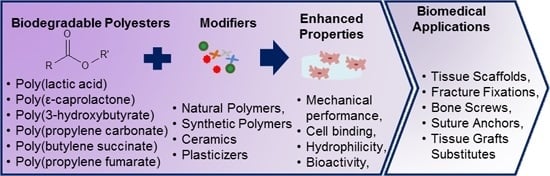
Biomedical Applications of Biodegradable Polyesters
Abstract
:1. Introduction
2. Synthesis of Polyesters
3. Properties of Polyesters
3.1. Mechanical Strength
| Material | Type | Tensile modulus (E, MPa) | Ultimate tensile strength (σm, MPa) | Elongation at break (εm, %) | Reference |
|---|---|---|---|---|---|
| Tissues | Bone (trabecular) | 483 | 2 | 2.5 | [24] |
| Cartilage | 10–100 | 10–40 | 15–20 | [25] | |
| Cardiovascular | 2–6 | 1 | 1200 | [26] | |
| Medical devices | Mg-based orthopaedic screw | Not reported | ~200 | ~9 | [27] |
| Suture | ~850 | ~37 | ~70 | [28] | |
| Medical mesh (Vicryl®) | 4.6 ± 0.6 (stiffness N/mm) | 78.2 ± 10.5 (maximum force N/cm) | 150 ± 6 | [29] | |
| Polyesters | PGA | 7000–8400 | 890 | 30 | [30] |
| PLGA(50:50) | ~2000 | 63.6 | 3–10 | [31,32] | |
| PLA | 3500 | 55 | 30–240 | [33] | |
| PHB | 3500 | ~40 | 5–8 | [34] | |
| PPF | 2000–3000 | 3–35 | 20.3 | [22,35,36] | |
| PCL | ~700 | 4–28 | 700–1000 | [30,31] | |
| PPC | 830 | 21.5 | 330 | [37] | |
| PBS | ~700 | ~17.5 | ~6 | [38] |
3.2. Degradation
| Polyesters | Degradation by-products (pKa) | In vivo degradation rate | Degradation mechanism |
|---|---|---|---|
| PLA (PLLA and PDLA) | Lactic acid (3.85) [60] (3.08) [61] | 50% in 1–2 years [62] 98% in 12 months [63] 100% in >12 months [64] 100% in 12–16 month [31] | Hydrolysis through the action of enzymes [33] |
| PGA | Glycolic acid (3.83) [61,65] | 100% in 2–3 months [62] 100% in 6–12 months [64] | Both enzymatic and non-enzymatic hydrolysis [62] |
| PLGA | Lactic acid (3.85)[60] (3.08) [61] Glycolic acid (3.83) [61,65] | 100% in 100 days (75% LA: 25%GA) [66] 100% in 50–100 days [62] | Hydrolysis through the action of enzymes [31] |
| PPC | CO2 and Water (pathway and intermediates unknown) | 6% in 200 days [67] No degradation after 2 months [68] | Hydrolysis, or enzyme mediation [69] |
| PHB | 3-Hydroxybutyric acid (4.41 [70] or 4.7 [71]) | 35% degradation of molecular weight after 6 months [72] 60% degradation via thickness of pellet after 24 weeks [73] | Hydrolysis via nonspecific esterase enzymes [74,75] |
| PHBV | 3-Hydroxybutyric acid (4.41 [70] or 4.7 [61,71]) 3-hydroxyvaleric acid (4.72 [61]) | 75% degradation via thickness of pellet after 24 weeks [73] | Hydrolysis via nonspecific esterase enzymes [74,75] |
| PBS | Succinic acid (4.21 and 5.64 for the first and second hydroxyl group) [76] | 5–10 wt % in 100 days (In vitro) [76] | Enzymatic hydrolytic degradation [77] |
| PCL | Caproic acid (4.88) [78] | 50% in 4 years [62] 1% in 6 months [79] | Hydrolytic degradation [79] |
| PPF | Fumaric acid (pKa2 = 4.44) [22] | Depends on the formulation and composition several months >24 [22] | Hydrolysis [80] |
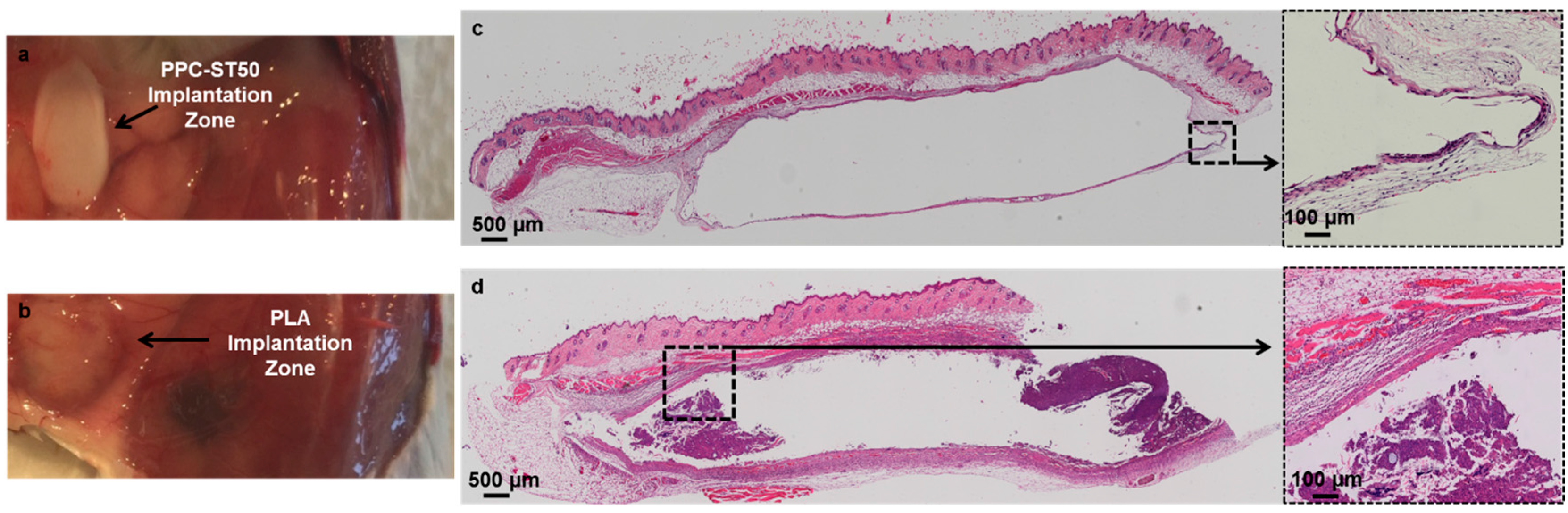
3.3. Commercial Application of Polyesters
| Polymers | Applications | Commercial products |
|---|---|---|
| PLA | Fracture fixation [25], interference screws [25], suture anchors, meniscus repair [25], reconstructive surgeries [2], Vascular grafts [27], Adhesion Barriers [28], Articular cartilage repair [29], Bone graft substitute [2,30], Dural substitutes [2], Skin substitutes [2], Tissue augmentation [30], Scaffolds [8] | Proceed™ Surgical Mesh (Ethicon Inc.) , Artisorb™ Bioabsorbable GTR Barrier (Atrix laboratories, Fort Collins, CO, USA) |
| PLGA | (Composition 85:15): Interference screws [25], plates [25], suture anchors [25], Stents [38]/(Composition 50:50): Suture [25], drug delivery [25], Articular cartilage repair [39]/(Composition 90:10):Artificial skin [25], wound healing [25], hernia repair [2], suture [2], tissue engineered vascular grafts [2] | Rapidsorb® plates (DePuy Synthes CMF, West Chester, PA,USA), Lactosorb® TraumaPlatingSystem (Biomet, Inc., Warsaw, IN, USA) [l-lactide/glycolide = 82/18], RFS™ Screw System (Tornier), RFS™ (Resorbable Fixation System) Pin System (Tornier), Xinsorb BRS™ stent (Huaan Biotechnology Group, Gansu, China) REF1, Dermagraft®, Vicryl® woven mesh (Ethicon Inc.) (Composition 90:10) |
| PCL | Suture coating [25], dental orthopedic implants [25], Tissue repair [2], hybrid tissue-engineered heart valves [2], Surgical meshes [2], cardiac patches [31], Vascular grafts [32], Adhesion Barriers [33], Dural substitutes [2], Stents [34], Ear implants [2], Tissue engineering scaffolds [16,35] | Tissue repair patches (Ethicon Inc.), Bulking and Filling agents (Angelo, 1996), DermaGraft™ (Organogenesis Inc., Canton, MD, USA) |
| PPF | Orthopedic implants [25], dental [25], foam coatings [25], drug delivery [25], Scaffolds [8,12] | ----- |
| PPC | Scaffolds [87,88] | ----- |
| PHB | Sutures (P4HB polymer) [2], screw fasteners for meniscal cartilage repair, Scaffold for tendon repair [2], Reconstructive surgeries (Surgical meshes) [2], Vascular grafts [32], Nerve repair [36,37], Bone tissue scaffold (P3HB) [26], Wound dressing (P3HB) [2], hemostats (P4HB) [2], Stents [38] | Phantom Fiber™ suture (Tornier Co.), MonoMax® suture (Braun Surgical Co.), BioFiber™ scaffold (P4HB polymer) (Tornier Co.), TephaFlex® mesh (Tepha Inc.) (P4HB polymer), GalaFLEX mesh (Galatea Corp.), Tornier® surgical mesh (Tornier Co.) |
| PHBV | Scaffolds [89,90] | ----- |
| PBS | Stents [2], Sterilization wrap [2], Diagnostic or Therapeutic Imaging | Disposable Medical Products-Bionolle® 1000 and 3000 (Showa Highpolymer Co. Ltd.) |
4. Modification of Polyesters
4.1. PLA
| Polyester | Modifier | Concentration (wt %) | Porosity (%) | Mechanical properties (MPa) | Enhanced properties | Reference |
|---|---|---|---|---|---|---|
| PLA | PU | 50 | 79 | 80 (C-M) | Mechanical performances | [96] |
| PCL | 50 | 81.5 ± 1.2 | 0.3 (C-S) | [97] | ||
| PEG | 20 | 86.75 | 1830 (Y-M) (nano-indentation method) | [98] | ||
| Triclosan | 20 | Solid structure | 61.98 ± 0.3 (T-S) | Cell binding | [99] | |
| Chitosan and keratin | 30% chitosan and 4% keratin | Solid structure | 35 (T-S) | [100] | ||
| BG | 40 | 0.211 (cm3/g) | 0.3 (C-S) | Bioactivity and neutralize the acidic degradation | [101] | |
| Carbonated apatite | 30 | 70 | 2.2 (R) | [102] | ||
| HA | 50 | 85 | 857 ± 0.268 (E-M) | [103] | ||
| Calcium phosphate | 50 | 96.58 ± 0.85 | 0.147 ± 0.02 (S) | [104] | ||
| Halloysite nanotube | 10 | Solid fibers | 10.4 (T-M) | [105] | ||
| PLGA | PHBV | 50 | 81.273 ± 2.192 | 1.5 (C-M) | Mechanical performances | [106] |
| Gelatin | 30 | 78.41 | 6.43 ± 0.37 (T-S) | Hydrophilicity | [107] | |
| Nano HA | 5 | 89.3 ± 1.4 | 1.3546 ± 0.053 (C-M) | Bioactivity | [108] | |
| BG | 1 | 93 ± 2 | 0.412 ± 0.057 (C-S) | [109] | ||
| Silica nanoparticles | 10 | Solid fibers | 114 ± 18.6 (Y-M) | [110] |
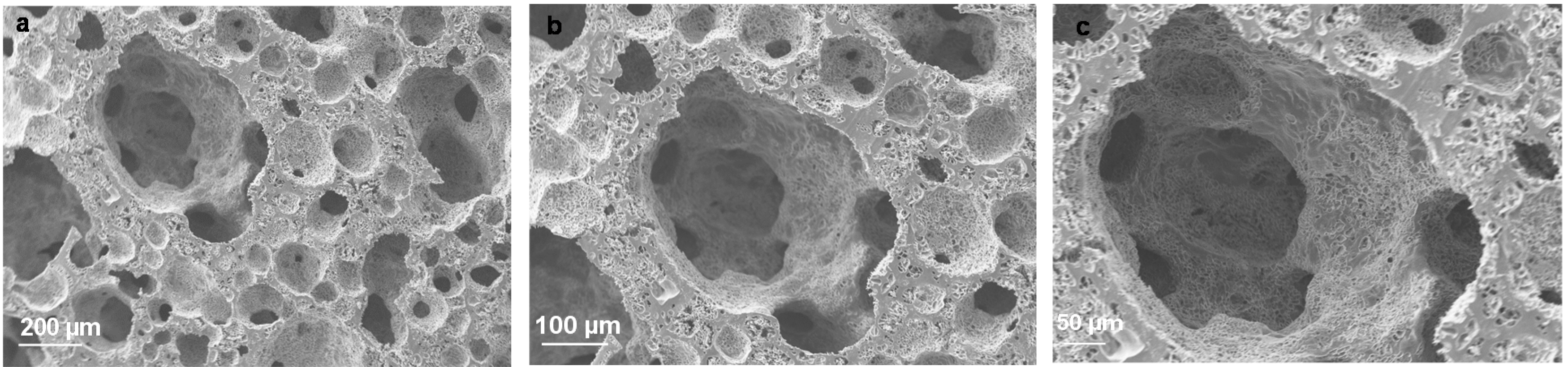
4.2. PHA Family
| Polyester | Modifier | Concentration (wt %) | Porosity (%) | Mechanical properties (MPa) | Enhanced properties | Reference |
|---|---|---|---|---|---|---|
| PHB | HA | 30 | Solid film | 1400 (S-M) | Bioactivity | [147] |
| Herafill | 30 | Solid film | 2800 (Y-M) | [148] | ||
| BG | 10 | 85 | Not reported | [149] | ||
