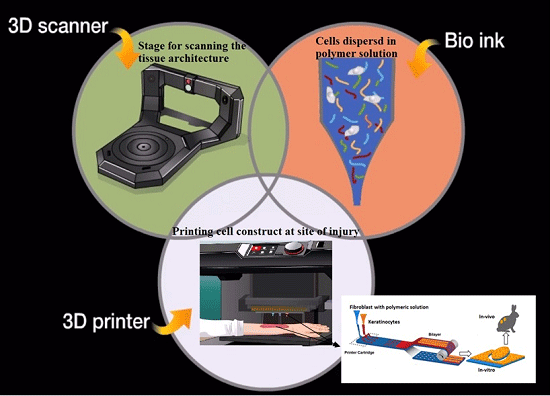
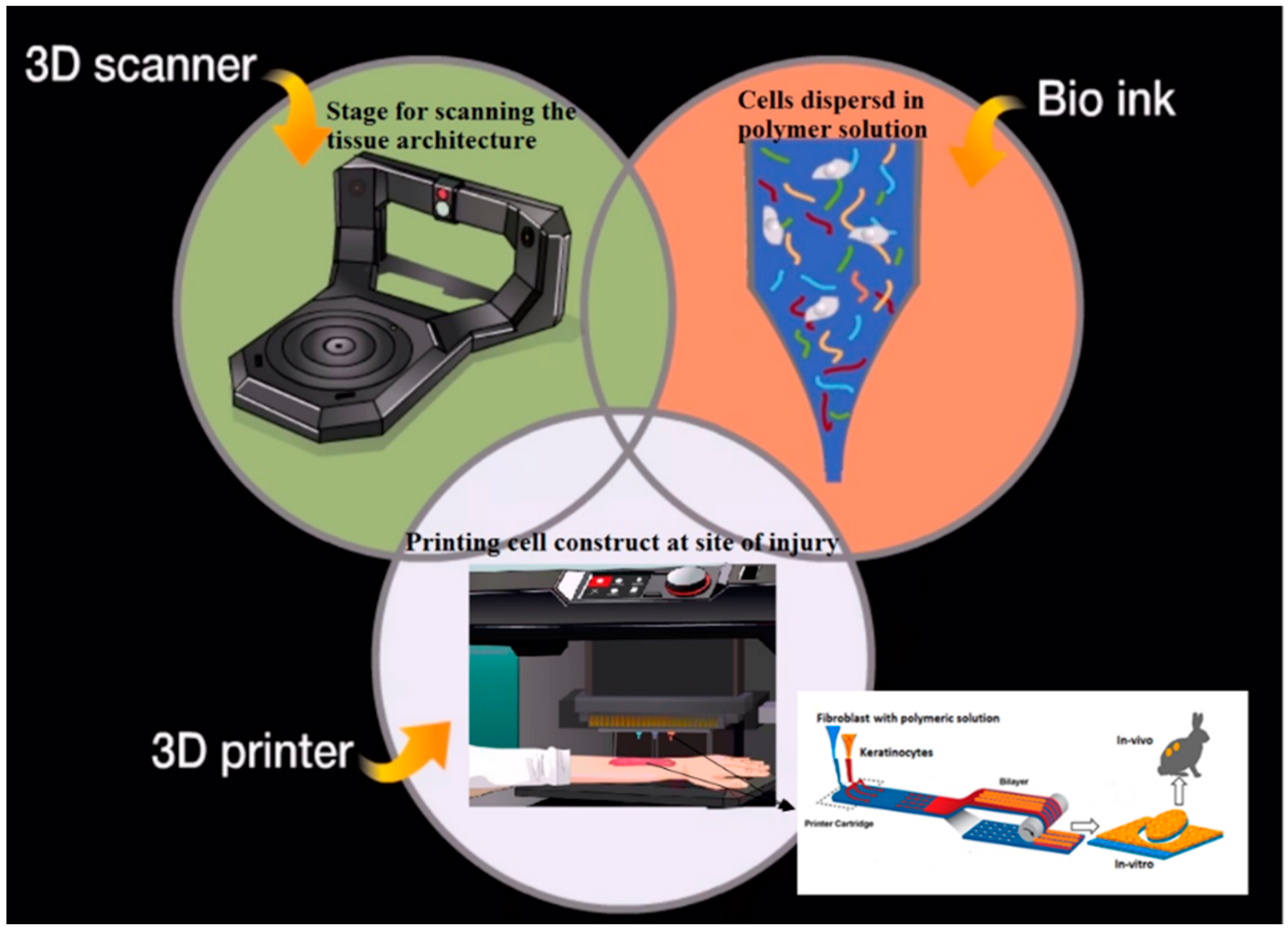
3D Printing of Scaffold for Cells Delivery: Advances in Skin Tissue Engineering
Abstract
:1. Introduction
2. Overview of Bio-Printing
2.1. Bioprinting of Hydrogels
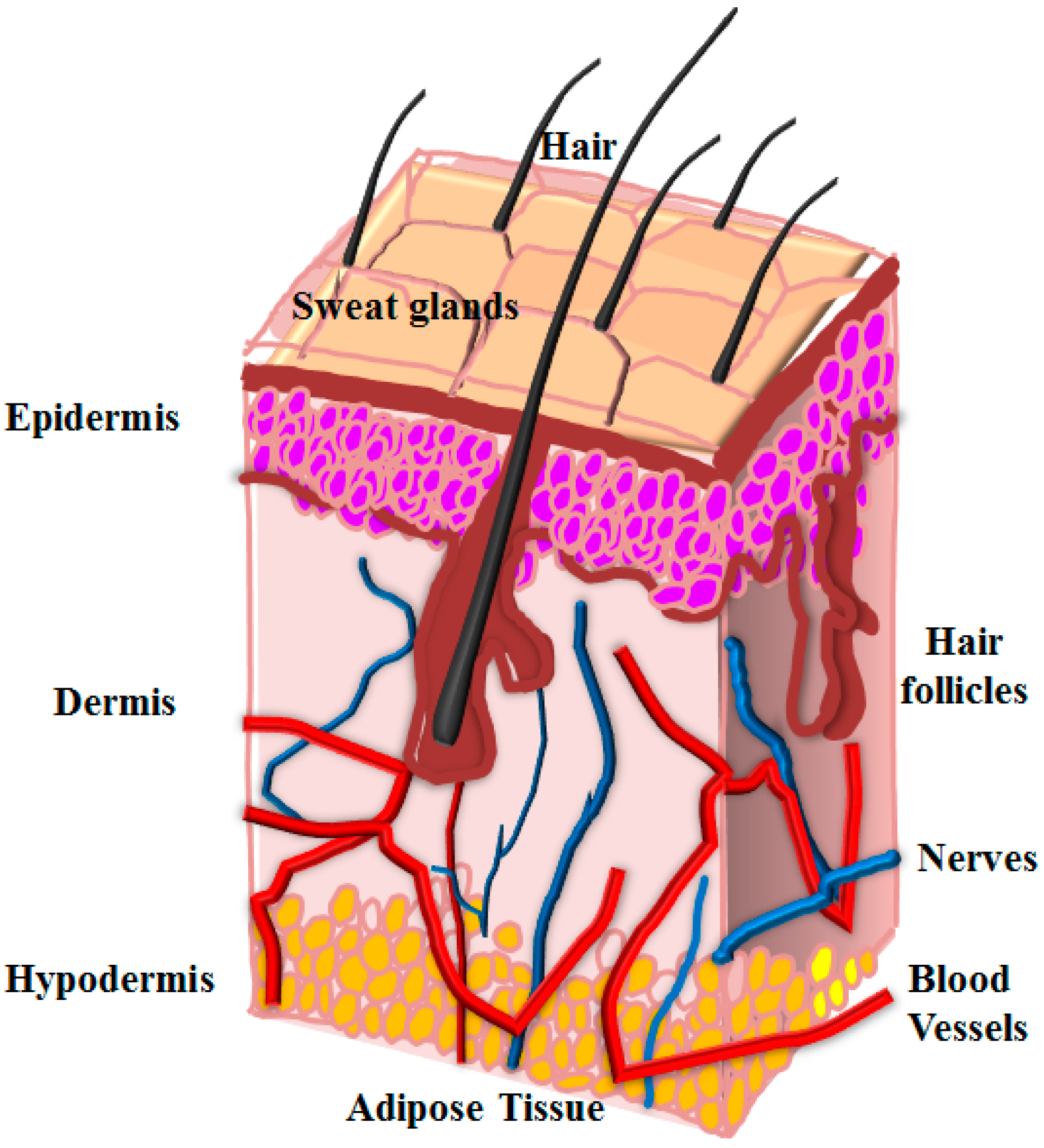
2.2. Emergence of 3D Printing for Skin Repair
2.2.1. Biomimicry
2.2.2. Autonomous Self-Assembly
2.2.3. Mini-Tissue
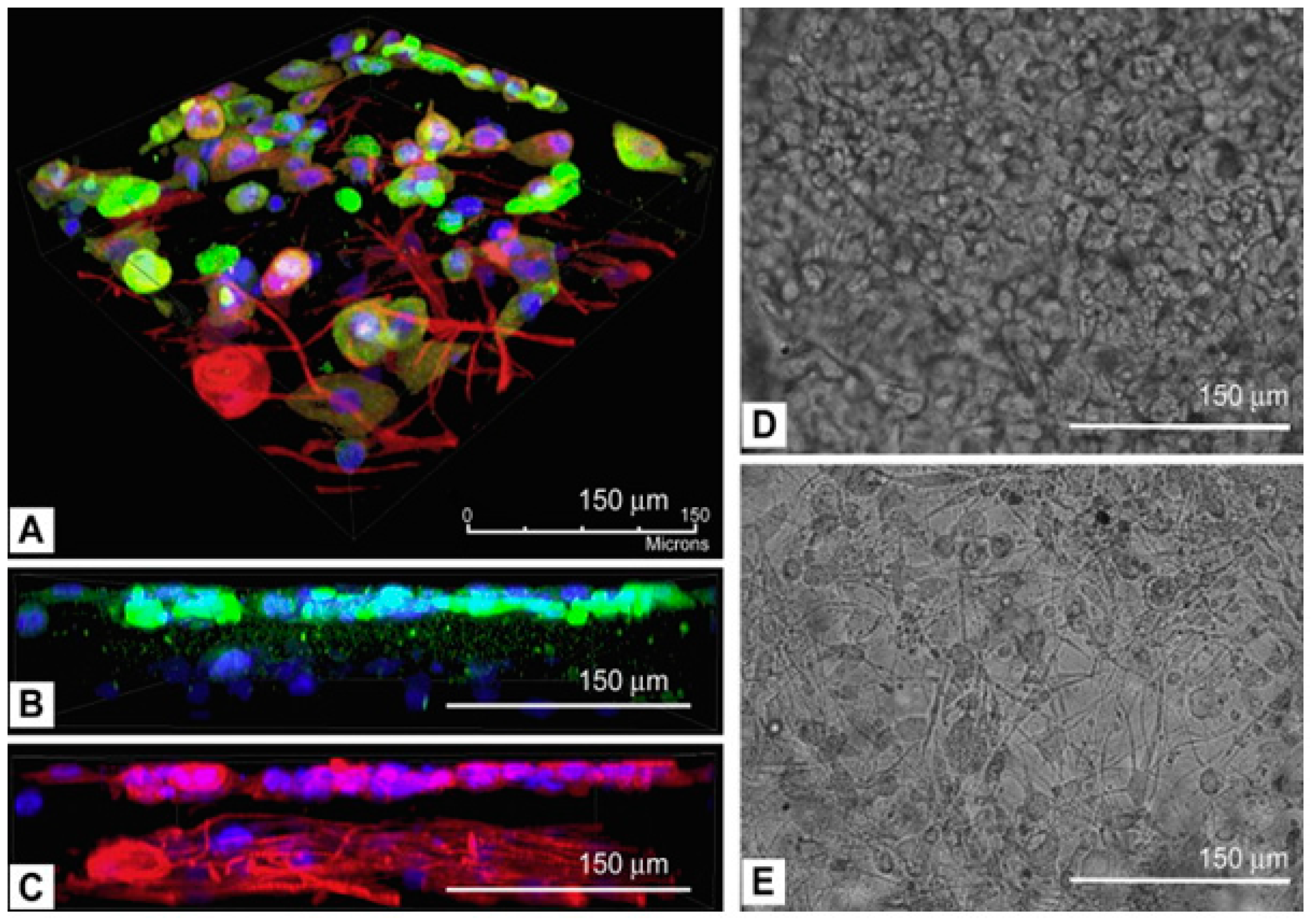
2.3. Laser Assisted Bioprinting
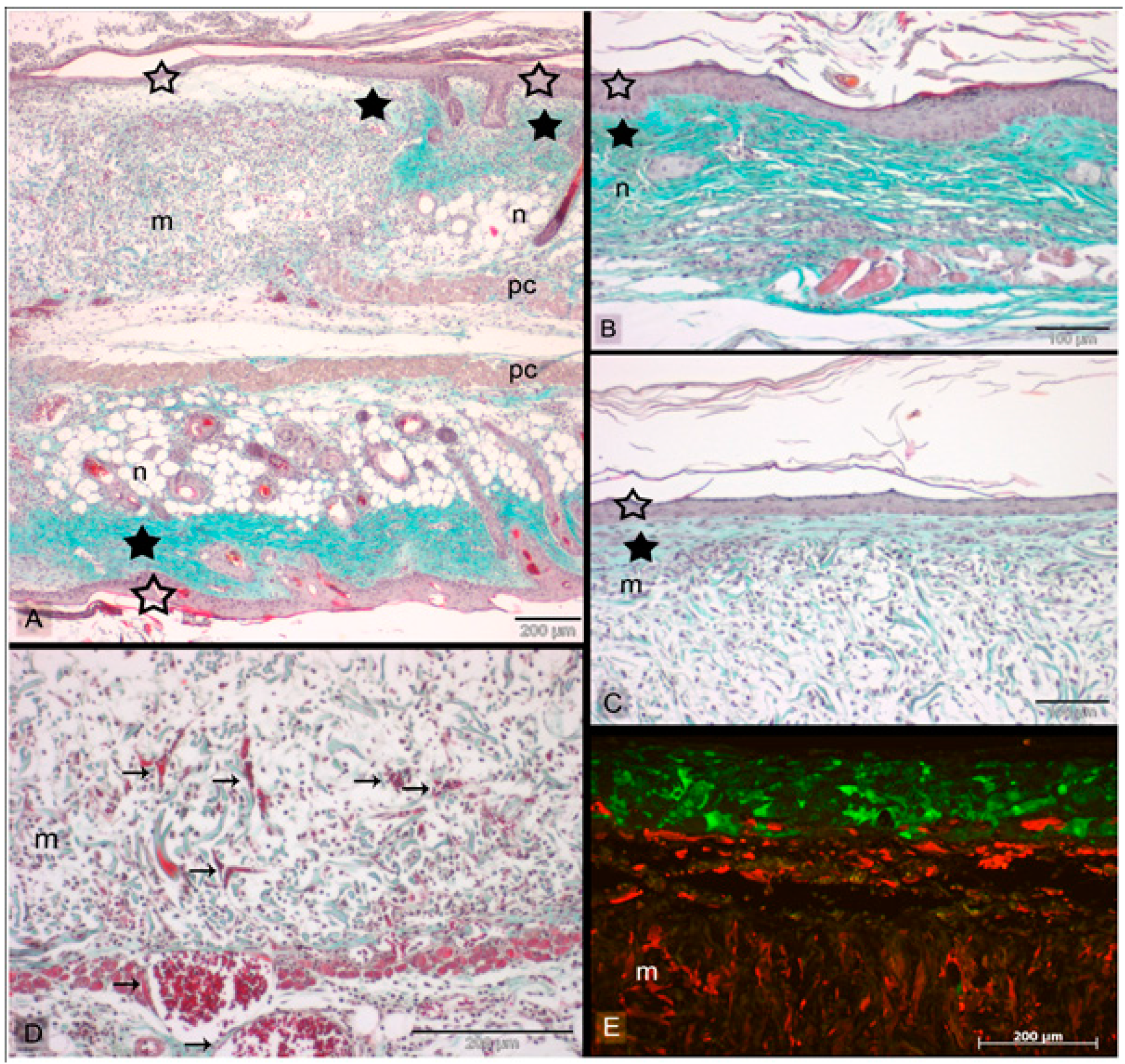
3. Advances in Stem Cells for Skin Substitute
3.1. Role of Induced Pluripotent (iPSCs) and Embryonic Stem Cells
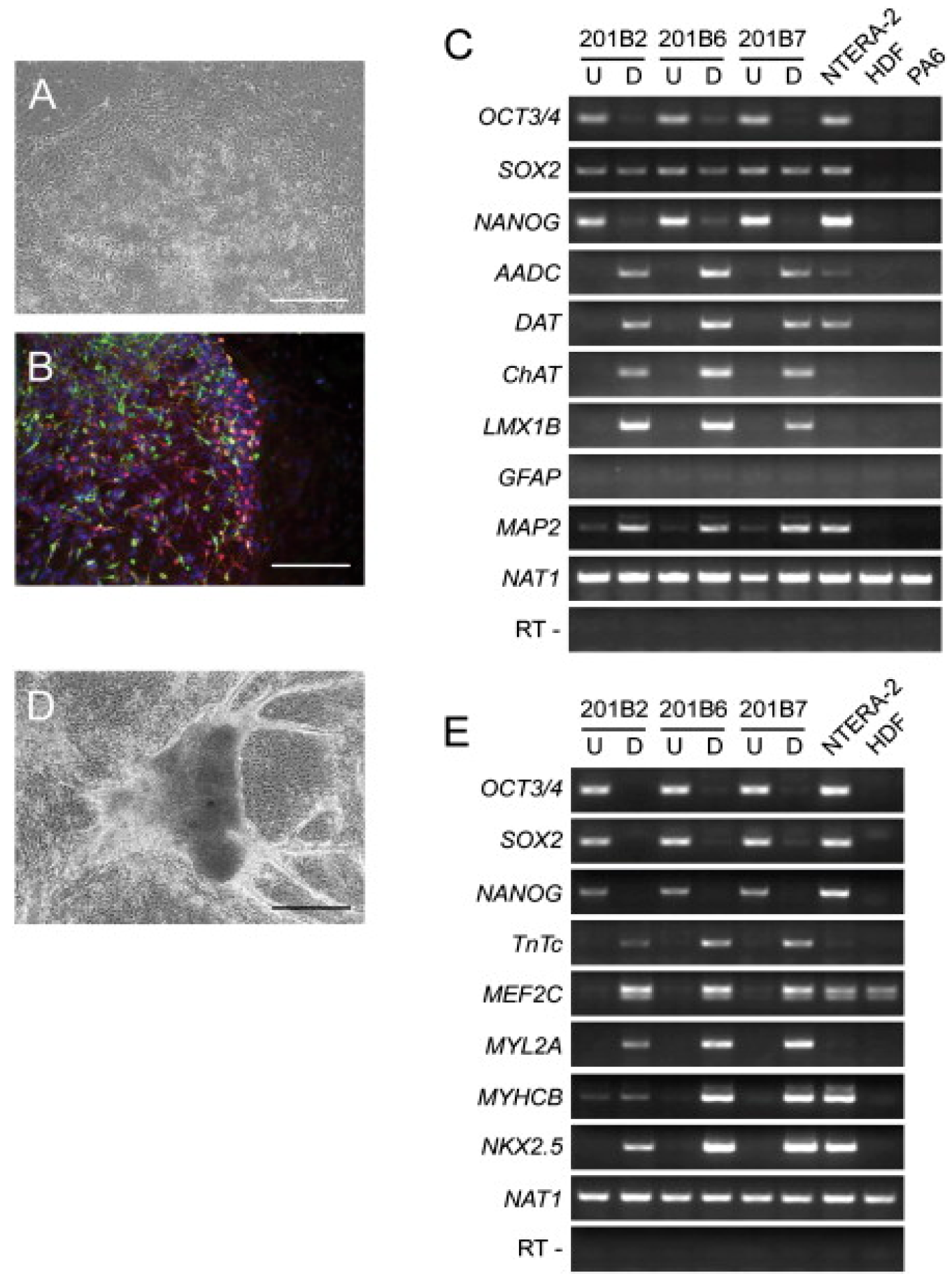
3.2. Manipulation of Mesenchymal Stem Cells for Skin Repair
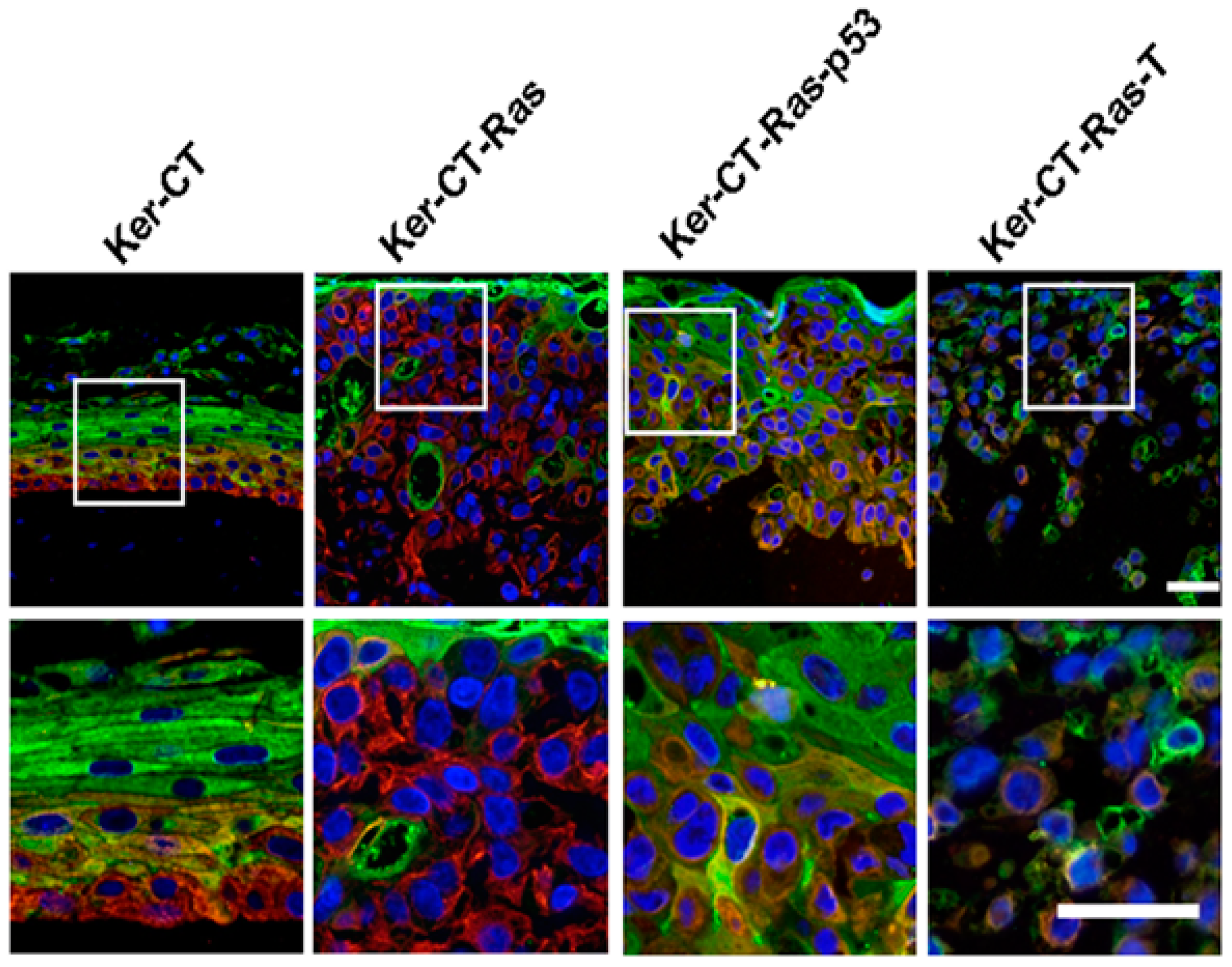
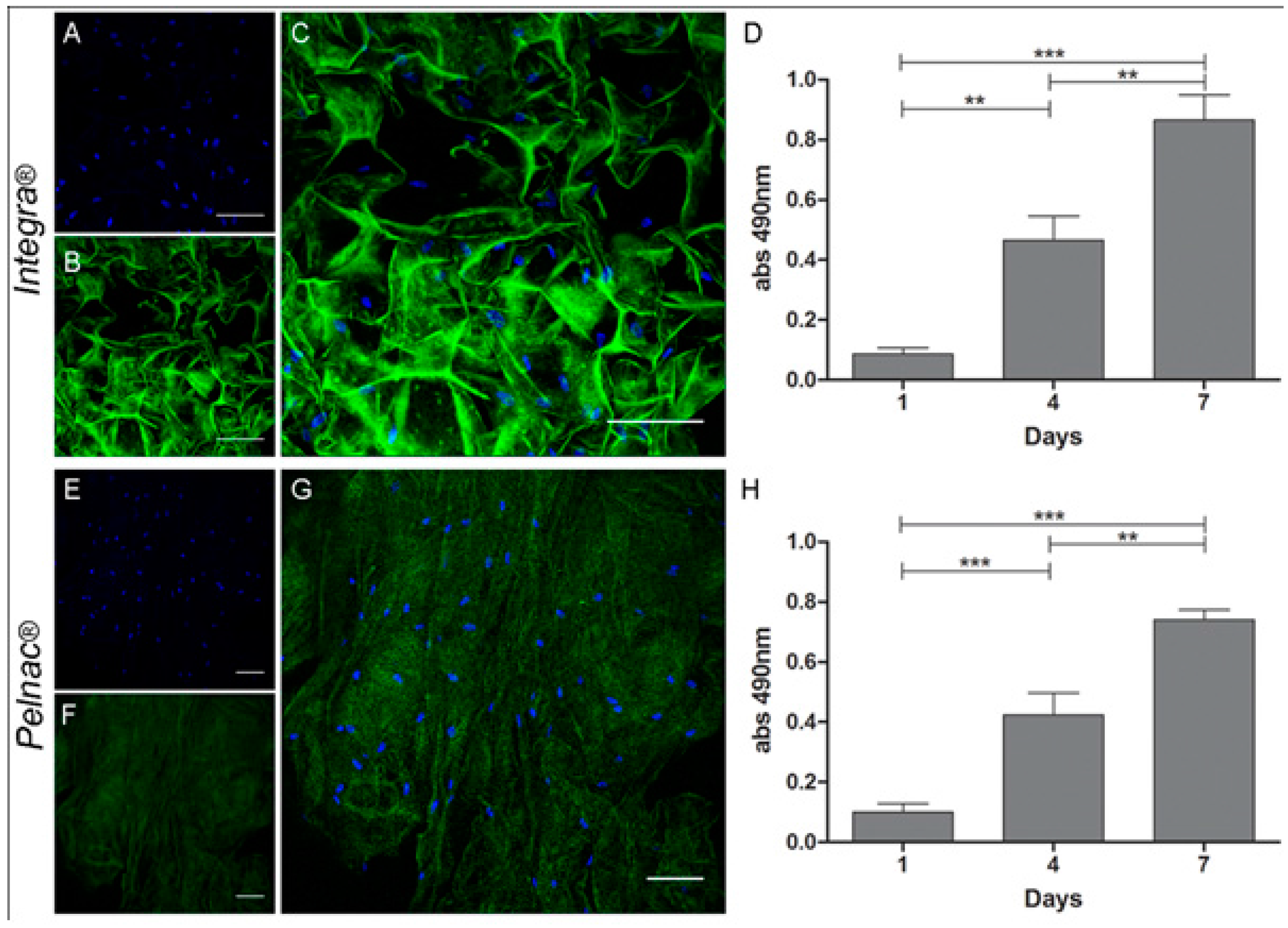
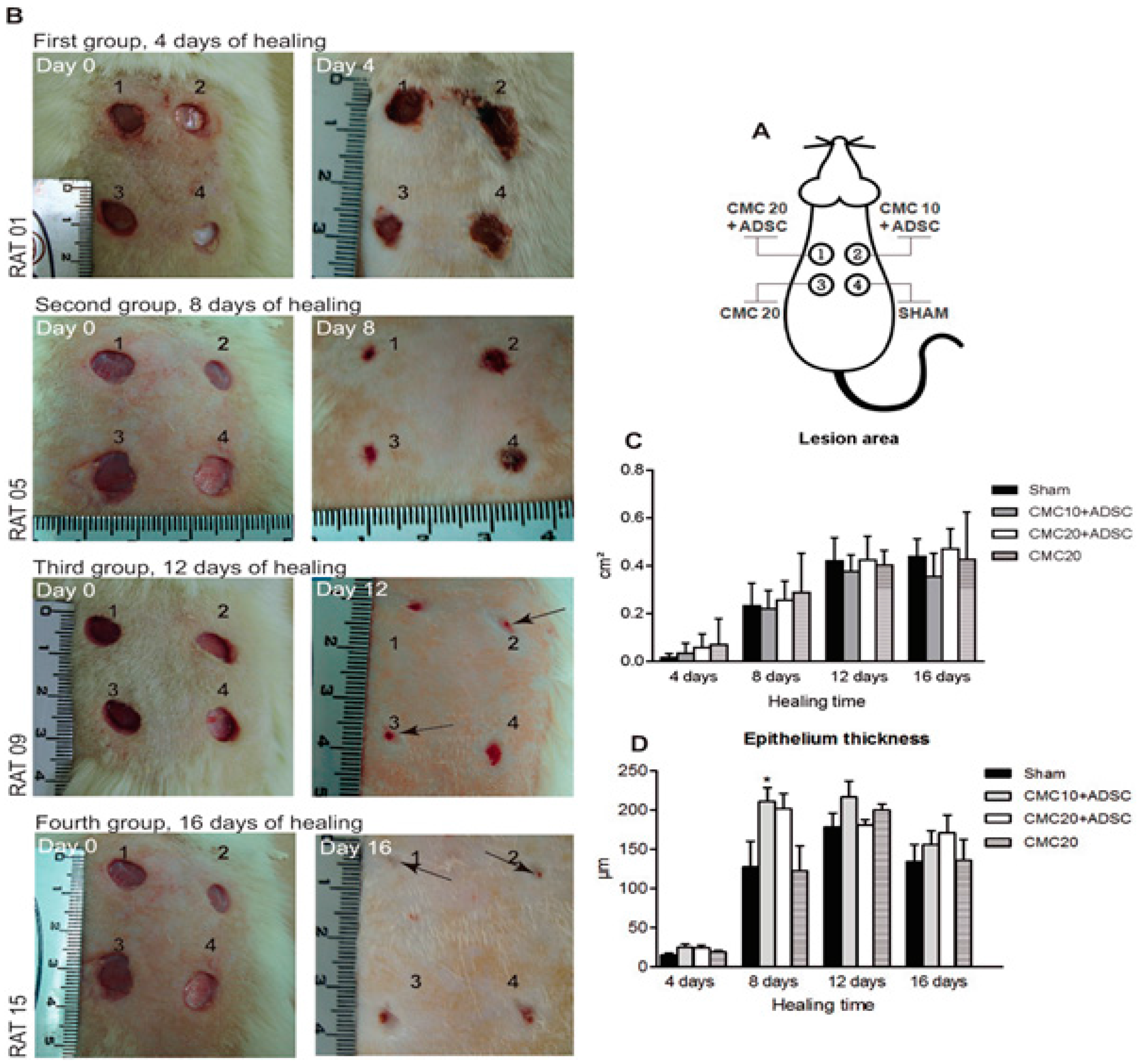
3.3. Adipose-Derived Stem Cells for Skin Regeneration
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Q.; Liang, S.; Thomas, G.A. Elastomeric biomaterials for tissue engineering. Prog. Polym. Sci. 2013, 38, 584–671. [Google Scholar] [CrossRef]
