The Effects of in Situ-Formed Silver Nanoparticles on the Electrical Properties of Epoxy Resin Filled with Silver Nanowires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
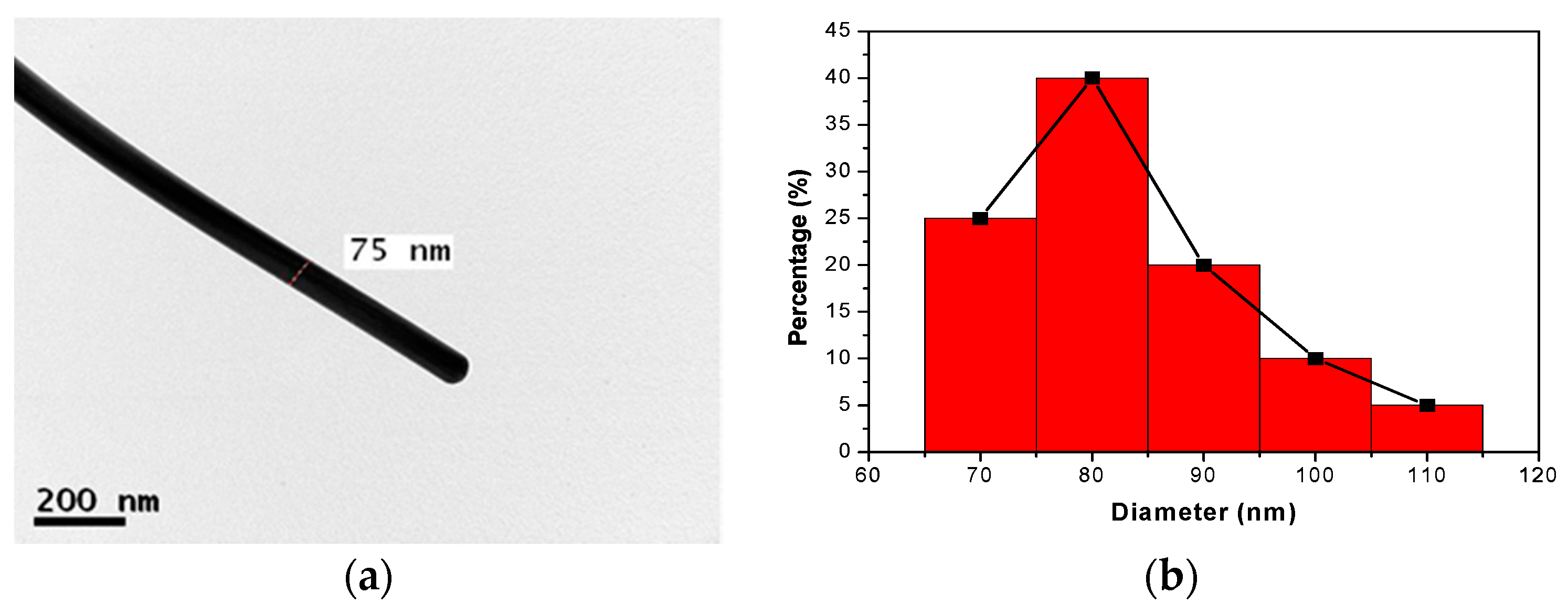
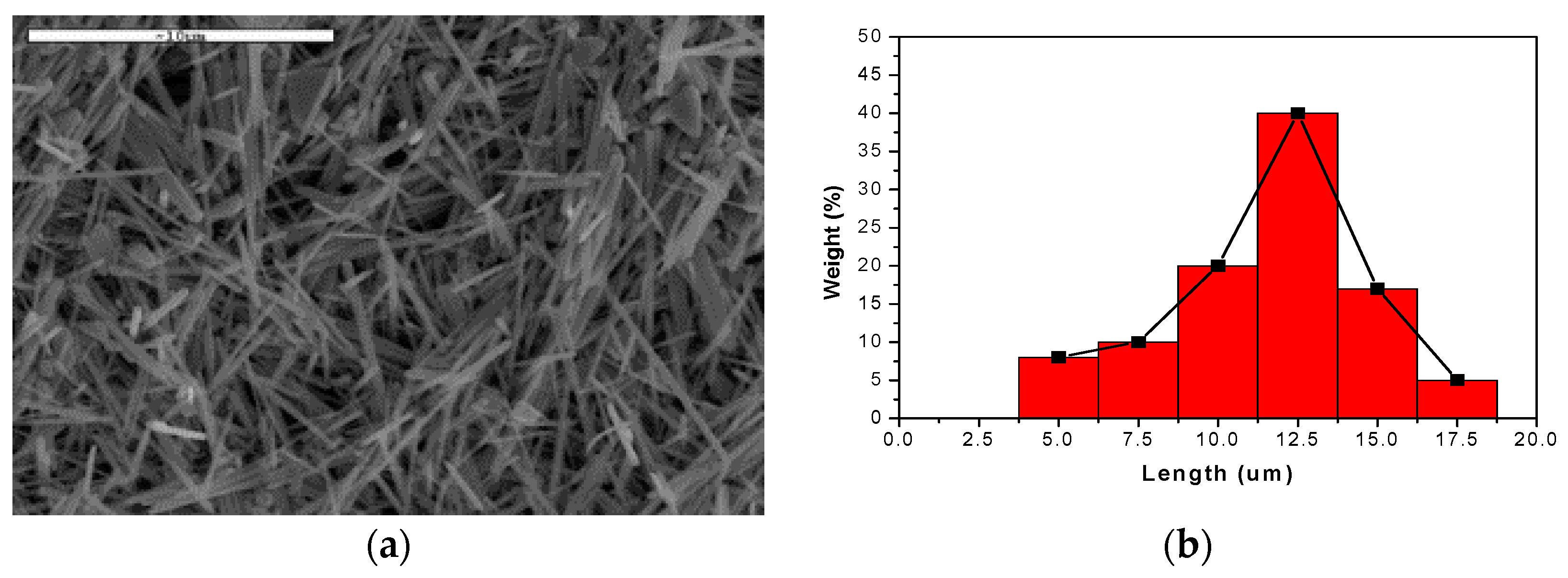
2.2. Synthesis of Silver Nanowires (AgNWs) Using a Microwave Process
2.3. Preparation of Silver–Imidazole Complex
2.4. Preparation of Epoxy/Silver Nanocomposites with AgNWs
2.5. Characterization
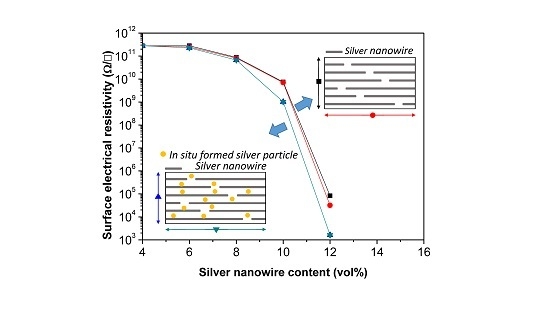
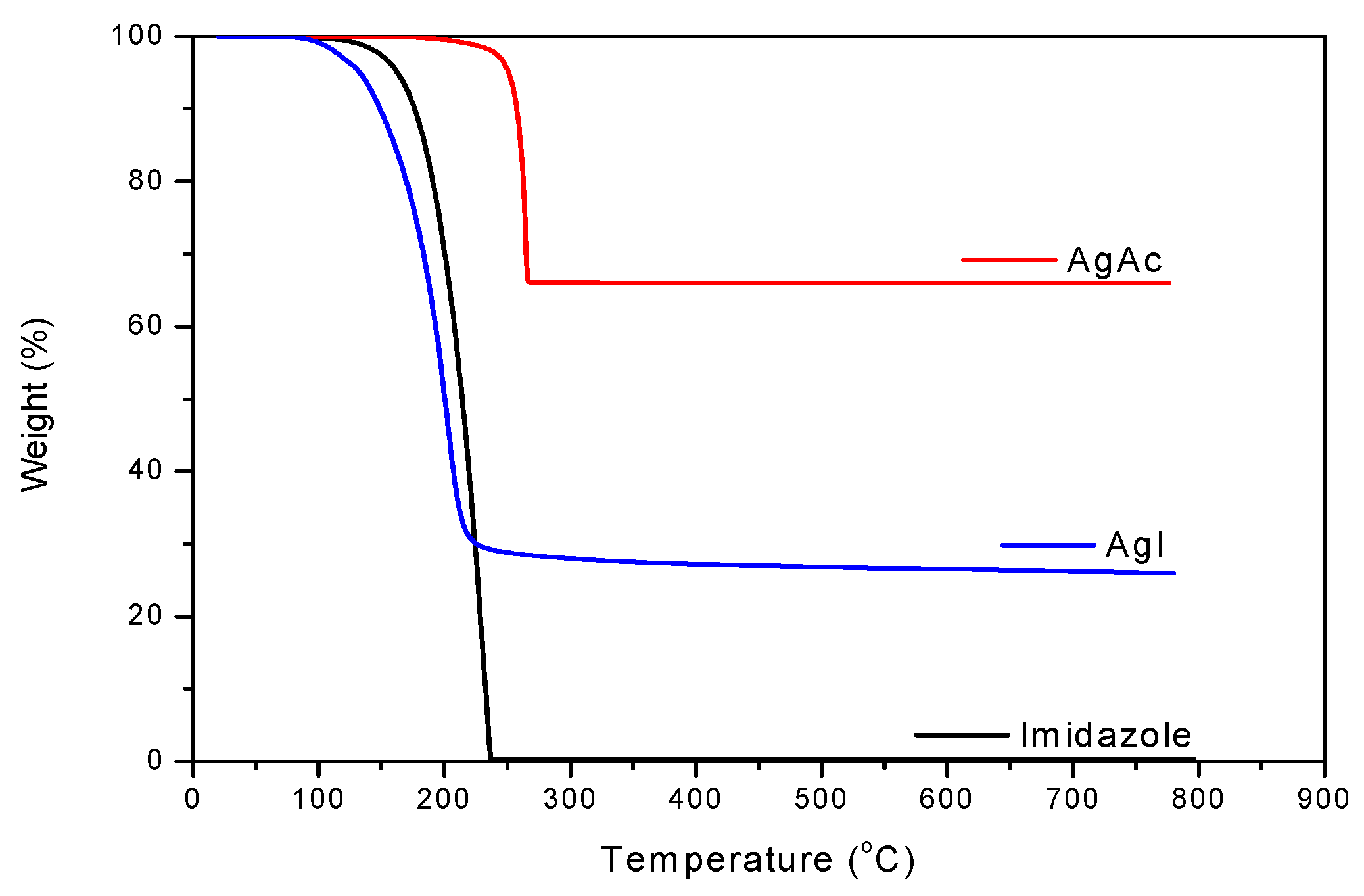
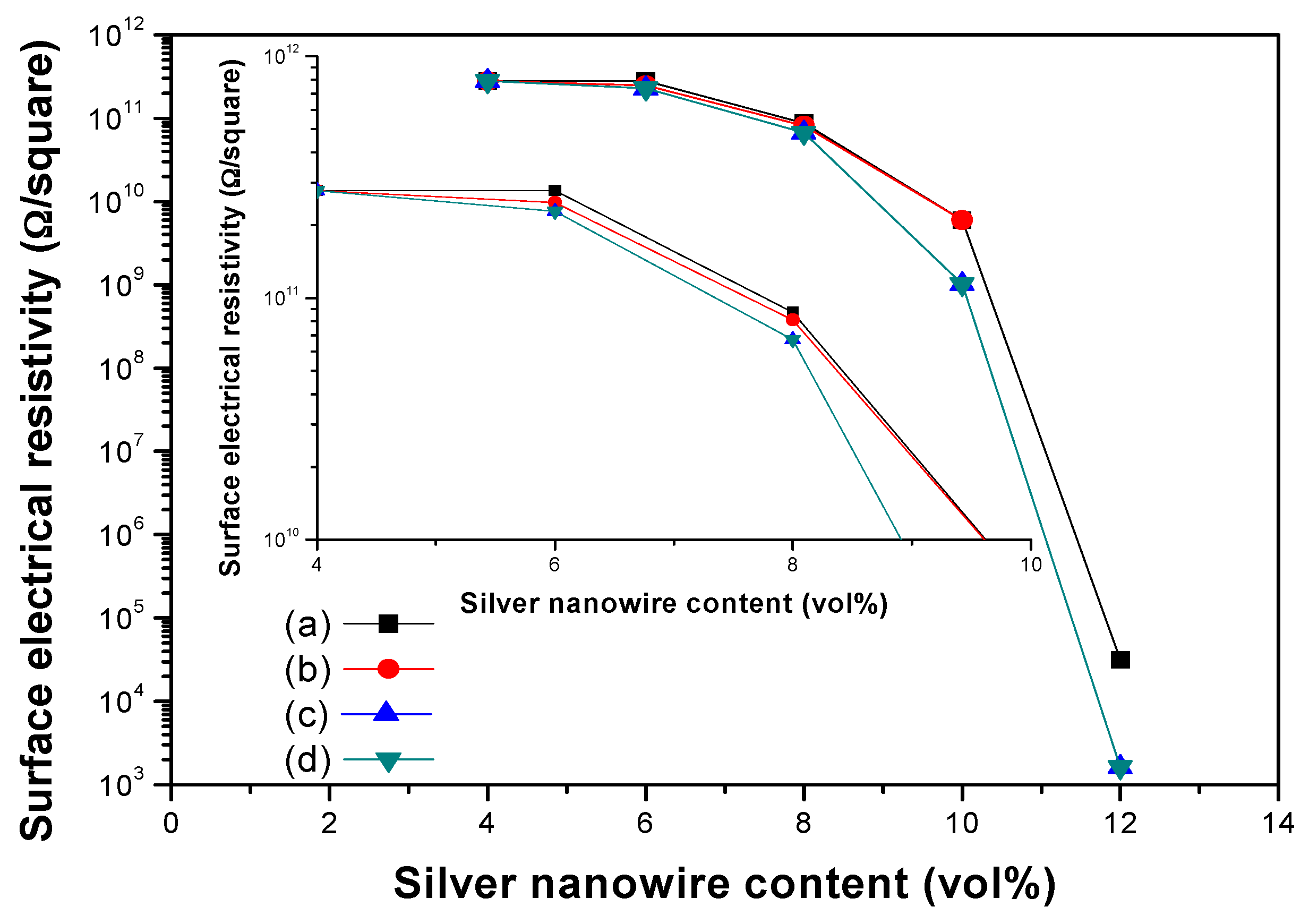
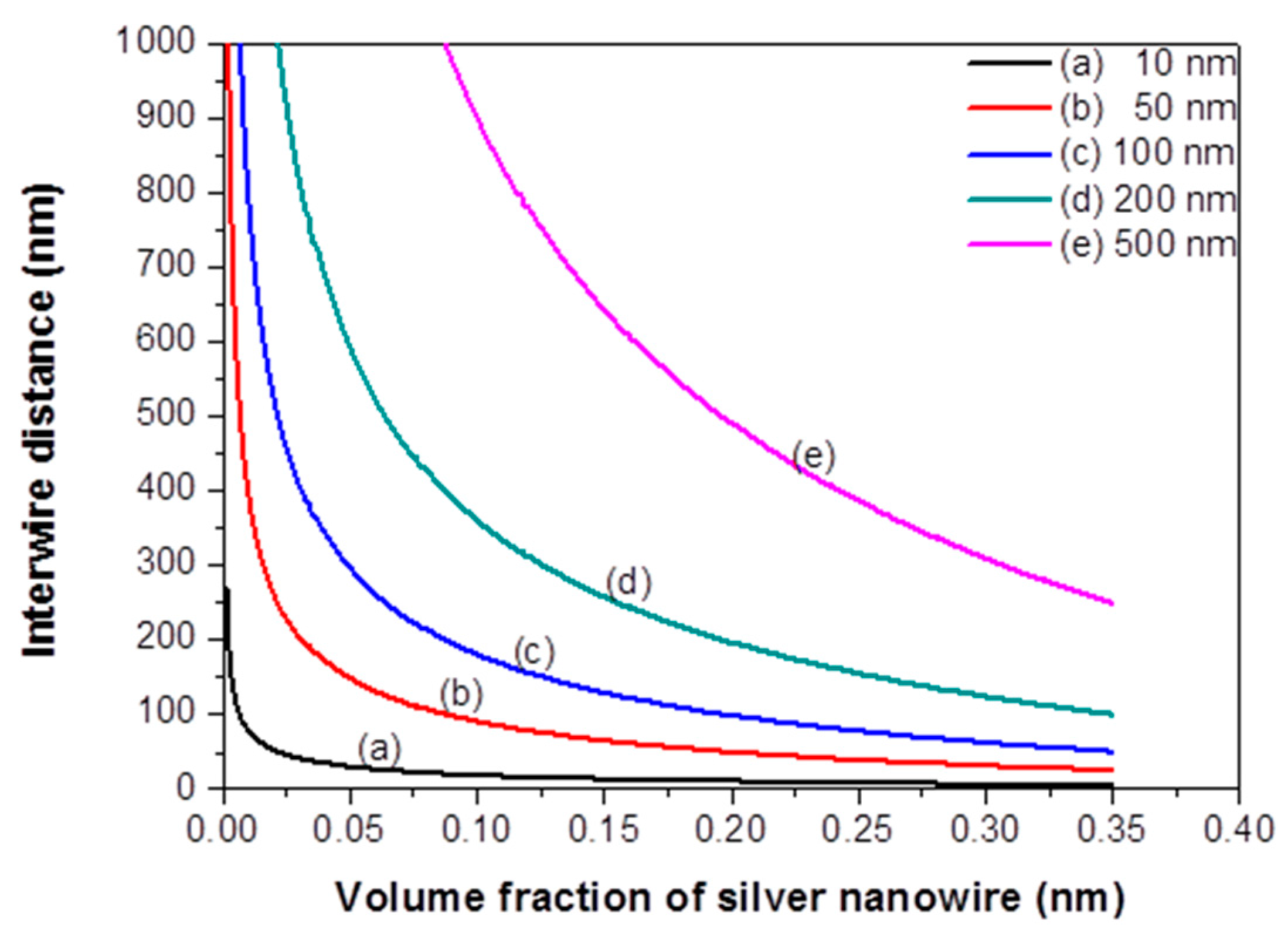
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yagci, Y.; Sangermano, M.; Rizza, G. Synthesis and Characterization of Gold-Epoxy Nanocomposites by Visible Light Photoinduced Electron Transfer and Cationic Polymerization Processes. Macromolecules 2008, 41, 7268–7270. [Google Scholar] [CrossRef]