| PHBV | Chitin | 10 | Not reported | 7.12 ± 0.24 (C-M) | Cell binding | [89] |
| Silk and nHA | 5 (w/v) % | 71.44 ± 0.81 | 0.72 ± 0.26 (Y-M (kPa)) | Bioactivity | [150] | |
| Calcium silicate | 20 | 80 | ~ 33 1 (C-M) | [151] | ||
| HA | 10 | Solid fibers | 4.19 ± 0.19 (U-S) | [152] |
4.3. PPC
| Polyester | Modifier | Concentration (wt %) | Porosity (%) | Mechanical properties (MPa) | Enhanced properties | Reference |
|---|---|---|---|---|---|---|
| PPC | Chitosan | 5 | 91.9 | 14.2 ± 0.56 (C-M) | Hydrophilicity and cell binding | [87] |
| Chitosan | 7 | Solid fibers | 5.0 ± 0.8 (T-S) | [168] | ||
| PEI and Gelatin | Coating | 92.3 | 0.4 (C-M) | [166,169] | ||
| Graphene oxide | 1 | 83.54 | 1 (C-M) | Physical characteristics such as mechanical performances and porosity | [170] | |
| Gelatin | 15 | Solid fibers | 2.88 ± 0.82 (T-S) | [88] | ||
| Starch | 50 | Solid disk | 33.9 (C-M) | [81] |
4.4. PBS
4.5. PCL
| Polyester | Modifier | Concentration (wt %) | Porosity (%) | Mechanical properties (MPa) | Enhanced properties | Reference |
|---|---|---|---|---|---|---|
| PCL | Chitosan | 25 | Solid fibers | 1.78 ± 0.25 (T-S) | Hydrophilicity and cell binding | [193] |
| Collagen | Coating | 93.9 ± 0.4 | 5 (Y-M) | [194] | ||
| Gelatin and Collagen | 20% gelatin and 1.5% collagen | Solid fibers | 1.29 (T-S) | [195] | ||
| Elastin | 30 | 91 | 1.30 ± 0.07 (C-M) | |||
| Alginate | 5 | 92 | 0.72 ± 0.04 (T-S) | [196] | ||
| Nanofiber PLA | 10 | 79.7 | Not reported | Physical characteristics such as mechanical properties and porosity | [197] | |
| MWNTs | 2 | Solid disk | 110 (T-M) | [198] | ||
| Phlorotannin nanofibers | 5 | Solid fibers | 57.8 ± 6.6 (Y-M) | [199] | ||
| Silica | 5.4 | 63.3 ± 2.0 | 13.6 ± 1.6 (Y-M) | Degradation behavior and bioactivity | [200] | |
| BG | 21 vol % | 0.1 (cm3/g) | 1310 (Y-M) | [201] | ||
| BG | 50 | Solid disk | ~ 190 (E-M) | [202] | ||
| nBG | 30 | 8 ± 5 vol % | 383 ± 50 (E-M) | [203] | ||
| Calcium phosphate | 10 | Solid fibers | 7.55 ± 0.70 (Y-M) | [204] |
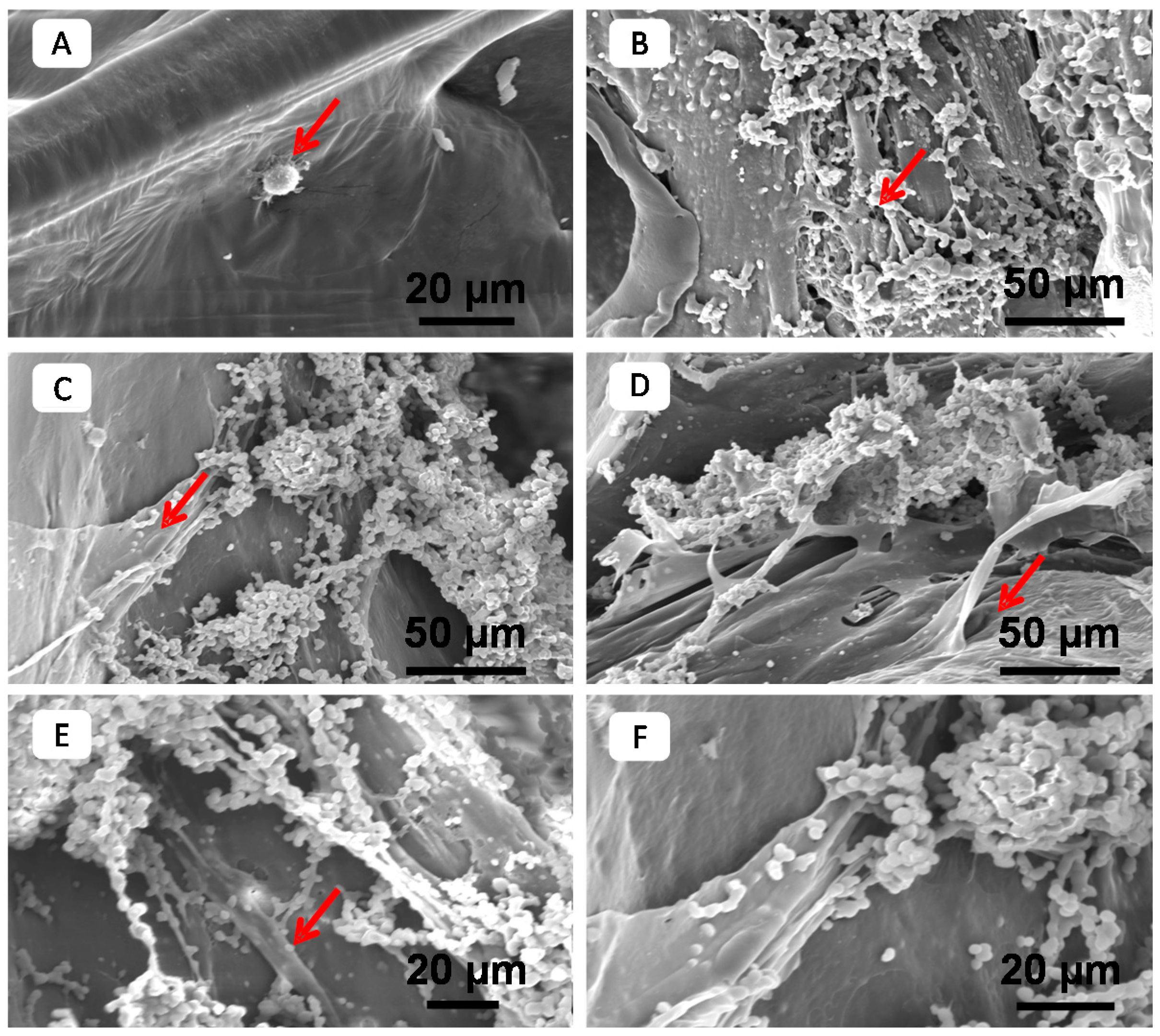
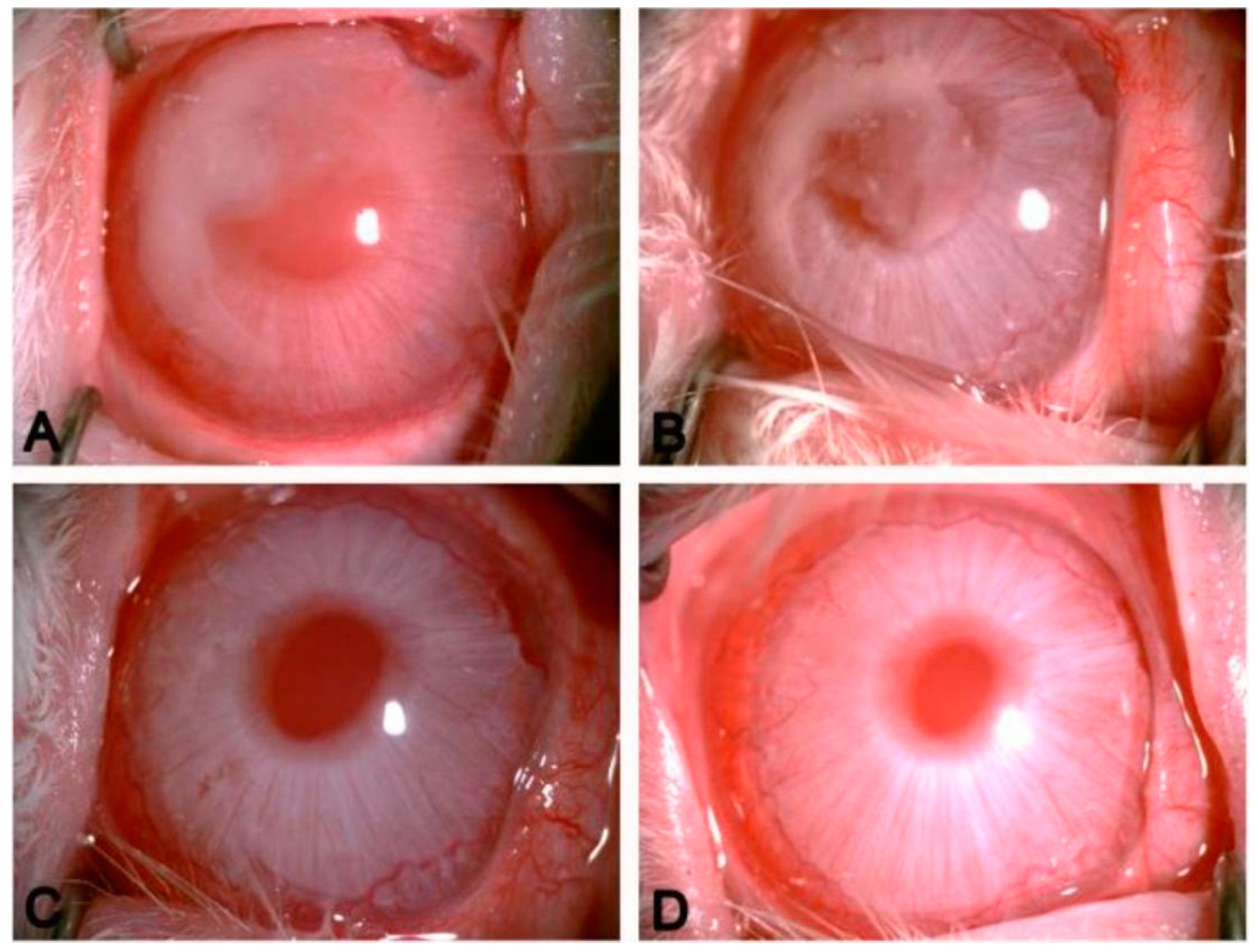
4.6. PPF
5. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
Abbreviations
| PLA | Poly(lactic acid) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PCL | Poly(ε-caprolactone) |
| PHB | Poly(3-hydroxybutyrate) or Poly(β-hydroxybutyric acid) |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PPC | Poly(propylene carbonate) |
| PBS | Poly(butylene succinate) |
| PPF | Poly(propylene fumarate) |
| TPU | Thermoplastic polyurethane |
| Y-M | Young’s modulus |
| T-S | Tensile strength |
| C-S | compressive strength |
| R | resistance |
| E-M | Elastic modulus |
| S | stiffness |
| T-M | Tensile modulus |
| C-M | Compressive modulus |
| S-M | storage modulus |
| PHAs | Polyhydroxyalkanoates |
| PEO | Polyethylene oxide |
| PEGM | Polyethylene glycol methacrylate |
| CO2 | Carbon dioxide |
| GO | Graphene oxide |
| NIPU | Non-isocyanate polyurethane |
| HA | Hydroxyapatite |
| TCP | β-tricalcium phosphates |
| MWCNTs | Multiwall carbon nanotubes |
| BG | Bioglass |
| PLEOF | Poly(lactide-ethylene oxide fumarate) |
| HEMA | Hydroxyethyl methacrylate |
| PEG | Polyethylene glycol |
References
- Research and Markets: Tissue Engineering: Technologies and Therapeutic Areas—A Global Market Overview to 2022. Available online: http://www.businesswire.com/news/home/20150915005908/en/Research-Markets-Tissue-Engineering-Technologies-Therapeutic-Areas (accessed on 19 November 2015).
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Introduction-biomaterials science: An evolving, multidisciplinary endeavor. In Biomaterials Science, 3rd ed.; Lemons, B.D., Ratner, A.S., Hoffman, F.J., Schoen, J.E., Eds.; Academic Press: Boston, MA, USA, 2013. [Google Scholar]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. 3-Applications of poly(lactic acid). In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 55–69. [Google Scholar]
- Diaz, A.; Katsarava, R.; Puiggali, J. Synthesis, properties and applications of biodegradable polymers derived from diols and dicarboxylic acids: From polyesters to poly(ester amide)s. Int. J Mol. Sci. 2014, 15, 7064–7123. [Google Scholar] [CrossRef] [PubMed]
- Sokolsky-Papkov, M.; Agashi, K.; Olaye, A.; Shakesheff, K.; Domb, A.J. Polymer carriers for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, K.; Azadpour, P.; Moztarzadeh, F.; Urbanska, A.M.; Mozafari, M. Tissue-engineered chitosan/bioactive glass bone scaffolds integrated with PLGA nanoparticles: A therapeutic design for on-demand drug delivery. Mater. Lett. 2015, 138, 16–20. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Seyednejad, H.; Gawlitta, D.; Dhert, W.J.A.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Preparation and characterization of a three-dimensional printed scaffold based on a functionalized polyester for bone tissue engineering applications. Acta Biomater. 2011, 7, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—polycaprolactone in the 21st century. Progress Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [PubMed]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Angela, L.S.; Chia-Chih, C.; Bryan, P.; Todd, E. Strategies in aliphatic polyester synthesis for biomaterial and drug delivery applications. In Degradable Polymers and Materials: Principles and Practice, 2nd ed.; American Chemical Society: Washington, DC, USA, 2012; Volume 1114, pp. 237–254. [Google Scholar]
- Yu, Y.; Wu, D.; Liu, C.; Zhao, Z.; Yang, Y.; Li, Q. Lipase/esterase-catalyzed synthesis of aliphatic polyesters via polycondensation: A review. Process Biochem. 2012, 47, 1027–1036. [Google Scholar] [CrossRef]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Dehghani, F. Solvent free synthesis of organometallic catalysts for the copolymerisation of carbon dioxide and propylene oxide. Appl. Catal. B Environ. 2010, 98, 101–111. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Chapter 1 PLA synthesis. From the monomer to the polymer. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; The Royal Society of Chemistry: London, UK, 2015; pp. 1–36. [Google Scholar]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, R.A.J.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Radecka, I. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 2007, 102, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.F.; Martin, D.P. Applications of polyhydroxyalkanoates (PHA) in medicine and pharmacy. In Biopolymers Online; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Seyednejad, H.; Ghassemi, A.H.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Control. Release 2011, 152, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases WJCC 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Røhl, L.; Larsen, E.; Linde, F.; Odgaard, A.; Jørgensen, J. Tensile and compressive properties of cancellous bone. J. Biomech. 1991, 24, 1143–1149. [Google Scholar] [CrossRef]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.P. Mechanical properties of normal and diseased cerebrovascular system. J. Vasc. Int. Neurol. 2009, 2, 155–162. [Google Scholar]
