Polylactide/Montmorillonite Hybrid Latex as a Barrier Coating for Paper Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Instruments
2.2. Preparation of the PLA/MMT Latex
2.3. Coating Procedures
3. Results and Discussion
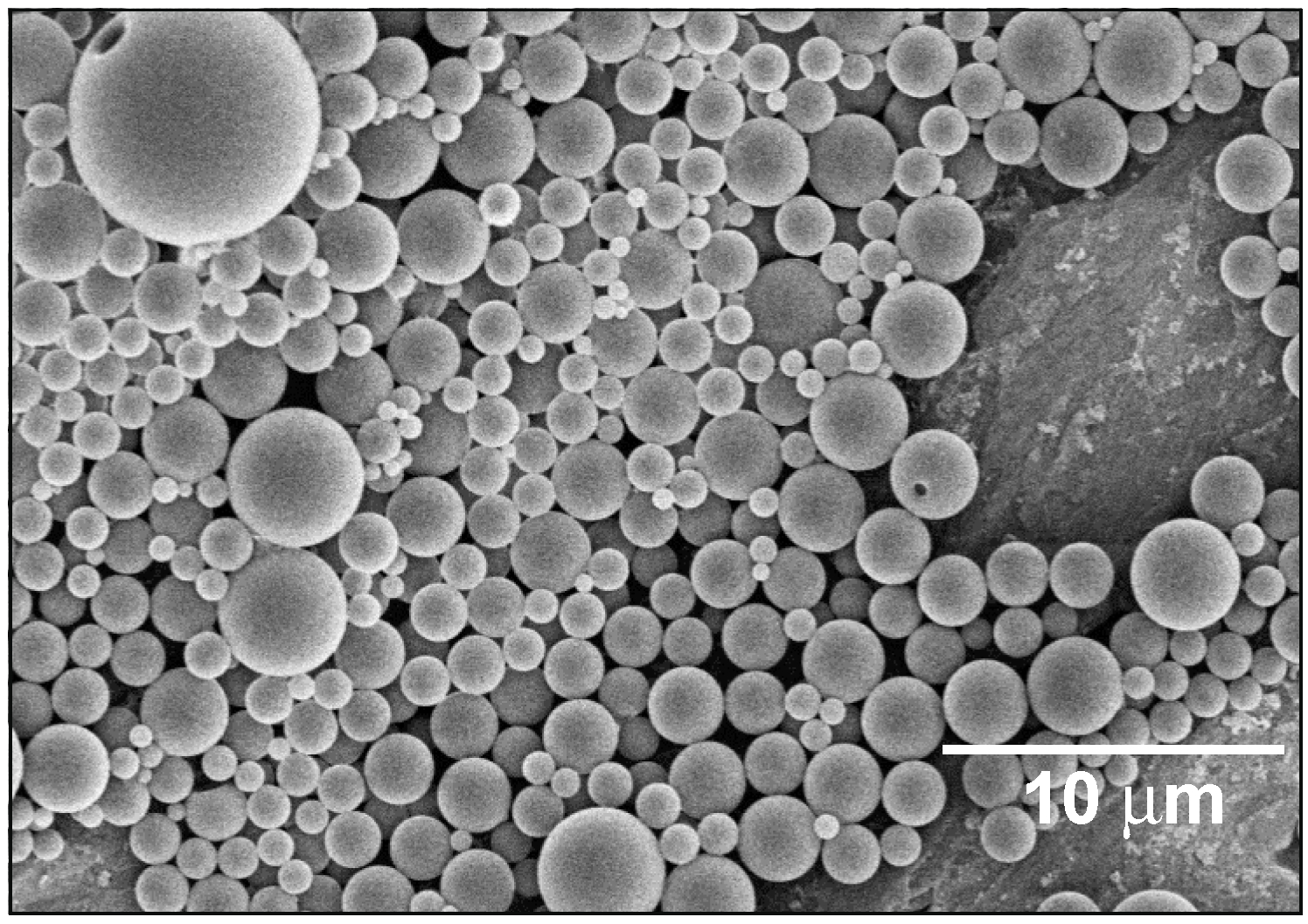
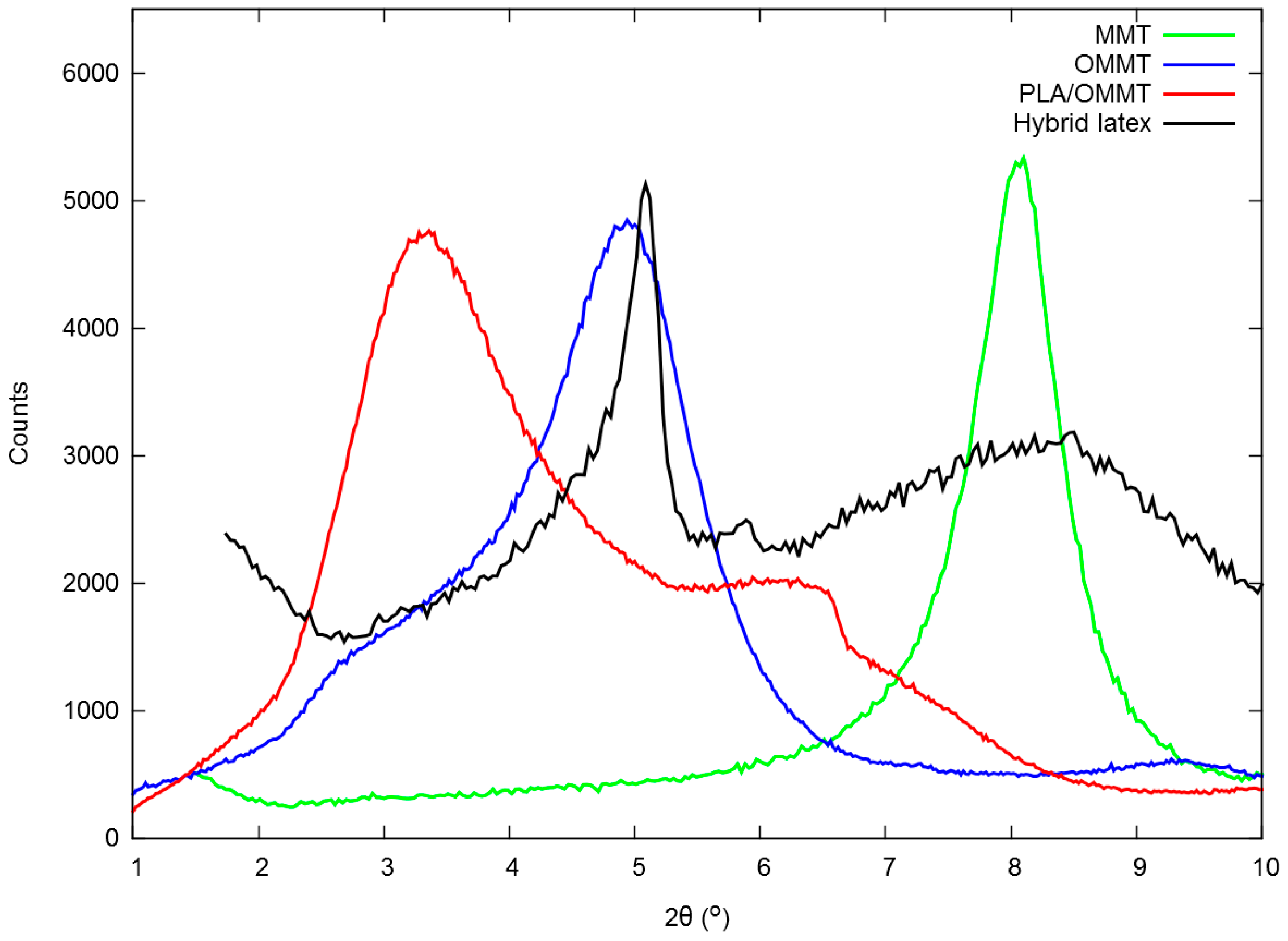
3.1. Properties of the PLA/MMT Latex
3.2. Properties of the Neat PLA/MMT Films
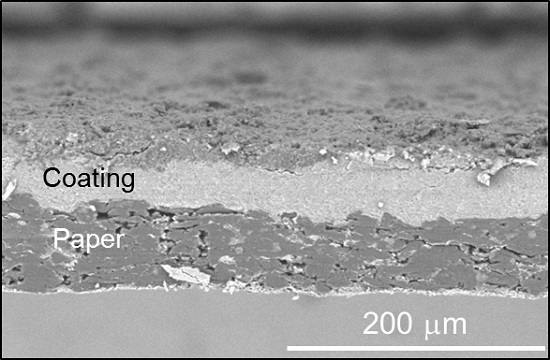
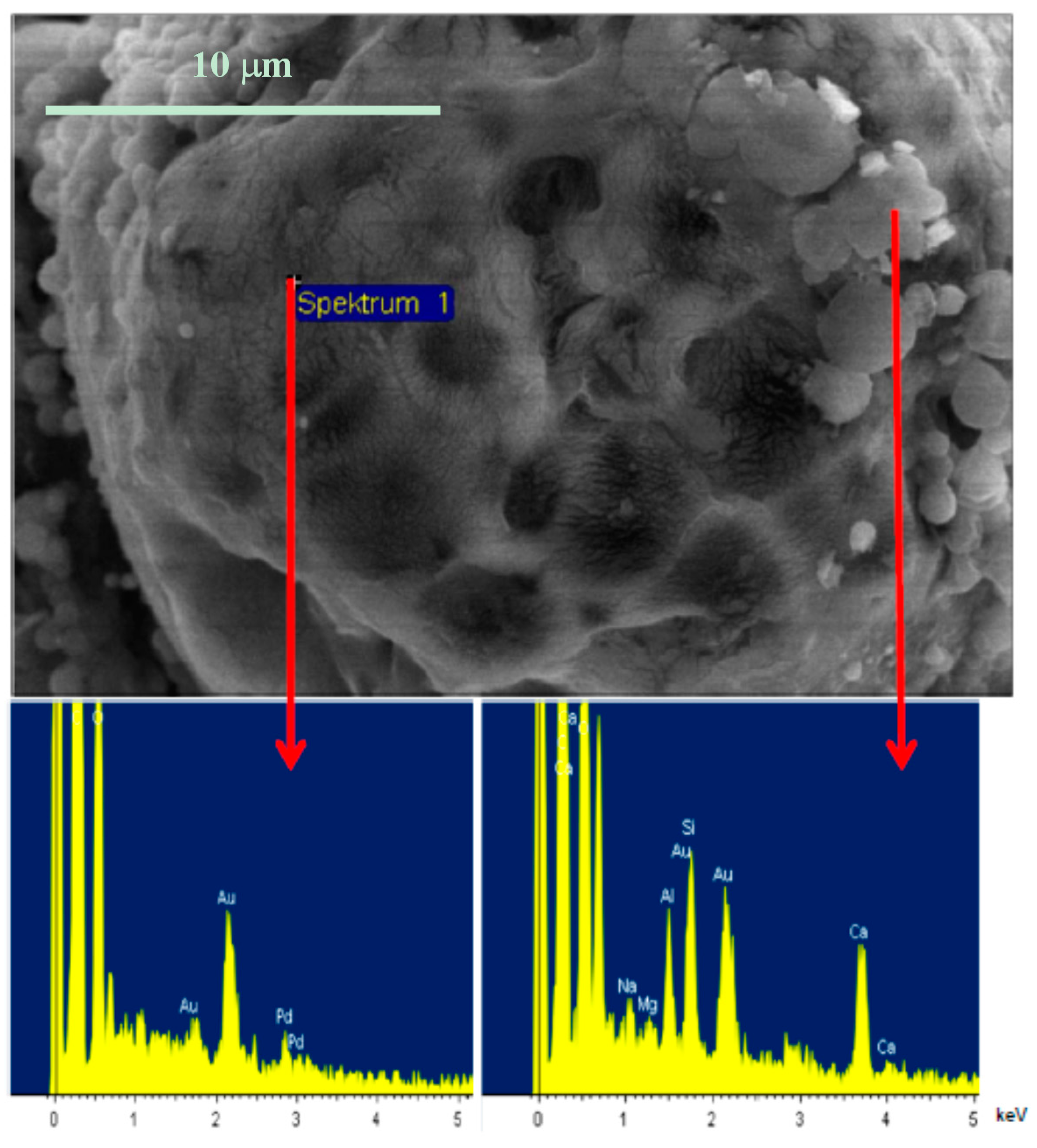
3.3. Properties of Papers Coated with PLA/MMT Latex
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duffy, E.; Gibney, M.J. Use of a food-consumption database with packaging information to estimate exposure to food-packaging migrants: Epoxidized soybean oil and styrene monomer. Food Add. Contamin. 2007, 24, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, M.; Grob, K. Is recycled newspaper suitable for food contact materials? Technical grade mineral oils from printing inks. Eur. Food Res. Technol. 2010, 230, 785–796. [Google Scholar] [CrossRef]