- Furth, M.E.; Atala, A.; van Dyke, M.E. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 2007, 28, 5068–5073. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Tripathi, A.; Zo, S.M.; Singh, D.; Han, S.S. Synthesis of composite gelatin-hyaluronic acid-alginate porous scaffold and evaluation for in vitro stem cell growth and in vivo tissue integration. Colloid Surf. B 2014, 116, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Puente, P.D.; Ludena, D. Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell. Res. 2014, 322, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffold. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Butcher, A.L.; Offeddu, G.S.; Oyen, M.L. Nanofibrous hydrogel composites as mechanically robust tissue engineering scaffolds. Trends Biotechnol. 2014, 32, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Utoh, R.; Nagase, K.; Okano, T. Cell sheet approach for tissue engineering and regenerative medicine. J. Control. Release 2014, 190, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Sheyn, D.; Mizrahi, O.; Bengamin, S.; Gazit, Z.; Pelled, G.; Gazit, D. Genetically modified cells in regenerative medicine and tissue engineering. Adv. Drug. Deliver. Rev. 2010, 62, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, J. Tissue engineering and regenerative medicine: From first principles to state of the art. J. Pediatr. Surg. 2010, 45, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Feric, N.T.; Thavandiran, N.; Nunes, S.S.; Radisic, M. The role of tissue engineering and biomaterials in cardiac regenerative medicine. Can. J. Cardiol. 2014, 30, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.; Wells, A. Dermal–epidermal communication in wound healing. Wounds 2001, 13, 183–189. [Google Scholar]
- Park, H.J.; Yu, S.J.; Yang, K.; Jin, Y.; Cho, A.N.; Kim, J.; Lee, B.; Yang, H.S.; Im, S.G.; Cho, S.W. Paper-based bioactive scaffolds for stem cell-mediated bone tissue engineering. Biomaterials 2014, 35, 9811–9823. [Google Scholar] [CrossRef] [PubMed]
- Bannasch, H.; Fohn, M.; Unterberg, T.; Bach, A.D.; Weyand, B.; Stark, G.B. Skin tissue engineering. Clin. Plast. Surg. 2003, 30, 573–579. [Google Scholar] [CrossRef]
- Raic, A.; Rodling, L.; Kalbacher, H.; Lee-Thedieck, C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematooietic stem and progenitor cells. Biomaterials 2014, 35, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, M.; Lizzo, G.; Gualandi, C.; Mangano, C.; Giuliani, A.; Focarete, M.L.; Calza, L. Influence