- Salahshoor, M.; Guo, Y. Biodegradable orthopedic magnesium-calcium (MGCA) alloys, processing, and corrosion performance. Materials 2012, 5, 135. [Google Scholar] [CrossRef]
- Haghighat, F.; Ravandi, S. Mechanical properties and in vitro degradation of PLGA suture manufactured via electrospinning. Fibers Polym. 2014, 15, 71–77. [Google Scholar] [CrossRef]
- Pott, P.P.; Schwarz, M.L.R.; Gundling, R.; Nowak, K.; Hohenberger, P.; Roessner, E.D. Mechanical properties of mesh materials used for hernia repair and soft tissue augmentation. PLoS ONE 2012, 7, e46978. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-lactic acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. 2-overview of poly(lactic acid). In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MD, USA, 2013; pp. 11–54. [Google Scholar]
- Jiang, L.; Zhang, J. 6-Biodegradable polymers and polymer blends. In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MD, USA, 2013; pp. 109–128. [Google Scholar]
- Shi, X.F.; Hudson, J.L.; Spicer, P.P.; Tour, J.M.; Krishnamoorti, R.; Mikos, A.G. Rheological behaviour and mechanical characterization of injectable poly(propylene fumarate)/single-walled carbon nanotube composites for bone tissue engineering. Nanotechnology 2005, 16, S531–S538. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hu, B.; Ning, Q.; Ye, C.; Xie, J.; Ye, J.; Gao, C. A primary study of poly(propylene fumarate)-2-hydroxyethyl methacrylate copolymer scaffolds for tarsal plate repair and reconstruction in rabbit eyelids. J. Mater. Chem. B 2015, 3, 4052–4062. [Google Scholar] [CrossRef]
- Du, L.; Qu, B.; Meng, Y.; Zhu, Q. Structural characterization and thermal and mechanical properties of poly(propylene carbonate)/MGAL-LDH exfoliation nanocomposite via solution intercalation. Compos. Sci. Technol. 2006, 66, 913–918. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Qu, W. Mechanical properties of poly(butylene succinate) (PBS) biocomposites reinforced with surface modified jute fibre. Compos. Appl. Sci. Manuf. 2009, 40, 669–674. [Google Scholar] [CrossRef]
- Contiliano, J.H.; Yuan, J.J.; Tenhuisen, K.S. Polymer-Based Orthopedic Screw and Driver System with Increased Insertion Torque Tolerance and Associated Method for Making and Using Same. U.S. Patents US20050216016 A1, 29 September 2005. [Google Scholar]
- Schwach, G.; Vert, M. In vitro and in vivo degradation of lactic acid-based interference screws used in cruciate ligament reconstruction. Int. J. Biol. Macromol. 1999, 25, 283–291. [Google Scholar] [CrossRef]
- Tenhuisen, K.S.; Janas, V.F.; Cooper, K.L.; Overaker, D.W.; Yuan, J.J. Self-Tapping Resorbable Two-Piece Bone Screw. U.S. Patents US6916321 B2, 12 July 2005. [Google Scholar]
- Herrmann, J.B.; Kelly, R.J.; Higgins, G.A. Polyglycolic acid sutures: Laboratory and clinical evaluation of a new absorbable suture material. Arch. Surg. 1970, 100, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Progress Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Wilkerson, J.P.; Zvijac, J.E.; Uribe, J.W.; Schurhoff, M.R.; Green, J.B. Failure of polymerized lactic acid tacks in shoulder surgery. J. Shoulder Elb. Surg. 2003, 12, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Liggins, R.T.; Burt, H.M. Paclitaxel loaded poly(l-lactic acid) microspheres: Properties of microspheres made with low molecular weight polymers. Int. J. Pharm. 2001, 222, 19–33. [Google Scholar] [CrossRef]
- Park, T.G. Degradation of poly(d,l-lactic acid) microspheres: effect of molecular weight. J. Control. Release 1994, 30, 161–173. [Google Scholar] [CrossRef]
- Alexis, F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)]. Polym. Int. 2005, 54, 36–46. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly(l-lactide). III. Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight poly(l-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Schliecker, G.; Schmidt, C.; Fuchs, S.; Wombacher, R.; Kissel, T. Hydrolytic degradation of poly(lactide-co-glycolide) films: Effect of oligomers on degradation rate and crystallinity. Int. J. Pharm. 2003, 266, 39–49. [Google Scholar] [CrossRef]
- Lee, J.W.; Gardella, J.A. In vitro hydrolytic surface degradation of poly(glycolic acid): Role of the surface segregated amorphous region in the induction period of bulk erosion. Macromolecules 2001, 34, 3928–3937. [Google Scholar] [CrossRef]
- Pitt, C.G.; Chasalow, F.I.; Hibionada, Y.M.; Klimas, D.M.; Schindler, A. Aliphatic polyesters. I. The degradation of poly(ε-caprolactone) in vivo. J. Appl. Polym. Sci. 1981, 26, 3779–3787. [Google Scholar] [CrossRef]
- Castilla-Cortázar, I.; Más-Estellés, J.; Meseguer-Dueñas, J.M.; Escobar Ivirico, J.L.; Marí, B.; Vidaurre, A. Hydrolytic and enzymatic degradation of a poly(ε-caprolactone) network. Polym. Degrad. Stab. 2012, 97, 1241–1248. [Google Scholar] [CrossRef]
- Fukuzaki, H.; Yoshida, M.; Asano, M.; Kumakura, M.; Mashimo, T.; Yuasa, H.; Imai, K.; Hidetoshi, Y. Synthesis of low-molecular-weight copoly(l-lactic acid/ɛ-caprolactone) by direct copolycondensation in the absence of catalysts, and enzymatic degradation of the polymers. Polymer 1990, 31, 2006–2014. [Google Scholar] [CrossRef]
- Gan, Z.; Liang, Q.; Zhang, J.; Jing, X. Enzymatic degradation of poly(ε-caprolactone) film in phosphate buffer solution containing lipases. Polym. Degrad. Stab. 1997, 56, 209–213. [Google Scholar] [CrossRef]
- Gan, Z.; Yu, D.; Zhong, Z.; Liang, Q.; Jing, X. Enzymatic degradation of poly(ε-caprolactone)/poly(dl-lactide) blends in phosphate buffer solution. Polymer 1999, 40, 2859–2862. [Google Scholar] [CrossRef]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(dl-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, Z.; Wei, W.; Li, Z. Preparation and characterization of PLLA composite scaffolds by ScCo2-induced phase separation. Polym. Compos. 2012, 33, 1667–1671. [Google Scholar] [CrossRef]
- Danmark, S.; Finne-Wistrand, A.; Schander, K.; Hakkarainen, M.; Arvidson, K.; Mustafa, K.; Albertsson, A.C. In vitro and in vivo degradation profile of aliphatic polyesters subjected to electron beam sterilization. Acta Biomater. 2011, 7, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.; Yang, X.; Mei, F.; Hu, X.; Chen, G.; Deng, X.; Ryu, S. Poly-l-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J. Biomed. Mater. Res. 2007, 82A, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Surface physiological changes induced by lactic acid on pathogens in consideration of pKa and pH. Food Control 2014, 46, 525–531. [Google Scholar] [CrossRef]
- Volova, T.G. Polyhydroxyalkanoates—Plastic Materials of the 21st Century: Production, Properties, Applications; Nova Science Publishers: New York, NY, USA, 2004. [Google Scholar]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Curtis, R.; Gogolewski, S. Effect of in vivo and in vitro degradation on molecular and mechanical properties of various low-molecular-weight polylactides. J. Biomed. Mater. Res. 1997, 36, 360–380. [Google Scholar] [CrossRef]