- Poças, M.F.; Oliveira, J.C.; Pereira, J.R.; Hogg, T. Consumer exposure to phthalates from paper packaging: An integrated approach. Food Add. Contamin. Part. A 2010, 27, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, M.; Grob, K. On-line coupled high performance liquid chromatography-gas chromatography for the analysis of contamination by mineral oil. Part 2: Migration from paper board into dry foods: Interpretation of chromatograms. J. Chromatogr. A 2012, 1255, 76–99. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, T.; Fasano, E.; Esposito, F.; del Prete, E.; Amodio Cocchieri, R. Study on the influence of temperature, storage time and packaging type on di-n-butylphthalate and di(2-ethylhexyl)phthalate release into packed meals. Food Add. Contamin. Part A 2013, 30, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Aulin, C.; Lindström, T. Biopolymer coatings for paper and paperboard. In Biopolymers—New Materials for Sustainable Films and Coatings; Plackett, D., Ed.; Wiley: Chichester, UK, 2011; pp. 255–276. [Google Scholar]
- Guazzotti, V.; Marti, A.; Piergiovanni, L.; Limbo, S. Bio-based coatings as potential barriers to chemical contaminants from recycled paper and board for food packaging. Food Add. Contamin. Part A 2014, 31, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Boesel, L.F.; De Geus, M.; Thöny-Meyer, L. Effect of PLA crystallization on the structure of biomimetic composites of PLA and clay. J. Appl. Polym. Sci. 2013, 129, 1109–1116. [Google Scholar] [CrossRef]
- Cheng, Q.; Jiang, L.; Tang, Z. Bioinspired layered materials with superior mechanical performance. Accounts Chem. Res. 2014, 47, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Walther, A; Bjurhager, I.; Malho, J.-M.; Pere, J.; Ruokolainen, J.; Berglund, L.A.; Ikkala, O. Large-area, lightweight and thick biomimetic composites with superior material properties via fast, economic, and green pathways. Nano Lett. 2010, 10, 2742–2748. [Google Scholar]
- Pluta, M.; Galeski, A.; Alexandre, M.; Paul, M.-A.; Dubois, P. Polylactide/montmorillonite nanocomposites and microcomposites prepared by melt blending: Structure and some physical properties. J. Appl. Polym. Sci. 2002, 86, 1497–1506. [Google Scholar] [CrossRef]