of biological matrix and artificial electrospun scaffolds on proliferation, differentiation and trophic factor synthesis of rat embryonic stem cells. Matrix. Biol. 2014, 33, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Han, S.F.; Zhao, Y.; Xiao, Z.F.; Han, J.; Chen, B.; Chen, L.; Dai, J.W. The three-dimensional collagen scaffold improves the stemness of rat bone marrow mesenchymal stem cells. J. Genet. Genomics 2012, 39, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Dong, Y.; Su, Z.; Yin, R.; Song, A.; Li, S.M. Preparation, characteristics and assessment of a novel gelatin-chitosan sponge scaffold as skin tissue engineering material. Int. J. Pharm. 2014, 476, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, F.H.; Hussain, F.S.J.; Rasad, M.S.B.A.; Yusoff, M.M. In vitro degradation study of novel HEC/PVA/collagen nanofibrous scaffold for skin tissue engineering applications. Polym. Degrad. Stabil. 2014, 110, 473–481. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug. Delive. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Bottcher-Haberzeth, S.; Biedermann, T.; Reichmann, E. Tissue engineering of skin. Burns 2010, 36, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Wood, F. Tissue engineering of skin. Clin. Plast. Surg. 2012, 39, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.A.; Ong, J.F.; Eriksson, E.; Junker, J.P.E.; Caterson, E.J. Tissue engineering of skin. J. Am. Coll. Surgeons 2013, 217, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Black, A.F.; Bouez, C.; Perrier, E.; Schlotmann, K.; Chapuis, F.; Damour, O. Optimization and characterization of an engineered human skin equivalent. Tissue Eng. 2005, 11, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.; Lapointe, M.; Soucy, J.; Pouliot, R. Development of an in vitro psoriatic skin model by tissue engineering. J. Dermatol. Sci. 2009, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Seneschal, S.; Clark, R.A.; Gehad, A.; Baecher-Allan, C.M.; Kupper, T.S. Human epidermal langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T Cells. Immunity 2012, 36, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Zulkiffli, F.H.; Hussain, F.S.J.; Rasad, M.S.B.A.; Yusoff, M.M. Nanostructured materials from hydroxyethyl cellulose for skin tissue engineering. Carbohyd. Polym. 