- Pietrzak, W.S.; Sarver, D.R.; Verstynen, M.L. Bioabsorbable polymer science for the practicing surgeon. J. Craniofacial Surg. 1997, 8, 87–91. [Google Scholar] [CrossRef]
- El Mubarak, M.A.S.; Lamari, F.N.; Kontoyannis, C. Simultaneous determination of allantoin and glycolic acid in snail mucus and cosmetic creams with high performance liquid chromatography and ultraviolet detection. J. Chromatogr. A 2013, 1322, 49–53. [Google Scholar] [CrossRef] [PubMed]
- McBane, J.E.; Sharifpoor, S.; Cai, K.; Labow, R.S.; Santerre, J.P. Biodegradation and in vivo biocompatibility of a degradable, polar/hydrophobic/ionic polyurethane for tissue engineering applications. Biomaterials 2011, 32, 6034–6044. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Song, C.; Cao, M.; Hu, D.; Liu, L.; Liu, N.; Wang, S. Thermal properties and degradability of poly(propylene carbonate)/poly(β-hydroxybutyrate-co-β-hydroxyvalerate) (PPC/PHBV) blends. Polym. Degrad. Stab. 2009, 94, 575–583. [Google Scholar] [CrossRef]
- Jayachandran, J.P.; Reed, H.A.; Hongshi, Z.; Rhodes, L.F.; Henderson, C.L.; Allen, S.; Kohl, P.A. Air-channel fabrication for microelectromechanical systems via sacrificial photosensitive polycarbonates. Microelectromech. Syst. J. 2003, 12, 147–159. [Google Scholar] [CrossRef]
- Luinstra, G.A.; Borchardt, E. Material properties of poly (propylene carbonates). In Synthetic Biodegradable Polymers; Springer: Berlin, Germany, 2012; pp. 29–48. [Google Scholar]
- He, J.; Chen, S.; Yu, Z. Determination of poly-β-hydroxybutyric acid in bacillus thuringiensis by capillary zone electrophoresis with indirect ultraviolet absorbance detection. J. Chromatogr. A 2002, 973, 197–202. [Google Scholar] [CrossRef]
- Dahl, S.R.; Olsen, K.M.; Strand, D.H. Determination of γ-hydroxybutyrate (GHB), β-hydroxybutyrate (BHB), pregabalin, 1,4-butane-diol (1,4BD) and γ-butyrolactone (GBL) in whole blood and urine samples by UPLC–MSMS. J. Chromatogr. B 2012, 885–886, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kunze, C.; Edgar Bernd, H.; Androsch, R.; Nischan, C.; Freier, T.; Kramer, S.; Kramp, B.; Schmitz, K.-P. In vitro and in vivo studies on blends of isotactic and atactic poly(3-hydroxybutyrate) for development of a dura substitute material. Biomaterials 2006, 27, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Shishatskaya, E.; Sevastianov, V.; Efremov, S.; Mogilnaya, O. Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 2003, 16, 125–133. [Google Scholar] [CrossRef]
- Boskhomdzhiev, A.P.; Bonartsev, A.P.; Makhina, T.K.; Myshkina, V.L.; Ivanov, E.A.; Bagrov, D.V.; Filatova, E.V.; Iordanskii, A.L.; Bonartseva, G.A. Biodegradation kinetics of poly(3-hydroxybutyrate)-based biopolymer systems. Biochem. Suppl. Series B Biomed. Chem. 2010, 4, 177–183. [Google Scholar] [CrossRef]
- Bonartsev, A.; Myshkina, V.; Nikolaeva, D.; Furina, E.; Makhina, T.; Livshits, V.; Boskhomdzhiev, A.; Ivanov, E.; Iordanskii, A.; Bonartseva, G. Biosynthesis, biodegradation, and application of poly (3-hydroxybutyrate) and its copolymers-natural polyesters produced by diazotrophic bacteria. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 1, 295–307. [Google Scholar]
- Lindström, A.; Albertsson, A.-C.; Hakkarainen, M. Quantitative determination of degradation products an effective means to study early stages of degradation in linear and branched poly(butylene adipate) and poly(butylene succinate). Polym. Degrad. Stab. 2004, 83, 487–493. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, M.; Yang, J.; Qiu, J.-H. Study on the enzymatic degradation of PBS and its alcohol acid modified copolymer. Biodegradation 2012, 23, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Sandonis, I.; Valle, M.B. In vitro degradation of poly(caprolactone)/nHA composites. J. Nanomater. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.-T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo. J. Biomed. Mater. Res. 2009, 90A, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Timmer, M.D.; Shin, H.; Horch, R.A.; Ambrose, C.G.; Mikos, A.G. In vitro cytotoxicity of injectable and biodegradable poly(propylene fumarate)-based networks: Unreacted macromers, cross-linked networks, and degradation products. Biomacromolecules 2003, 4, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Manavitehrani, I.; Fathi, A.; Wang, Y.; Maitz, P.K.; Dehghani, F. Reinforced poly(propylene carbonate) composite with enhanced and tunable characteristics, an alternative for poly(lactic acid). ACS Appl. Mater. Interfaces 2015, 7, 22421–22430. [Google Scholar] [CrossRef] [PubMed]
- Eggers, J.; Steinbüchel, A. Poly(3-hydroxybutyrate) degradation in ralstonia eutropha h16 is mediated stereoselectively to (s)-3-hydroxybutyryl coenzyme a (CoA) via crotonyl-CoA. J. Bacteriol. 2013, 195, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Niaounakis, M. 7-Medical, dental, and pharmaceutical applications. In Biopolymers: Applications and Trends; Niaounakis, M., Ed.; William Andrew Publishing: Oxford, UK, 2015; pp. 291–405. [Google Scholar]
- Doğan, A.; Demirci, S.; Bayir, Y.; Halici, Z.; Karakus, E.; Aydin, A.; Cadirci, E.; Albayrak, A.; Demirci, E.; Karaman, A.; et al. Boron containing poly-(lactide-co-glycolide) (PLGA) scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2014, 44, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Chuenjitkuntaworn, B.; Supaphol, P.; Pavasant, P.; Damrongsri, D. Electrospun poly(l-lactic acid)/hydroxyapatite composite fibrous scaffolds for bone tissue engineering. Polym. Int. 2010, 59, 227–235. [Google Scholar] [CrossRef]
- Zhao, J.H.; Han, W.Q.; Chen, H.D.; Tu, M.; Huan, S.W.; Miao, G.Q.; Zeng, R.; Wu, H.; Cha, Z.G.; Zhou, C.R. Fabrication and in vivo osteogenesis of biomimetic poly(propylene carbonate) scaffold with nanofibrous chitosan network in macropores for bone tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Salick, M.R.; Cordie, T.; Mi, H.-Y.; Peng, X.-F.; Turng, L.-S. Electrospinning homogeneous nanofibrous poly(propylene carbonate)/gelatin composite scaffolds for tissue engineering. Ind. Eng. Chem. Res. 2014, 53, 9391–9400. [Google Scholar] [CrossRef]
- Li, H.-Y.; Li, H.; Wang, B.-J.; Gu, Q.; Jiang, Z.-Q.; Wu, X.-D. Synthesis and properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/chitin nanocrystals composite scaffolds for tissue engineering. Chin. Chem. Lett. 2014, 25, 1635–1638. [Google Scholar] [CrossRef]
- Nair, M.B.; Baranwal, G.; Vijayan, P.; Keyan, K.S.; Jayakumar, R. Composite hydrogel of chitosan–poly(hydroxybutyrate-co-valerate) with chondroitin sulfate nanoparticles for nucleus pulposus tissue engineering. Coll. Surf. B Biointerfaces 2015, 136, 84–92. [Google Scholar] [CrossRef] [PubMed]
- European Bioplastics, I.f.B., and Biocomposites, nova Institute. Global Production Capacities of Bioplastics. Available online: http://en.european-bioplastics.org/technologymaterials/materials/ (accessed on 27 September 2015).