- Boesel, L.F. Effect of plasticizers on the barrier and mechanical properties of biomimetic composites of chitosan and clay. Carbohydr. Polym. 2015, 115, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, S.E.; Chinellato, A.C.; Boesel, L.F.; Pessan, L.A. Poly(ethylene terephthalate)-organoclay nanocomposites: Morphological, thermal and barrier properties. J. Metastab. Nanocryst. Mater. 2004, 2, 57–64. [Google Scholar] [CrossRef]
- Ho, T.T.T.; Ko, Y.S.; Zimmermann, T.; Geiger, T.; Caseri, W. Processing and characterization of nanofibrillated cellulose/layered silicate systems. J. Mater. Sci. 2012, 47, 4370–4382. [Google Scholar] [CrossRef]
- Ho, T.T.T.; Zimmermann, T.; Ohr, S.; Caseri, W.R. Composites of cationic nanofibrillated cellulose and layered silicates: Water vapor barrier and mechanical properties. ACS Appl. Mater. Interf. 2012, 4, 4832–4840. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Walther, A.; Ikkala, O.; Belova, L.; Berglund, L. Clay nanopaper with tough cellulose nanofiber matrix for fire retardancy and gas barrier functions. Biomacromolecules. 2011, 12, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Boesel, L.F.; Pessan, L.A. Poly(ethylene terephthalate)-organoclay nanocomposites: Morphological characterization. Mater. Sci. Forum 2002, 403, 89–94. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carboh. Polym. 2012, 90, 735. [Google Scholar] [CrossRef] [PubMed]
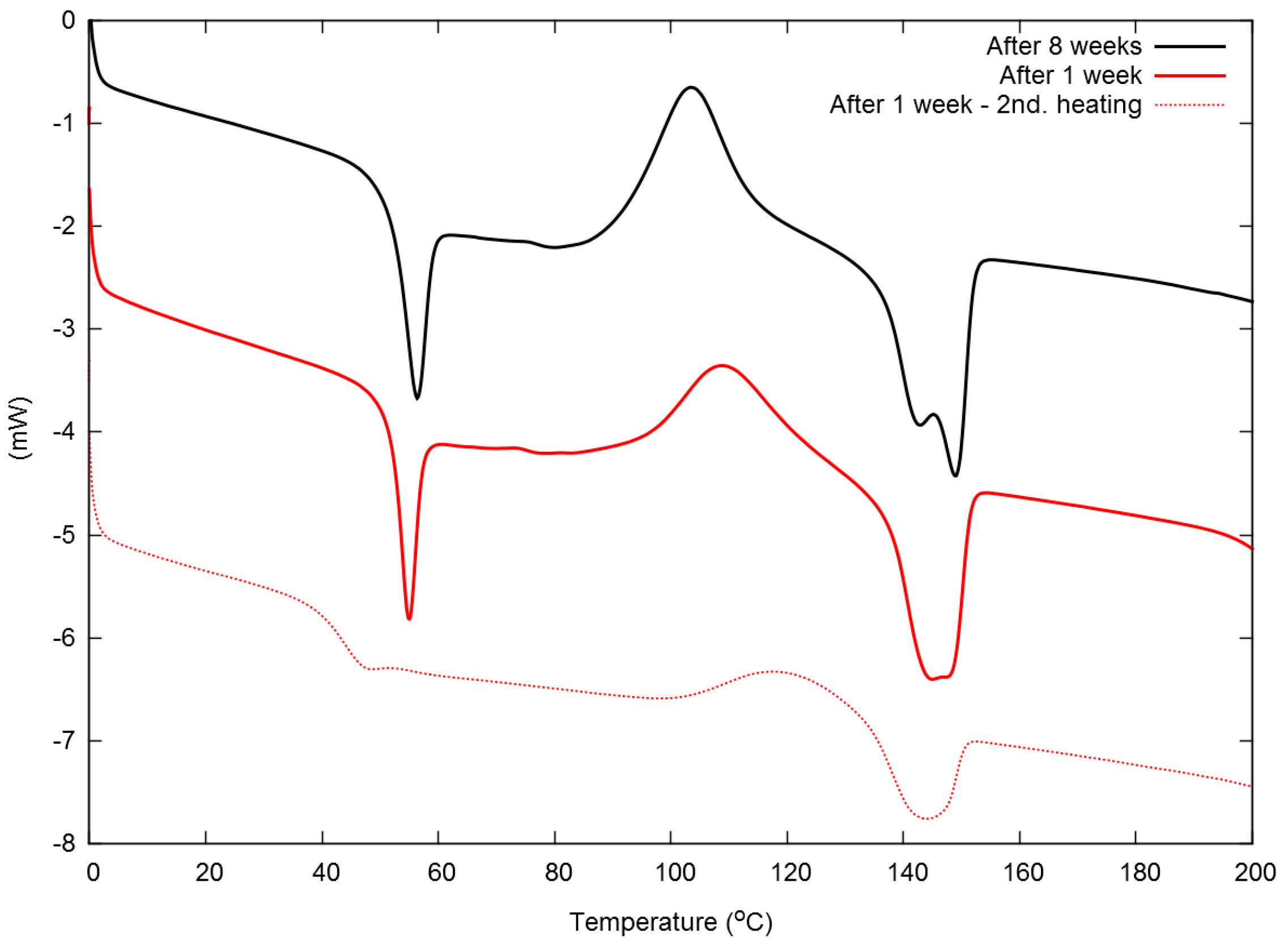
| Aging Time | Molecular Weight (103 g/mol) | Tg (°C) 1st/2nd Heating | Tc (°C) 1st/2nd Heating | Tm (°C) 1st/2nd Heating | Χc (%) 1st Heating |
|---|---|---|---|---|---|
| 1 week | 113 | 53/43 | 109/119 | 148/144 | 1.1 |
| 8 weeks | 102 | 54/43 | 103/113 | 148/147 | 1.0 |
| Solvent casted (from [8]) | – | 53/– | 105/– | 167/– | 2 |
| Total Solid Content of the Latex % | Amount of MMT in the Latex % | WVTR* g/(m2·day) | WVP ** (10−14 kg·m/(m2·Pa·s)) |
|---|---|---|---|
| 21 | 3 | 25 | 0.77 |
| 22 | 5 | 35 | 1.9 |
| 21 | 5 | 42 | 1.4 |
| 23 | 12 | 426 | 8.8 |
| 26 | 14 | 361 | 7.2 |
| Paper | Coating Weight g/m2 | WVTR g/(m2 day) |
|---|---|---|
| KL uncoated | – | 264 |
| KL with 6 layers PLA/MMT | 20 | 81 |
| KL with 6 layers, annealed at 150 °C | 20 | 42 |
