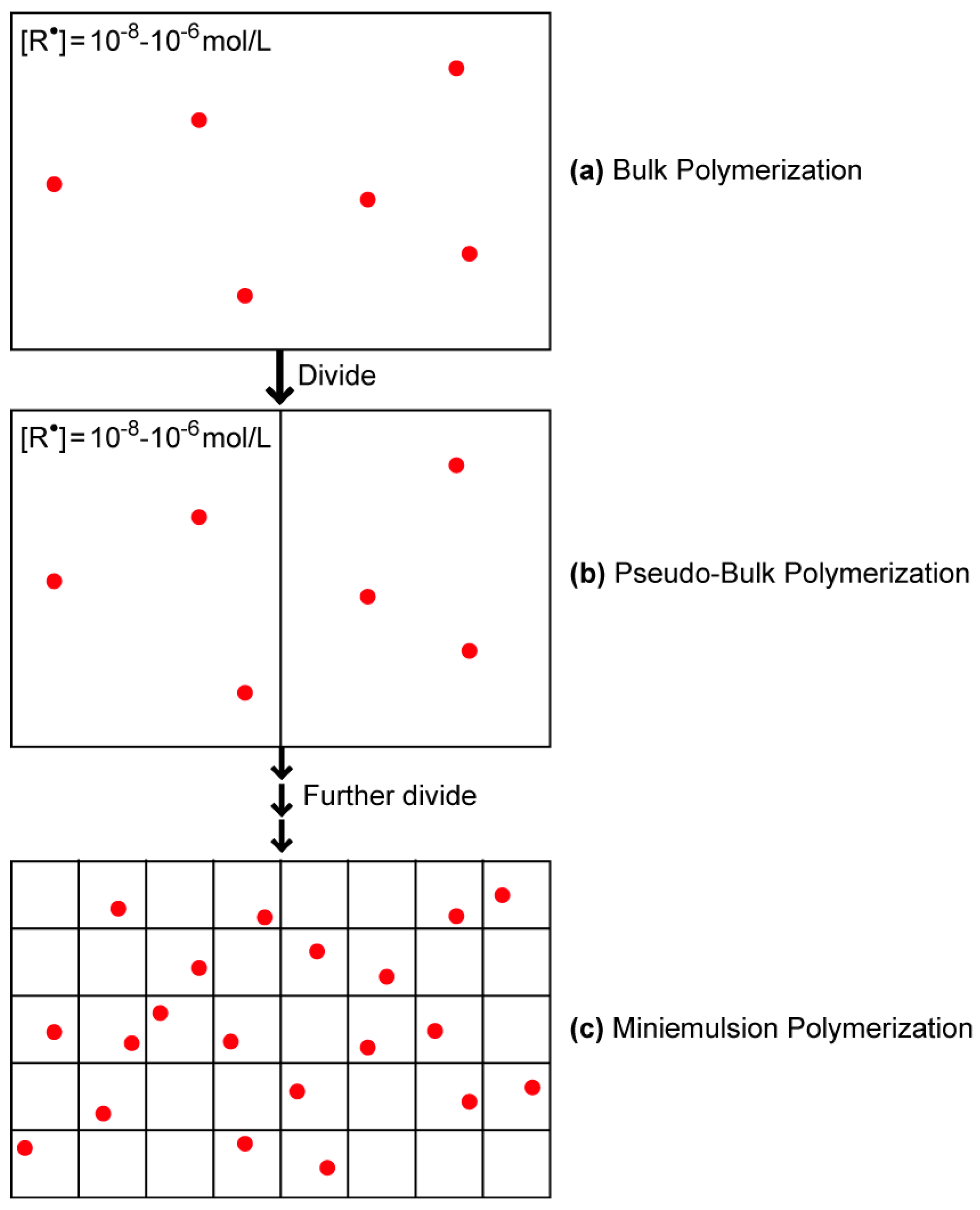
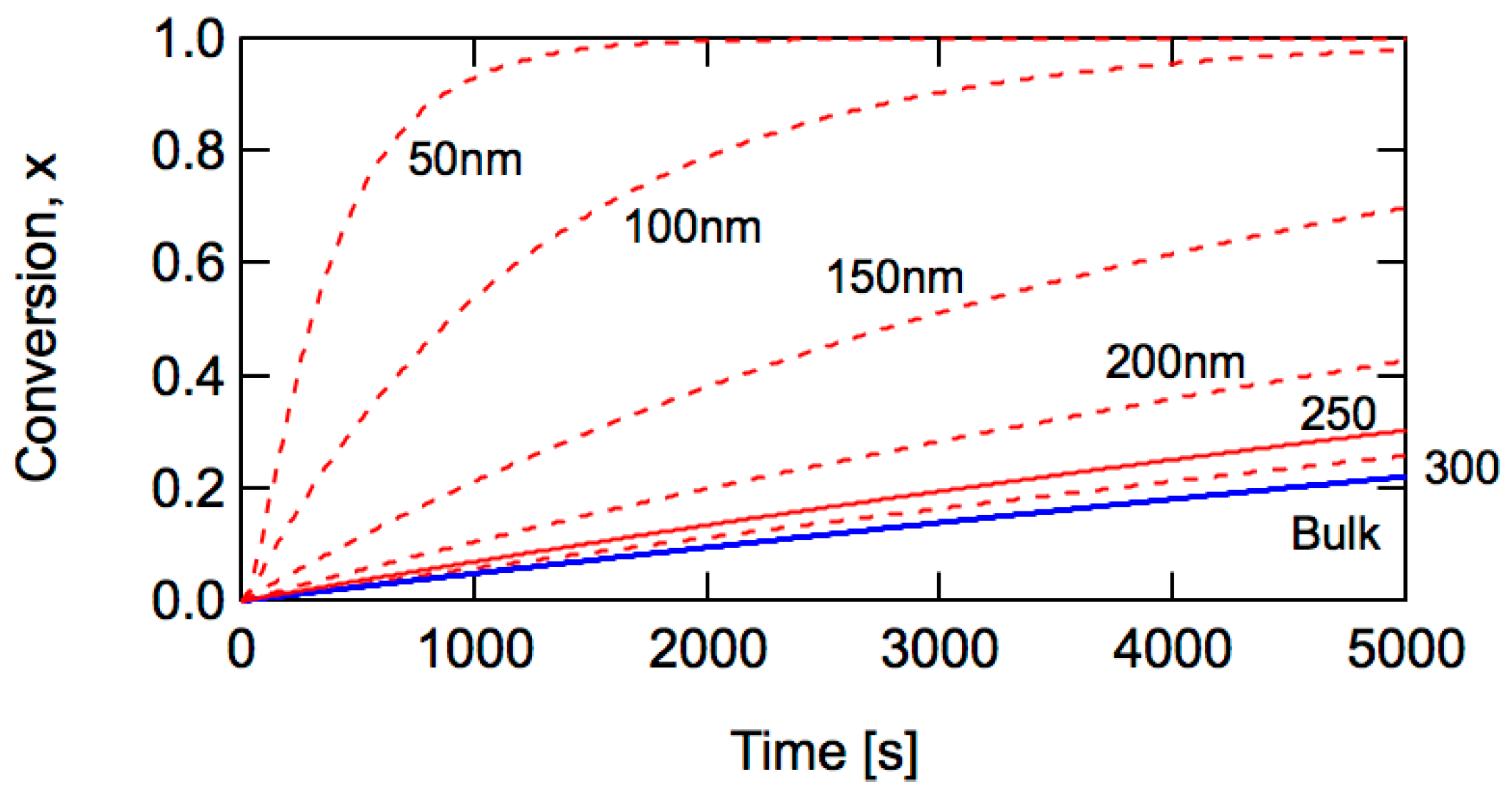


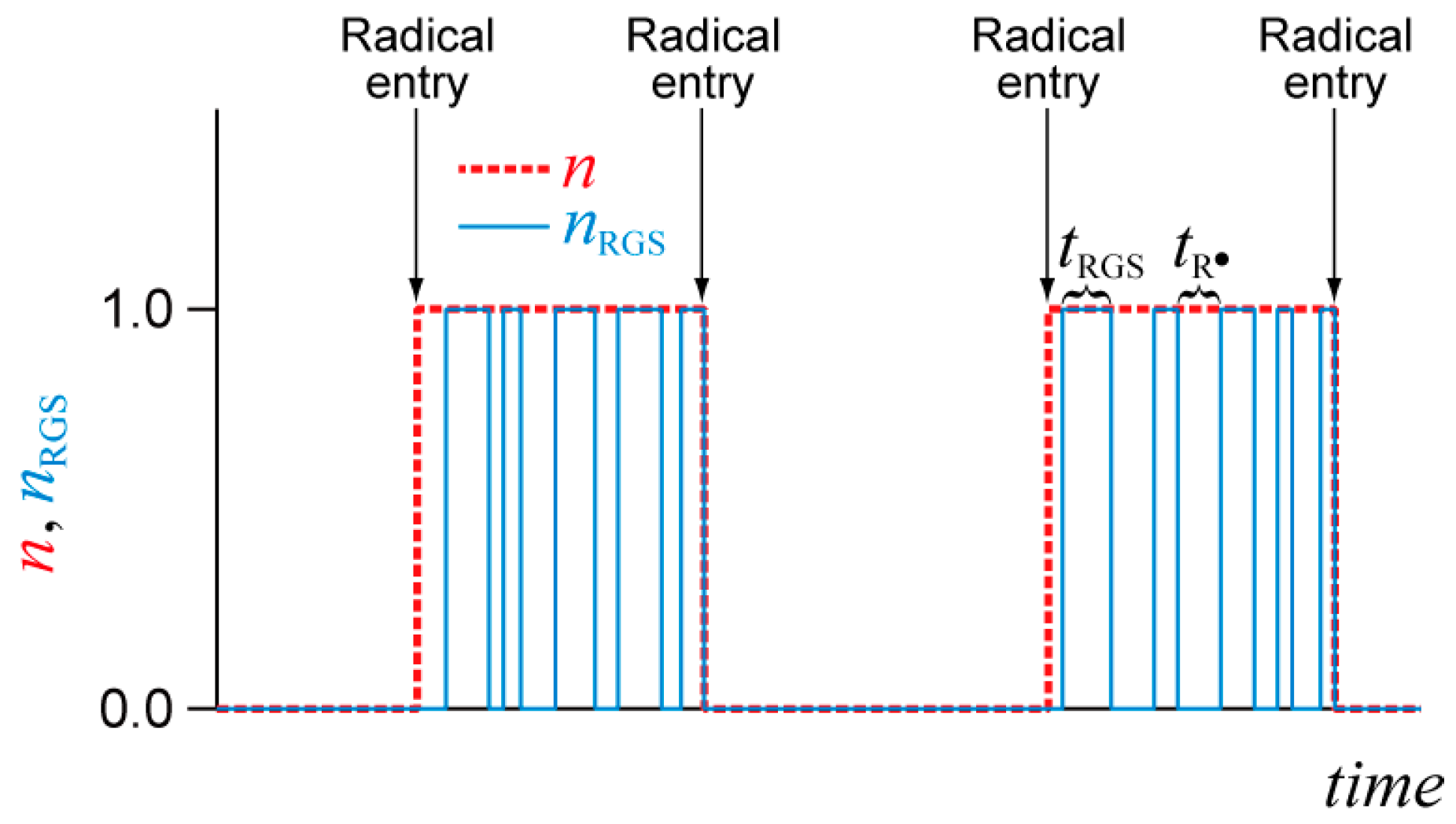
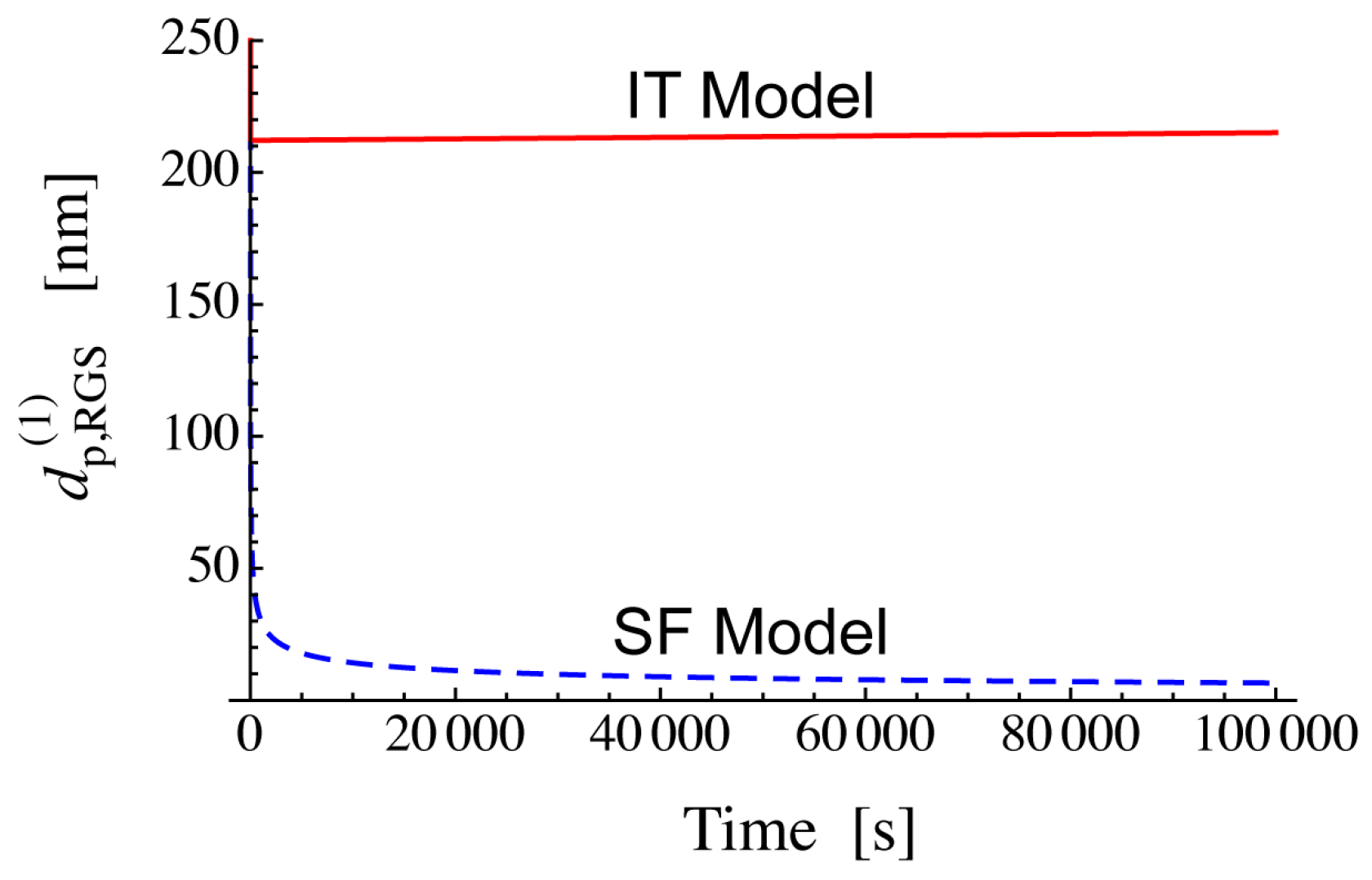

| Particle Diameter dp (nm) | Concentration (mol/L) |
|---|---|
| 1000 | 3.18 × 10−9 |
| 200 | 3.97 × 10−7 |
| 100 | 3.18 × 10−6 |
| 50 | 2.55 × 10−5 |
| 25 | 2.04 × 10−4 |
| Type of Polymerization | Threshold Diameter | Polymerization Rate | |
|---|---|---|---|
| Conventional FRP | Increases for dp smaller than |  | |
| SRMP, ATRP | Decreases for dp smaller than |  | |
| RAFT | Increases for dp smaller than |  | |
© 2016 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobita, H. Effect of Small Reaction Locus in Free-Radical Polymerization: Conventional and Reversible-Deactivation Radical Polymerization. Polymers 2016, 8, 155. https://doi.org/10.3390/polym8040155
Tobita H. Effect of Small Reaction Locus in Free-Radical Polymerization: Conventional and Reversible-Deactivation Radical Polymerization. Polymers. 2016; 8(4):155. https://doi.org/10.3390/polym8040155
Chicago/Turabian StyleTobita, Hidetaka. 2016. "Effect of Small Reaction Locus in Free-Radical Polymerization: Conventional and Reversible-Deactivation Radical Polymerization" Polymers 8, no. 4: 155. https://doi.org/10.3390/polym8040155
APA StyleTobita, H. (2016). Effect of Small Reaction Locus in Free-Radical Polymerization: Conventional and Reversible-Deactivation Radical Polymerization. Polymers, 8(4), 155. https://doi.org/10.3390/polym8040155