- Fambri, L.; Pegoretti, A.; Fenner, R.; Incardona, S.D.; Migliaresi, C. Biodegradable fibres of poly(l-lactic acid) produced by melt spinning. Polymer 1997, 38, 79–85. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, Y.; Ahire, J.; Chao, Y. Co-encapsulation of biodegradable nanoparticles with silicon quantum dots and quercetin for monitored delivery. Adv. Healthc. Mater. 2013, 2, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Avérous, L. 9-Synthesis, properties, environmental and biomedical applications of polylactic acid. In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 171–188. [Google Scholar]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.-F.; Turng, L.-S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- Kouya, T.; Tada, S.-i.; Minbu, H.; Nakajima, Y.; Horimizu, M.; Kawase, T.; Lloyd, D.R.; Tanaka, T. Microporous membranes of PLLA/PCL blends for periosteal tissue scaffold. Mater. Lett. 2013, 95, 103–106. [Google Scholar] [CrossRef]
- Serra, T.; Ortiz-Hernandez, M.; Engel, E.; Planell, J.A.; Navarro, M. Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds. Mater. Sci. Eng. C 2014, 38, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Davachi, S.M.; Kaffashi, B.; Zamanian, A.; Torabinejad, B.; Ziaeirad, Z. Investigating composite systems based on poly l-lactide and poly l-lactide/triclosan nanoparticles for tissue engineering and medical applications. Mater. Sci. Eng. C 2016, 58, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.E.; Spiridon, I. PLA/chitosan/keratin composites for biomedical applications. Mater. Sci. Eng. C 2014, 40, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, D.; Chen, X.; Lin, H.; Qu, F. One-pot synthesis of macro-mesoporous bioactive glasses/polylactic acid for bone tissue engineering. Mater. Sci. Eng. C 2014, 43, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Le Bolay, N.; Santran, V.; Dechambre, G.; Combes, C.; Drouet, C.; Lamure, A.; Rey, C. Production, by co-grinding in a media mill, of porous biodegradable polylactic acid–apatite composite materials for bone tissue engineering. Powder Technol. 2009, 190, 89–94. [Google Scholar] [CrossRef]
- Cai, X.; Tong, H.; Shen, X.; Chen, W.; Yan, J.; Hu, J. Preparation and characterization of homogeneous chitosan–polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomater. 2009, 5, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Charles-Harris, M.; del Valle, S.; Hentges, E.; Bleuet, P.; Lacroix, D.; Planell, J.A. Mechanical and structural characterisation of completely degradable polylactic acid/calcium phosphate glass scaffolds. Biomaterials 2007, 28, 4429–4438. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Marshall, J.; Haroosh, H.J.; Mohammadzadehmoghadam, S.; Liu, D.; Qi, X.; Lau, K.-T. Polylactic acid (PLA)/halloysite nanotube (HNT) composite mats: Influence of hnt content and modification. Compos. Appl. Sci. Manuf. 2015, 76, 28–36. [Google Scholar] [CrossRef]
- Huang, W.; Shi, X.; Ren, L.; Du, C.; Wang, Y. PHBV microspheres–PLGA matrix composite scaffold for bone tissue engineering. Biomaterials 2010, 31, 4278–4285. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.X.; Wang, Y.S.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y.F. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Qian, J.; Xu, W.; Yong, X.; Jin, X.; Zhang, W. Fabrication and in vitro biocompatibility of biomorphic PLGA/nHA composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2014, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Blaker, J.J.; Maquet, V.; Day, R.M.; Jérôme, R. Preparation and characterisation of poly(lactide-co-glycolide) (PLGA) and PLGA/bioglass® composite tubular foam scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2005, 25, 23–31. [Google Scholar] [CrossRef]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Kawazoe, N.; Lin, X.; Dong, J.; Chen, G. The influence of structural design of PLGA/collagen hybrid scaffolds in cartilage tissue engineering. Biomaterials 2010, 31, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tanaka, J.; Tateishi, T. Osteochondral tissue engineering using a PLGA–collagen hybrid mesh. Mater. Sci. Eng. C 2006, 26, 124–129. [Google Scholar] [CrossRef]
- Nojehdehian, H.; Moztarzadeh, F.; Baharvand, H.; Nazarian, H.; Tahriri, M. Preparation and surface characterization of poly-l-lysine-coated PLGA microsphere scaffolds containing retinoic acid for nerve tissue engineering: In vitro study. Coll. Surf. B Biointerfaces 2009, 73, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Lorite, G.S.; Kokkonen, H.E.; Cho, S.-W.; Lehenkari, P.P.; Skrifvars, M.; Tuukkanen, J. Effect of bioactive extruded PLGA/HA composite films on focal adhesion formation of preosteoblastic cells. Coll. Surf. B Biointerfaces 2014, 121, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Dickerman, R.D.; Schneider, S.J. New method of pediatric cranioplasty for skull defect utilizing polylactic acid absorbable plates and carbonated apatite bone cement. J. Craniofac. Surg. 2004, 15, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.S.; Carpenter, N.; Wei, T.L.; McNally, D.; Ahmed, I.; Boszczyk, B.M. Effects of adding resorbable phosphate glass fibres and PLA to calcium phosphate bone cements. J. Appl. Biomater. Funct. Mater. 2014, 12, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Losee, J.E.; Karmacharya, J.; Gannon, F.H.; Slemp, A.E.; Ong, G.; Hunenko, O.; Gorden, A.D.; Bartlett, S.P.; Kirschner, R.E. Reconstruction of the immature craniofacial skeleton with a carbonated calcium phosphate bone cement: Interaction with bioresorbable mesh. J. Craniofacial Surg. 2003, 14, 117–124. [Google Scholar] [CrossRef]
- Stupack, D.G.; Puente, X.S.; Boutsaboualoy, S.; Storgard, C.M.; Cheresh, D.A. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 2001, 155, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Lieb, E.; Hacker, M.; Tessmar, J.; Kunz-Schughart, L.A.; Fiedler, J.; Dahmen, C.; Hersel, U.; Kessler, H.; Schulz, M.B.; Gopferich, A. Mediating specific cell adhesion to low-adhesive diblock copolymers by instant modification with cyclic RGD peptides. Biomaterials 2005, 26, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.M.; Vasile, M.J.; Pearce, E.M.; Waszczak, J.V. Copper chloride complexes with poly(2-vinylpyridine): Preparation and redox properties. Macromolecules 1988, 21, 3125–3134. [Google Scholar] [CrossRef]
- Lecomte, P.; Detrembleur, C.; Lou, X.; Mazza, M.; Halleux, O.; Jerome, R. Novel functionalization routes of poly(epsilon-caprolactone). Macromol. Symp. 2000, 157, 47–60. [Google Scholar] [CrossRef]
- Lou, X.D.; Detrembleur, C.; Jerome, R. Novel aliphatic polyesters based on functional cyclic (di)esters. Macromol. Rapid Commun. 2003, 24, 161–172. [Google Scholar] [CrossRef]
- Jerome, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.K. Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 2007, 36, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Metters, A.T.; Anseth, K.S.; Bowman, C.N. Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel. Polymer 2000, 41, 3993–4004. [Google Scholar] [CrossRef]
- Riley, T.; Stolnik, S.; Heald, C.R.; Xiong, C.D.; Garnett, M.C.; Illum, L.; Davis, S.S.; Purkiss, S.C.; Barlow, R.J.; Gellert, P.R. Physicochemical evaluation of nanoparticles assembled from poly(lactic acid)-poly(ethylene glycol) (PLA-PEG) block copolymers as drug delivery vehicles. Langmuir 2001, 17, 3168–3174. [Google Scholar] [CrossRef]
- Fu, B.; Xiao, L.; Yu, L.J.; Yang, G. Preparation of lactic acid based polyurethanes modified by castor oil. Multi-Funct. Mater. Struct. 2008, 47–50, 1458–1461. [Google Scholar] [CrossRef]
- Hu, Y.F.; Liu, Y.F.; Qi, X.; Liu, P.; Fan, Z.Y.; Li, S.M. Novel bioresorbable hydrogels prepared from chitosan-graft-polylactide copolymers. Polym. Int. 2012, 61, 74–81. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Copinet, A.; Legin-Copinet, E.; Fricoteaux, F.; Coma, V. Different PLA grafting techniques on chitosan. J. Polym. Environ. 2011, 19, 166–171. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Park, H.; Jabbari, E.; Conway, D.E.; Sheffield, T.L.; Ambrose, C.G.; Mikos, A.G. Thermally cross-linked oligo(poly(ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro. Biomacromolecules 2004, 5, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, A.S.; He, X.; Jabbari, E. Viscoelastic characterization and modeling of gelation kinetics of injectable in situ cross-linkable poly(lactide-co-ethylene oxide-co-fumarate) hydrogels. Biomacromolecules 2007, 8, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, A.S.; Xu, W.; He, X.; Jabbari, E. Gelation and degradation characteristics of in situ photo-crosslinked poly(l-lactide-co-ethylene oxide-co-fumarate) hydrogels. Polymer 2007, 48, 7113–7120. [Google Scholar] [CrossRef]
- He, X.; Ma, J.; Jabbari, E. Migration of marrow stromal cells in response to sustained release of stromal-derived factor-1α from poly(lactide ethylene oxide fumarate) hydrogels. Int. J. Pharm. 2010, 390, 107–116. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ma, J.; Mercado, A.; Xu, W.; Jabbari, E. Cytotoxicity of paclitaxel in biodegradable self-assembled core-shell poly(lactide-co-glycolide ethylene oxide fumarate) nanoparticles. Pharm. Res. 2008, 25, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Zhong, X.; Valtchev, P.; Dehghani, F. Synthesis of a biodegradable polymer in gas expanded solution: Effect of the process on cytocompatibility. Green Chem. 2013, 15, 1280–1291. [Google Scholar] [CrossRef]