| SiNQ | – | 308 |
| SiNQ with 6 layers PLA/MMT | 22 | 11 |
| SiNQ with 6 layers, annealed at 150 °C | 22 | 93 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandera, D.; Meyer, V.R.; Prevost, D.; Zimmermann, T.; Boesel, L.F. Polylactide/Montmorillonite Hybrid Latex as a Barrier Coating for Paper Applications. Polymers 2016, 8, 75. https://doi.org/10.3390/polym8030075
Bandera D, Meyer VR, Prevost D, Zimmermann T, Boesel LF. Polylactide/Montmorillonite Hybrid Latex as a Barrier Coating for Paper Applications. Polymers. 2016; 8(3):75. https://doi.org/10.3390/polym8030075
Chicago/Turabian StyleBandera, Davide, Veronika R. Meyer, David Prevost, Tanja Zimmermann, and Luciano F. Boesel. 2016. "Polylactide/Montmorillonite Hybrid Latex as a Barrier Coating for Paper Applications" Polymers 8, no. 3: 75. https://doi.org/10.3390/polym8030075
APA StyleBandera, D., Meyer, V. R., Prevost, D., Zimmermann, T., & Boesel, L. F. (2016). Polylactide/Montmorillonite Hybrid Latex as a Barrier Coating for Paper Applications. Polymers, 8(3), 75. https://doi.org/10.3390/polym8030075