- Maruo, M.; Acocella, M.R.; Corcione, C.S.; Maffezzoli, A.; Guera, G. Catalytic activity of graphite-based nanofillers on cure reaction of epoxy resins. Polymer 2014, 55, 5612–5615. [Google Scholar] [CrossRef]
- Greco, A.; Corcione, C.S.; Maffezzoli, A. Effect of multi-scale diffusion on the permeability behavior of intercalated nanocomposites. J. Membr. Sci. 2016, 505, 92–99. [Google Scholar] [CrossRef]
- Lee, H.; Chou, K.; Shih, Z. Effect of nano-sized silver particles on the resistivity of polymeric conductive adhesives. Int. J. Adhes. Adhes. 2005, 25, 437–441. [Google Scholar] [CrossRef]
- Jiang, H.; Moon, K.; Li, Y.; Wong, C.P. Surface functionalized silver nanoparticles for ultrahigh conductive polymer composites. Chem. Mater. 2006, 18, 2969–2973. [Google Scholar] [CrossRef]
- Lu, J.; Moon, K.; Wong, C.P. Silver/polymer nanocomposite as a high-k polymer matrix for dielectric composites with improved dielectric performance. J. Mater. Chem. 2008, 18, 4821–4826. [Google Scholar] [CrossRef]
- Konghua, L.; Lan, L.; Yuanfang, L.; Demin, J. One-step synthesis of metal nanoparticle decorated graphene by liquid phase exfoliation. J. Mater. Chem. 2012, 22, 20342–20352. [Google Scholar]