- Lee, S.; Zhong, X.; Ravarian, R.; Valtchev, P.; Dehghani, F. An approach to improve the efficiency of polymerization and enhance biological activity of poly(lactide-co-ethylene oxide fumarate) hydrogels. Polym. Sci. Polym. Chem. 2014, 52, 1291–1299. [Google Scholar] [CrossRef]
- Fathi, A.; Lee, S.; Zhong, X.; Hon, N.; Valtchev, P.; Dehghani, F. Fabrication of interpenetrating polymer network to enhance the biological activity of synthetic hydrogels. Polymer 2013, 54, 5534–5542. [Google Scholar] [CrossRef]
- Fathi, A.; Lee, S.; Breen, A.; Shirazi, A.N.; Valtchev, P.; Dehghani, F. Enhancing the mechanical properties and physical stability of biomimetic polymer hydrogels for micro-patterning and tissue engineering applications. Eur. Polym. J. 2014, 59, 161–170. [Google Scholar] [CrossRef]
- Fathi, A.; Mithieux, S.M.; Wei, H.; Chrzanowski, W.; Valtchev, P.; Weiss, A.S.; Dehghani, F. Elastin based cell-laden injectable hydrogels with tunable gelation, mechanical and biodegradation properties. Biomaterials 2014, 35, 5425–5435. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Dehghani, F. Antiseptic Polymer and Synthesis Thereof. AUP2015902943, 24 July 2015. [Google Scholar]
- Fathi, A.; Dehghani, F.; Weiss, A.; Mithieux, S. Application of Thermoresponsive Bioactive Injectable Hydrogel for Bone Regeneration. AUP2015903552, 1 September 2015. [Google Scholar]
- Dehghani, F.; Weiss, A.; Wei, H.; Mithieux, S.; Fathi, A. A Peptide-Hydrogel Composite. WO2013091001 A1, 27 June 2013. [Google Scholar]
- Hazer, B.; Steinbüchel, A. Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl. Microbiol. Biotechnol. 2007, 74, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Hajnal, I.; Wu, H.; Lv, L.; Ye, J. Engineering biosynthesis mechanisms for diversifying polyhydroxyalkanoates. Trends Biotechnol. 2015, 33, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-Q.; Zhao, Y.; Chen, G.-Q. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 2007, 28, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Igari, T.; Nishimura, D.; Nakamura, M.; Satoh, Y.; Munekata, M. Isolation and characterization ofbacillus sp. Int005 accumulating polyhydroxyalkanoate (PHA) from gas field soil. J. Biosci. Bioeng. 2003, 95, 77–81. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Khlusov, I.A.; Volova, T.G. A hybrid PHB-hydroxyapatite composite for biomedical application: production, in vitro and in vivo investigation. J. Biomater. Sci. Polym. Ed. 2006, 17, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Meischel, M.; Eichler, J.; Martinelli, E.; Karr, U.; Weigel, J.; Schmöller, G.; Tschegg, E.K.; Fischerauer, S.; Weinberg, A.M.; Stanzl-Tschegg, S.E. Adhesive strength of bone-implant interfaces and in vivo degradation of PHB composites for load-bearing applications. J. Mech. Behav. Biomed. Mater. 2016, 53, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Ansari, T.I.; Valappil, S.P.; Mohn, D.; Philip, S.E.; Stark, W.J.; Roy, I.; Knowles, J.C.; Salih, V.; Boccaccini, A.R. Poly(3-hydroxybutyrate) multifunctional composite scaffolds for tissue engineering applications. Biomaterials 2010, 31, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Paşcu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, J.; Liu, J. Evaluation of PHBV/calcium silicate composite scaffolds for cartilage tissue engineering. Appl. Surf. Sci. 2014, 317, 278–283. [Google Scholar] [CrossRef]
- Zhang, S.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Biocomposite scaffolds for bone regeneration: Role of chitosan and hydroxyapatite within poly-3-hydroxybutyrate-co-3-hydroxyvalerate on mechanical properties and in vitro evaluation. J. Mech. Behav. Biomed. Mater. 2015, 51, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Avella, M.; Martuscelli, E.; Raimo, M. The fractionated crystallization phenomenon in poly(3-hydroxybutyrate) poly(ethylene oxide) blends. Polymer 1993, 34, 3234–3240. [Google Scholar] [CrossRef]
- Francis, L.; Meng, D.; Knowles, J.C.; Roy, I.; Boccaccini, A.R. Multi-functional P(3HB) microsphere/45s5 bioglass®-based composite scaffolds for bone tissue engineering. Acta Biomater. 2010, 6, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Amrita, A.A.; Sharma, P.; Katti, D.S. Pullulan-based composite scaffolds for bone tissue engineering: Improved osteoconductivity by pore wall mineralization. Carbohydr. Polym. 2015, 123, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.T.; Jamshidi, A.; Irani, S. Nano-hydroxyapatite reinforced polyhydroxybutyrate composites: A comprehensive study on the structural and in vitro biological properties. Mat. Sci. Eng. C Mater. 2013, 33, 2776–2787. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Probst, F.; Schwartz, C.; Cornelsen, M.; Seitz, H.; Ehrenfeld, M.; Otto, S. A concept for scaffold-based tissue engineering in alveolar cleft osteoplasty. J. Cranio-Maxillofac. Surg. 2015, 43, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.Y.; Deng, X.M. Semi-interpenetrating networks of bacterial poly(3-hydroxybutyrate) with net-poly(ethylene glycol). Polymer 2001, 42, 4091–4097. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Inoue, S.; Koinuma, H.; Tsuruta, T. Copolymerization of carbon dioxide and epoxide. J. Polym. Sci. Polym. Lett. 1969, 7, 287–292. [Google Scholar] [CrossRef]
- Luinstra, G.A. Poly(propylene carbonate), old copolymers of propylene oxide and carbon dioxide with new interests: Catalysis and material properties. Polym. Rev. 2008, 48, 192–219. [Google Scholar] [CrossRef]
- Li, X.H.; Meng, Y.Z.; Zhu, Q.; Xu, Y.; Tjong, S.C. Melt processable and biodegradable aliphatic polycarbonate derived from carbon dioxide and propylene oxide. J. Appl. Polym. Sci. 2003, 89, 3301–3308. [Google Scholar] [CrossRef]
- Kim, G.; Ree, M.; Kim, H.; Kim, I.; Kim, J.; Lee, J. Biological affinity and biodegradability of poly(propylene carbonate) prepared from copolymerization of carbon dioxide with propylene oxide. Macromol. Res. 2008, 16, 473–480. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nakano, M.; Juni, K.; Inoue, S.; Yoshida, Y. Examination of biodegradability of poly(ethylene carbonate) and poly(propylene carbonate) in the peritoneal cavity in rats. Chem. Pharm. Bull. 1983, 31, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Dehghani, F. Fabrication of biomimetic poly(propylene carbonate) scaffolds by using carbon dioxide as a solvent, monomer and foaming agent. Green Chem. 2012, 14, 2523–2533. [Google Scholar] [CrossRef]
- Welle, A.; Kröger, M.; Döring, M.; Niederer, K.; Pindel, E.; Chronakis, I.S. Electrospun aliphatic polycarbonates as tailored tissue scaffold materials. Biomaterials 2007, 28, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.-Y.; Peng, J.; Peng, X.-F.; Turng, L.-S. Electrospun aligned poly(propylene carbonate) microfibers with chitosan nanofibers as tissue engineering scaffolds. Carbohydr. Polym. 2015, 117, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Lu, Z.; Valtchev, P.; Wei, H.; Zreiqat, H.; Dehghani, F. Surface modification of poly(propylene carbonate) by aminolysis and layer-by-layer assembly for enhanced cytocompatibility. Coll. Surf. B Biointerfaces 2012, 93, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Su, J.J.; Gao, J.; Hu, X.; Geng, C.Z.; Fu, Q. Fabrication of well-controlled porous foams of graphene oxide modified poly(propylene-carbonate) using supercritical carbon dioxide and its potential tissue engineering applications. J Supercrit. Fluid 2013, 73, 1–9. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, D.; Seo, J.; Han, H.; Khan, S.B. Preparation and characterization of poly(propylene carbonate)/exfoliated graphite nanocomposite films with improved thermal stability, mechanical properties and barrier properties. Polym. Int. 2013, 62, 1386–1394. [Google Scholar] [CrossRef]
- Gao, G.; Feng, C.; Wang, K.; Deng, H.; Zhang, Q.; Bai, H.; Fu, Q. A promising alternative to conventional polyethylene with poly(propylene carbonate) reinforced by graphene oxide nanosheets. J. Mater. Chem. 2011, 21, 17627–17630. [Google Scholar] [CrossRef]
- Ren, G.J.; Sheng, X.F.; Qin, Y.S.; Chen, X.S.; Wang, X.H.; Wang, F.S. Toughening of poly(propylene carbonate) using rubbery non-isocyanate polyurethane: Transition from brittle to marginally tough. Polymer 2014, 55, 5460–5468. [Google Scholar] [CrossRef]
- Qin, Y.S.; Chen, L.J.; Wang, X.H.; Zhao, X.J.; Wang, F.S. Enhanced mechanical performance of poly(propylene carbonate) via hydrogen bonding interaction with o-lauroyl chitosan. Carbohydr. Polym. 2011, 84, 329–334. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, R.Y.; Cao, Y.X.; Meng, Y.Z. Essential work of fracture analysis for starch filled poly(propylene carbonate) composites. Mater. Des. 2007, 28, 1934–1939. [Google Scholar] [CrossRef]
- Zeng, S.S.; Wang, S.J.; Xiao, M.; Han, D.M.; Meng, Y.Z. Preparation and properties of biodegradable blend containing poly (propylene carbonate) and starch acetate with different degrees of substitution. Carbohydr. Polym. 2011, 86, 1260–1265. [Google Scholar] [CrossRef]
- Ma, X.F.; Chang, P.R.; Yu, J.G.; Wang, N. Preparation and properties of biodegradable poly(propylene carbonate)/thermoplastic dried starch composites. Carbohydr. Polym. 2008, 71, 229–234. [Google Scholar] [CrossRef]
- Flynn, A.; Torres, L.F.; Orts, W.J.; Klamczynski, A. Bioderived Compatibilizer for Biopolymers. U.S. Patents US20090253871 A1, 8 October 2015. [Google Scholar]
- Chen, C.; John Scheirs, J. Polymer/Thermoplastic Starch Compositions. EP2473542 B1, 29 July 2015. [Google Scholar]
- Tian, L.; Wang, P.; Zhao, Z.; Ji, J. Antimicrobial activity of electrospun poly(butylenes succinate) fiber mats containing PVP-capped silver nanoparticles. Appl. Biochem. Biotechnol. 2013, 171, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.H.; Im, S.S.; Youk, J.H. Electrospinning and structural characterization of ultrafine poly(butylene succinate) fibers. Polymer 2005, 46, 9538–9543. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Correlo, V.M.; Sol, P.C.; Bhattacharya, M.; Srouji, S.; Livne, E.; Reis, R.L.; Neves, N.M. Chitosan–poly(butylene succinate) scaffolds and human bone marrow stromal cells induce bone repair in a mouse calvaria model. J. Tissue Eng. Regen. Med. 2012, 6, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-B.; Huang, C.-L.; Jiao, L.; Lu, X.; Wang, Y.-Z.; Wang, X.-L. Synthesis and properties of biodegradable poly(butylene succinate-co-diethylene glycol succinate) copolymers. Ind. Eng. Chem. Res. 2012, 51, 12258–12265. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Correlo, V.M.; Sol, P.C.; Bhattacharya, M.; Charbord, P.; Delorme, B.; Reis, R.L.; Neves, N.M. Osteogenic differentiation of human bone marrow mesenchymal stem cells seeded on melt based chitosan scaffolds for bone tissue engineering applications. Biomacromolecules 2009, 10, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Correlo, V.M.; Sol, P.C.; Costa-Pinto, A.R.; Malafaya, P.B.; Salgado, A.J.; Bhattacharya, M.; Charbord, P.; Neves, N.M.; Reis, R.L. Assessment of the suitability of chitosan/polybutylene succinate scaffolds seeded with mouse mesenchymal progenitor cells for a cartilage tissue engineering approach. Tissue Eng. 2008, 14, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Llorens, E.; Ibañez, H.; del Valle, L.J.; Puiggalí, J. Biocompatibility and drug release behavior of scaffolds prepared by coaxial electrospinning of poly(butylene succinate) and polyethylene glycol. Mater. Sci. Eng. C 2015, 49, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Viha, C.; Thitirat, C.; Furuike, T.; Tamura, H.; Jayakumar, R. Fabrication of chitin/poly(butylene succinate)/chondroitin sulfate nanoparticles ternary composite hydrogel scaffold for skin tissue engineering. Polymers 2014, 6, 2974. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, J.H. New application of poly(butylene succinate) (PBS) based ionomer as biopolymer: A role of ion group for hydroxyapatite (HAP) crystal formation. J. Mater. Sci. 2009, 44, 6398–6403. [Google Scholar] [CrossRef]
- Ngamviriyavong, P.; Patntirapong, S.; Janvikul, W.; Arphavasin, S.; Meesap, P.; Singhatanadgit, W. Development of poly(butylene succinate)/calcium phosphate composites for bone engineering. Compos. Interfaces 2014, 21, 431–441. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ε-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Cooper, A.; Bhattarai, N.; Zhang, M. Fabrication and cellular compatibility of aligned chitosan–PCL fibers for nerve tissue regeneration. Carbohydr. Polym. 2011, 85, 149–156. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.H. Electrohydrodynamic direct printing of PCL/collagen fibrous scaffolds with a core/shell structure for tissue engineering applications. Chem. Eng. J. 2015, 279, 317–326. [Google Scholar] [CrossRef]
- Gautam, S.; Chou, C.-F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater. Sci. Eng. C 2014, 34, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, G. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr. Polym. 2014, 114, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Causa, F.; Taddei, P.; di Foggia, M.; Ciapetti, G.; Martini, D.; Fagnano, C.; Baldini, N.; Ambrosio, L. Polylactic acid fibre-reinforced polycaprolactone scaffolds for bone tissue engineering. Biomaterials 2008, 29, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Pei, X.; He, R.; Wan, Q.; Wang, J. Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Coll. Surf. B Biointerfaces 2012, 93, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, G. Electrospun PCL/phlorotannin nanofibres for tissue engineering: Physical properties and cellular activities. Carbohydr. Polym. 2012, 90, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hwang, H.; Kim, Y.; Jeon, H.; Kim, G. Physical and bioactive properties of multi-layered PCL/silica composite scaffolds for bone tissue regeneration. Chem. Eng. J. 2014, 250, 399–408. [Google Scholar] [CrossRef]
- Dziadek, M.; Menaszek, E.; Zagrajczuk, B.; Pawlik, J.; Cholewa-Kowalska, K. New generation poly(ε-caprolactone)/gel-derived bioactive glass composites for bone tissue engineering: Part I. Material properties. Mater. Sci. Eng. C 2015, 56, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhah, A.; Marquardt, L.M.; Sakiyama-Elbert, S.E.; Day, D.E.; Harkins, A.B. Fabrication and characterization of poly-(ε)-caprolactone and bioactive glass composites for tissue engineering applications. Mater. Sci. Eng. C 2015, 49, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Shin, K.-H.; Noh, D.-Y.; Jo, I.-H.; Koh, Y.-H.; Kim, H.-E.; Kim, S.E. Sol–gel derived nanoscale bioactive glass (nBG) particles reinforced poly(ε-caprolactone) composites for bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lui, Y.S.; Choo, C.K.C.; Sow, W.T.; Huang, C.L.; Ng, K.W.; Tan, L.P.; Loo, J.S.C. Calcium phosphate coated keratin–PCL scaffolds for potential bone tissue regeneration. Mater. Sci. Eng. C 2015, 49, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.; Myers, J.D.; Madihally, S.V.; Jacot, J.G. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013, 9, 5630–5642. [Google Scholar] [CrossRef] [PubMed]
- Biscaia, S.I.; Viana, T.F.; Almeida, H.A.; Bártolo, P.J. Production and characterisation of PCL/es scaffolds for bone tissue engineering. Mater. Today Proc. 2015, 2, 208–216. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Weiss, A.S.; Dehghani, F. Fabrication of porous PCL/elastin composite scaffolds for tissue engineering applications. J. Supercrit. Fluids 2011, 59, 157–167. [Google Scholar] [CrossRef]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Martens, P.; Weiss, A.S.; Dehghani, F. The effect of elastin on chondrocyte adhesion and proliferation on poly (ɛ-caprolactone)/elastin composites. Biomaterials 2011, 32, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Patrício, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Procedia CIRP 2013, 5, 110–114. [Google Scholar] [CrossRef]
- Williamson, M.R.; Black, R.; Kielty, C. PCL–PU composite vascular scaffold production for vascular tissue engineering: Attachment, proliferation and bioactivity of human vascular endothelial cells. Biomaterials 2006, 27, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Meng, T.T.H.; Chuan, Y.L.; Chowdhury, M.; Prasad, R.G.S.V. Fabrication and characterization of hybrid PCL/PEG 3D scaffolds for potential tissue engineering applications. Mater. Lett. 2014, 131, 255–258. [Google Scholar] [CrossRef]
- Mabrouk, M.; Bijukumar, D.; Mulla, J.A.S.; Chejara, D.R.; Badhe, R.V.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. Enhancement of the biomineralization and cellular adhesivity of polycaprolactone-based hollow porous microspheres via dopamine bio-activation for tissue engineering applications. Mater. Lett. 2015, 161, 503–507. [Google Scholar] [CrossRef]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, P.; Cannillo, V.; Sola, A.; Dorigato, A.; Chiellini, F. Highly porous polycaprolactone-45s5 bioglass® scaffolds for bone tissue engineering. Compos. Sci. Technol. 2010, 70, 1869–1878. [Google Scholar] [CrossRef]
- Shim, W.S.; Yoo, J.S.; Bae, Y.H.; Lee, D.S. Novel injectable pH and temperature sensitive block copolymer hydrogel. Biomacromolecules 2005, 6, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Zheng, L.; Luo, F.; Luo, J.; Qian, Z. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.J.; Joo, M.K.; Choi, B.G.; Park, M.H.; Hamley, I.W.; Jeong, B. Multiple sol-gel transitions of PEG-PCL-PEG triblock copolymer aqueous solution. Macromol. Rapid Commun. 2010, 31, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.J.; Suh, J.M.; Bae, Y.H.; Kim, S.W.; Jeong, B. Caprolactonic poloxamer analog: PEG-PCL-PEG. Biomacromolecules 2005, 6, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.; Song, C.; Ma, G. Injectable thermosensitive hydrogels for intra-articular delivery of methotrexate. J. Appl. Polym. Sci. 2011, 122, 2139–2145. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C 2014, 45, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, S. Poly(ɛ-caprolactone) acrylates synthesized using a facile method for fabricating networks to achieve controllable physicochemical properties and tunable cell responses. Polymer 2010, 51, 164–177. [Google Scholar] [CrossRef]