- Ghosh, K.; Maiti, S.N. Mechanical properties of silver-powder-filled polypropylene composites. J. Appl. Polym. Sci. 1996, 60, 323–331. [Google Scholar] [CrossRef]
- Sastry, M.; Mayya, K.S.; Bandyopadhyay, K. pH dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 1997, 127, 221–228. [Google Scholar] [CrossRef]
- Shipway, A.; Lahav, M.; Gabai, R.; Willner, I. Investigations into the Electrostatically Induced Aggregation of Au Nanoparticles. Langmuir 2000, 16, 8789–8795. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, X.; Cui, Y.; Lauhon, L.; Kim, K.; Lieber, C. Logic gates and computation from assembled nanowire building blocks. Science 2001, 294, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Gole, A.; Lala, L.; Gonnade, R.; Ganvir, V.; Sastry, M. Studies on the Reversible Aggregation of Cysteine-Capped Colloidal Silver Particles Interconnected via Hydrogen Bonds. Langmuir 2001, 17, 6262–6268. [Google Scholar] [CrossRef]
- Gudiksen, M.S.; Lauhon, L.J.; Wang, J.; Smith, D.C.; Lieber, C.M. Growth of nanowire superlattice structures for nanoscale photonics and electronics. Nature 2002, 415, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Sauer, G.; Brehm, G.; Schneider, S. Highly ordered monocrystalline silver nanowire arrays. J. Appl. Phys. 2002, 91, 3243–3247. [Google Scholar] [CrossRef]
- Dang, M.; Shen, Y.; Nan, C.W. Dielectric behavior of three-phase percolative Ni–BaTiO3/polyvinylidene fluoride composites. Appl. Phys. Lett. 2002, 81, 4814–4816. [Google Scholar] [CrossRef]
- Tao, A.; Kim, F.; Hess, C.; Goldberger, J.; He, R.; Sun, Y.; Xia, Y.; Yang, P. Langmuir-Blodgett silver nanowire monolayers for molecular sensing using surface-enhanced Raman spectroscopy. Nano Lett. 2003, 3, 1229–1233. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Langmuir-Blodgett silver nanowire monolayers for molecular sensing using surface-enhanced Raman spectroscopy. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yin, Y.; Mayers, B.; Herricks, T.; Xia, Y. Uniform Silver Nanowires Synthesis by Reducing AgNO3 with Ethylene Glycol in the Presence of Seeds and Poly(Vinyl Pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nakamoto, M. Novel preparation of monodispersed silver nanoparticles via amine adducts derived from insoluble silver myristate in tertiary alkylamine. J. Mater. Chem. 2003, 13, 2064–2065. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Polyol Synthesis of Uniform Silver Nanowires: A Plausible Growth Mechanism and the Supporting Evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Pothukuchi, S.; Li, Y.; Wong, C.P. Development of a novel polymer–metal nanocomposite obtained through the route of in situ reduction for integral capacitor application. J. Appl. Polym. Sci. 2004, 93, 1531–1538. [Google Scholar] [CrossRef]
- Wiley, B.; Herricks, T.; Sun, Y.; Xia, Y. Polyol Synthesis of Silver Nanoparticles: Use of Chloride and Oxygen to Promote the Formation of Single-Crystal, Truncated Cubes and Tetrahedrons. Nano Lett. 2004, 4, 1733–1739. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y.; Zeng, X.; Zeng, Y.; Wang, Y. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci. 2005, 288, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Xiong, Y.; Li, Z.; Yin, Y.; Xia, Y. Right Bipyramids of Silver: A New Shape Derived from Single Twinned Seeds. Nano Lett. 2006, 6, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M.; Yagci, Y.; Rizza, G. In Situ Synthesis of Silver-Epoxy Nanocomposites by Photoinduced Electron Transfer and Cationic Polymerization Processes. Macromolecules 2007, 40, 8827–8829. [Google Scholar] [CrossRef]
- Zhang, R.; Moon, K.; Lin, W.; Wong, C.P. Preparation of highly conductive polymer nanocomposites by low temperature sintering of silver nanoparticles. J. Mater. Chem. 2010, 20, 2018–2023. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Tsuji, T. Synthesis of gold nanorods and nanowires by a microwave–polyol method. Mater. Lett. 2004, 58, 2326–2330. [Google Scholar] [CrossRef]