- Ranjha, N.; Mudassir, J.; Majeed, S. Synthesis and characterization of polycaprolactone/acrylic acid (PCL/AA) hydrogel for controlled drug delivery. Bull. Mater. Sci. 2011, 34, 1537–1547. [Google Scholar] [CrossRef]
- Rieger, J.; Van Butsele, K.; Lecomte, P.; Detrembleur, C.; Jerome, R.; Jerome, C. Versatile functionalization and grafting of poly(ε-caprolactone) by michael-type addition. Chem. Commun. 2005, 2, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, L.; Gruetzmacher, J.A.; Currier, B.L.; Yaszemski, M.J. A biodegradable and cross-linkable multiblock copolymer consisting of poly(propylene fumarate) and poly(ε-caprolactone): Synthesis, characterization, and physical properties. Macromolecules 2005, 38, 7358–7370. [Google Scholar] [CrossRef]
- Boffito, M.; Sirianni, P.; Di Rienzo, A.M.; Chiono, V. Thermosensitive block copolymer hydrogels based on poly(ɛ-caprolactone) and polyethylene glycol for biomedical applications: State of the art and future perspectives. J. Biomed. Mater. Res. 2015, 103, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Qin, G.; Li, X.; Lv, H.; Qian, Z.; Yu, L. The PEG-PCL-PEG hydrogel as an implanted ophthalmic delivery system after glaucoma filtration surgery; a pilot study. Med. Hypothesis Discov. Innov. Ophthalmol. 2014, 3, 3–8. [Google Scholar] [PubMed]
- Lin, T.-C.; Hung, K.-H.; Peng, C.-H.; Liu, J.-H.; Woung, L.-C.; Tsai, C.-Y.; Chen, S.-J.; Chen, Y.-T.; Hsu, C.-C. Nanotechnology-based drug delivery treatments and specific targeting therapy for age-related macular degeneration. J. Chin. Med. Assoc. 2015, 78, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-W.; Elkasabi, Y.; Chen, H.-Y.; Zhang, Y.; Lahann, J.; Hollister, S.J.; Krebsbach, P.H. The use of reactive polymer coatings to facilitate gene delivery from poly (ɛ-caprolactone) scaffolds. Biomaterials 2009, 30, 5785–5792. [Google Scholar] [CrossRef] [PubMed]
- Riva, R.; Lenoir, S.; Jérôme, R.; Lecomte, P. Functionalization of poly(ε-caprolactone) by pendant hydroxyl, carboxylic acid and epoxide groups by atom transfer radical addition. Polymer 2005, 46, 8511–8518. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Xie, Z.; Cheng, H.; Jing, X. Aliphatic poly(ester-carbonate)s bearing amino groups and its rgd peptide grafting. J. Polym. Sci. Polym. Chem. 2008, 46, 7022–7032. [Google Scholar] [CrossRef]
- Kasper, F.K.; Tanahashi, K.; Fisher, J.P.; Mikos, A.G. Synthesis of poly(propylene fumarate). Nat. Protoc. 2009, 4, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Piard, C.M.; Melchiorri, A.; Dreher, M.L.; Fisher, J.P. Evaluating changes in structure and cytotoxicity during in vitro degradation of three-dimensional printed scaffolds. Tissue Eng. 2015, 21, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Etheridge, J.M.; Thompson, J.A.; Vorwald, C.E.; Dean, D.; Fisher, J.P. Evaluation of the in vitro cytotoxicity of cross-linked biomaterials. Biomacromolecules 2013, 14, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, L.; Yaszemski, M.J. Bone-tissue-engineering material poly(propylene fumarate): Correlation between molecular weight, chain dimensions, and physical properties. Biomacromolecules 2006, 7, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Vehof, J.W.M.; Dean, D.; van, d.W.J.P.C.M.; Holland, T.A.; Mikos, A.G.; Jansen, J.A. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J. Biomed. Mater. Res. 2002, 59, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, L.G.; Yu, L.; Hibino, N.; Fisher, J.P. Reinforced pericardium as a hybrid material for cardiovascular applications. Tissue Eng. 2014, 20, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- He, S.; J. Yaszemski, M.; Yasko, A.W.; Engel, P.S.; Mikos, A.G. Injectable biodegradable polymer composites based on poly(propylene fumarate) crosslinked with poly(ethylene glycol)-dimethacrylate. Biomaterials 2000, 21, 2389–2394. [Google Scholar] [CrossRef]
- He, S.; Timmer, M.D.; Yaszemski, M.J.; Yasko, A.W.; Engel, P.S.; Mikos, A.G. Synthesis of biodegradable poly(propylene fumarate) networks with poly(propylene fumarate)–diacrylate macromers as crosslinking agents and characterization of their degradation products. Polymer 2001, 42, 1251–1260. [Google Scholar] [CrossRef]
- Timmer, M.D.; Ambrose, C.G.; Mikos, A.G. In vitro degradation of polymeric networks of poly(propylene fumarate) and the crosslinking macromer poly(propylene fumarate)-diacrylate. Biomaterials 2003, 24, 571–577. [Google Scholar] [CrossRef]
- Horch, R.A.; Shahid, N.; Mistry, A.S.; Timmer, M.D.; Mikos, A.G.; Barron, A.R. Nanoreinforcement of poly(propylene fumarate)-based networks with surface modified alumoxane nanoparticles for bone tissue engineering. Biomacromolecules 2004, 5, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Dean, D.; Mikos, A.G. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials 2002, 23, 4333–4343. [Google Scholar] [CrossRef]
- Wang, K.; Cai, L.; Wang, S. Methacryl-polyhedral oligomeric silsesquioxane as a crosslinker for expediting photo-crosslinking of poly(propylene fumarate): Material properties and bone cell behavior. Polymer 2011, 52, 2827–2839. [Google Scholar] [CrossRef]
- Kim, K.; Dean, D.; Wallace, J.; Breithaupt, R.; Mikos, A.G.; Fisher, J.P. The influence of stereolithographic scaffold architecture and composition on osteogenic signal expression with rat bone marrow stromal cells. Biomaterials 2011, 32, 3750–3763. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, J.; Runge, M.B.; Dadsetan, M.; Chen, Q.; Lu, L.; Yaszemski, M.J. Cross-linking characteristics and mechanical properties of an injectable biomaterial composed of polypropylene fumarate and polycaprolactone co-polymer. J. Biomater. Sci. Polym. Ed. 2011, 22, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Brandi, F.; Anjum, F.; Ceseracciu, L.; Barone, A.C.; Athanassiou, A. Rigid biodegradable photopolymer structures of high resolution using deep-UV laser photocuring. J. Micromech. Microeng. 2011. [Google Scholar] [CrossRef]
- Cai, L.; Wang, K.; Wang, S. Poly(ethylene glycol)-grafted poly(propylene fumarate) networks and parabolic dependence of MC3T3 cell behavior on the network composition. Biomaterials 2010, 31, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Danti, S.; D'Alessandro, D.; Pietrabissa, A.; Petrini, M.; Berrettini, S. Development of tissue-engineered substitutes of the ear ossicles: Porp-shaped poly(propylene fumarate)-based scaffolds cultured with human mesenchymal stromal cells. J. Biomed. Mater. Res. 2010, 92, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, S. Parabolic dependence of material properties and cell behavior on the composition of polymer networks via simultaneously controlling crosslinking density and crystallinity. Biomaterials 2010, 31, 7423–7434. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Muthu, J. Biosynthetic hydrogels—Studies on chemical and physical characteristics on long-term cellular response for tissue engineering. J. Biomed. Mater. Res. 2014, 102, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Thankam, F.G.; Muthu, J. Infiltration and sustenance of viability of cells by amphiphilic biosynthetic biodegradable hydrogels. J. Mater. Sci. Mater. Med. 2014, 25, 1953–1965. [Google Scholar] [CrossRef] [PubMed]
- Shung, A.K.; Behravesh, E.; Jo, S.; Mikos, A.G. Crosslinking characteristics of and cell adhesion to an injectable poly(propylene fumarate-co-ethylene glycol) hydrogel using a water-soluble crosslinking system. Tissue Eng. 2003, 9, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, K.; Mikos, A.G. Protein adsorption and smooth muscle cell adhesion on biodegradable agmatine-modified poly(propylene fumarate-co-ethylene glycol) hydrogels. J. Biomed. Mater. Res. 2003, 67, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, K.; Mikos, A.G. Cell adhesion on poly(propylene fumarate-co-ethylene glycol) hydrogels. J. Biomed. Mater. Res. 2002, 62, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, K.; Jo, S.; Mikos, A.G. Synthesis and characterization of biodegradable cationic poly(propylene fumarate-co-ethylene glycol) copolymer hydrogels modified with agmatine for enhanced cell adhesion. Biomacromolecules 2002, 3, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, K.; Mikos, A.G. Effect of hydrophilicity and agmatine modification on degradation of poly(propylene fumarate-co-ethylene glycol) hydrogels. J. Biomed. Mater. Res. 2003, 67, 1148–1154. [Google Scholar]
- Cai, L.; Chen, J.; Rondinone, A.J.; Wang, S. Injectable and biodegradable nanohybrid polymers with simultaneously enhanced stiffness and toughness for bone repair. Adv. Funct. Mater. 2012, 22, 3181–3190. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. https://doi.org/10.3390/polym8010020
Manavitehrani I, Fathi A, Badr H, Daly S, Negahi Shirazi A, Dehghani F. Biomedical Applications of Biodegradable Polyesters. Polymers. 2016; 8(1):20. https://doi.org/10.3390/polym8010020
Chicago/Turabian StyleManavitehrani, Iman, Ali Fathi, Hesham Badr, Sean Daly, Ali Negahi Shirazi, and Fariba Dehghani. 2016. "Biomedical Applications of Biodegradable Polyesters" Polymers 8, no. 1: 20. https://doi.org/10.3390/polym8010020
APA StyleManavitehrani, I., Fathi, A., Badr, H., Daly, S., Negahi Shirazi, A., & Dehghani, F. (2016). Biomedical Applications of Biodegradable Polyesters. Polymers, 8(1), 20. https://doi.org/10.3390/polym8010020