- Judd, M.D.; Plunkett, B.A.; Pope, M.I. The thermal decomposition of calcium, sodium, silver and copper(II) acetate. J. Therm. Anal. Calorim. 1974, 6, 555–563. [Google Scholar] [CrossRef]
- Corcione, C.E.; Maffezzoli, A. Transport properties of graphite/epoxy composites: Thermal, permeability and dielectric characterization. Polym. Test. 2013, 32, 880–888. [Google Scholar] [CrossRef]
- Hao, T.; Riman, R.E. Calculation of interparticle spacing in colloidal systems. J. Colloid Interface Sci. 2006, 297, 374–377. [Google Scholar] [CrossRef] [PubMed]
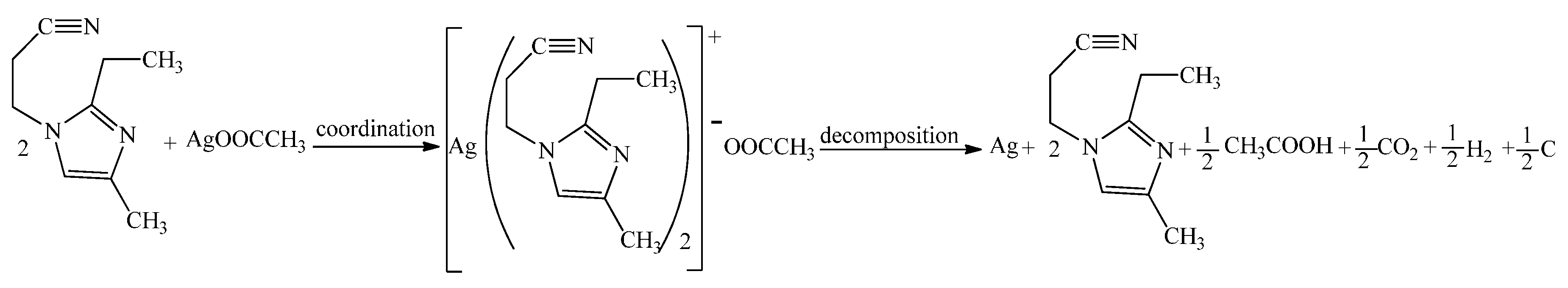
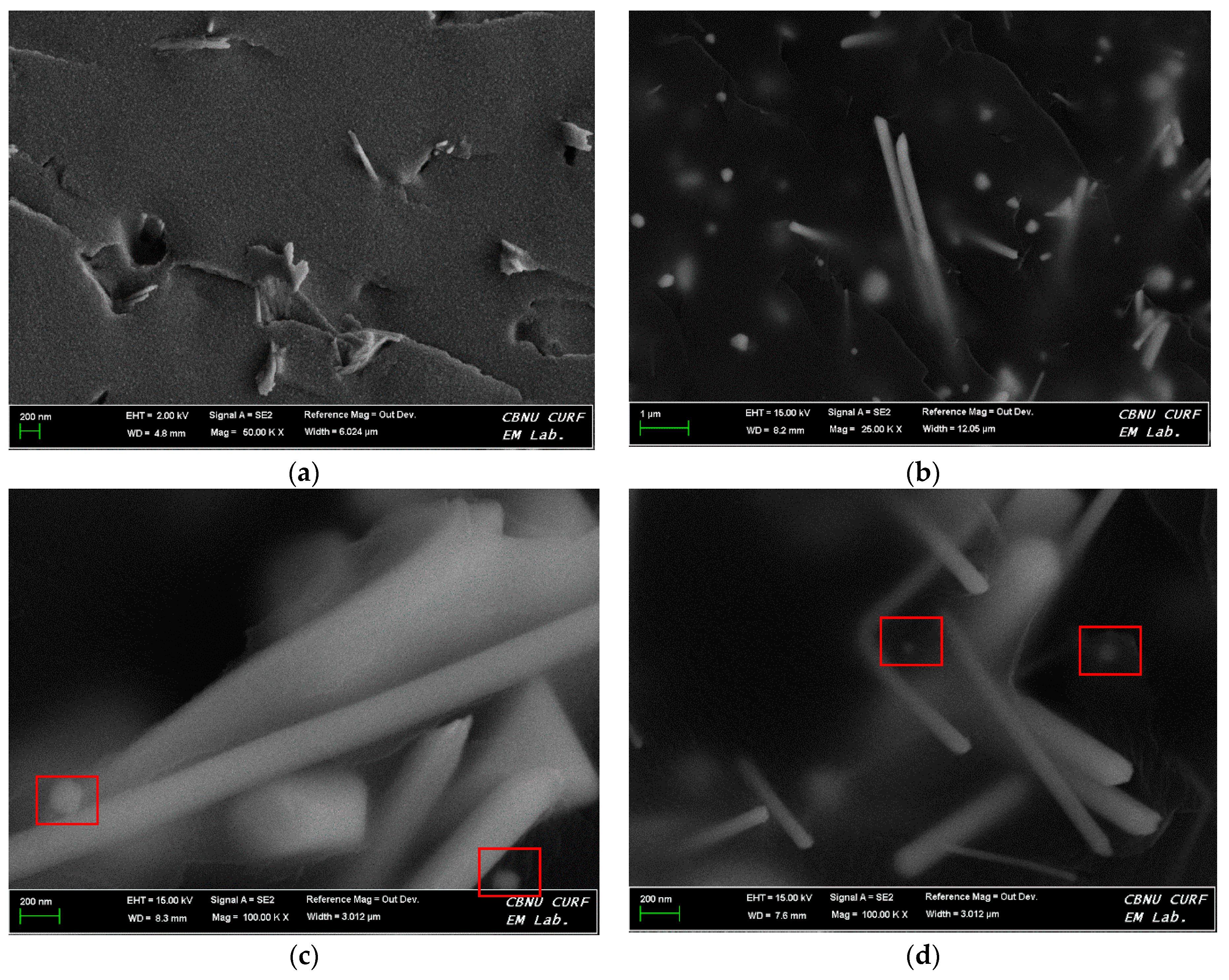
| Element | Epoxy Resin Cured with the Silver–Imidazole Complex | Epoxy Resin Cured with Imidazole | ||
|---|---|---|---|---|
| Wt % | At % | Wt % | At % | |
| C | 67.86 | 75.50 | 69.48 | 75.62 |
| O | 28.85 | 24.09 | 30.52 | 24.38 |
| Ag | 3.29 | 00.41 | 0.00 | 0.00 |
| Sample | (a) | (b) | (c) | (d) |
|---|---|---|---|---|
| Pc (vol %) | 12.60 | 12.80 | 12.50 | 12.50 |
| t | 4.29 | 4.57 | 4.49 | 4.49 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.-S.; Lee, D.S.; Kang, I. The Effects of in Situ-Formed Silver Nanoparticles on the Electrical Properties of Epoxy Resin Filled with Silver Nanowires. Polymers 2016, 8, 157. https://doi.org/10.3390/polym8040157
Song G-S, Lee DS, Kang I. The Effects of in Situ-Formed Silver Nanoparticles on the Electrical Properties of Epoxy Resin Filled with Silver Nanowires. Polymers. 2016; 8(4):157. https://doi.org/10.3390/polym8040157
Chicago/Turabian StyleSong, Gwang-Seok, Dai Soo Lee, and Ilho Kang. 2016. "The Effects of in Situ-Formed Silver Nanoparticles on the Electrical Properties of Epoxy Resin Filled with Silver Nanowires" Polymers 8, no. 4: 157. https://doi.org/10.3390/polym8040157
APA StyleSong, G.-S., Lee, D. S., & Kang, I. (2016). The Effects of in Situ-Formed Silver Nanoparticles on the Electrical Properties of Epoxy Resin Filled with Silver Nanowires. Polymers, 8(4), 157. https://doi.org/10.3390/polym8040157