2014, 114, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Chou, C.F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mat. Sci. Eng. C 2014, 34, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Singh, D.; Singh, D.; Han, S.S. Surfactant role in modifying architecture of functional polymeric gelatin scaffolds. Int. J. Polym. Mater. Po. 2014, 63, 951–956. [Google Scholar] [CrossRef]
- Singh, D.; Singh, D.; Choi, S.; Zo, S.M.; Painuli, R.K.; Kwon, S.W.; Han, S.S. Effect of extracts of terminalia chebula on proliferation of keratinocytes and fibroblasts cells: An alternative approach for wound healing. Evid. Based Compl. Alt. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Jin, G.R.; Prabhakaran, M.P.; Ramakrishna, S. Stem cell differentiation to epidermal lineages on electrospun nanofibrous substrates for skin tissue engineering. Acta. Biomater. 2011, 7, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Varkey, M.; Ding, J.; Tredget, E.E. The effect of keratinocytes on the biomechanical characteristics and pore microstructure of tissue engineered skin using deep dermal fibroblasts. Biomaterials 2014, 35, 9591–9598. [Google Scholar] [CrossRef] [PubMed]
- Fauza, D.O.; Fishman, S.J.; Mehegan, K.; Atala, A. Videofetoscopically assisted fetal tissue engineering: Skin replacement. J. Pediatr. Surg. 1998, 33, 357–361. [Google Scholar] [CrossRef]
- Gillette, B.M.; Rossen, N.S.; Das, N.; Leong, D.; Wang, M.; Dugar, A.; Sia, S.K. Engineering extracellular matrix structure in 3D multiphase tissues. Biomaterials 2011, 32, 8067–8076. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Farach-Carson, M.C. Mining the extracellular matrix for tissue engineering applications. Regen. Med. 2010, 5, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–533. [Google Scholar] [CrossRef]
- Kuschel, C.; Steuer, H.; Maurer, A.N.; Kanzok, B.; Stoop, R.; Angres, B. Cell adhesion profiling using extracellular matrix protein microarrays. Biotechniques 2006, 40, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Huck, W.T.S. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell. Bio. 2013, 14, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Panetti, T.S.; Hannah, D.F.; Avraamides, C.; Gaughan, J.P.; Marcinkiewicz, C.; Huttenlocher, A.; Mosher, D.F. Extracellular matrix molecules regulate endothelial cell migration stimulated by lysophosphatidic acid. J. Thromb. Haemost. 2004, 2, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Trappmann, B.; Chen, C.S. How cells sense extracellular matrix stiffness: A material’s perspective. Curr. Opin. Biotech. 2013, 24, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta. Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 11, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Barrias, C.C.; Granja, P.L.; Bartolo, P.J. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 2013, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Reis, N.; Derby, B. Bioprinting: A beginning. Tissue Eng. 2006, 12, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. 2009, 4, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Tao, X.; Damon, B.J.; Manley, B.; Kesari, P.; Jalota, S.; Bhaduri, S. Drop-on-demand printing of cells and materials for designer tissue constructs. Mat. Sci. Eng. C 2007, 27, 372–376. [Google Scholar] [CrossRef]
- Lee, W.G.; Demirci, U.; Khademhosseini, A. Microscale electroporation: Challenges and perspectives for clinical applications. Integr. Biol. 2009, 1, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Allain, L.R.; Askari, M.; Stokes, D.L.; Vo-Dinh, T. Microarray sampling-platform fabrication using bubble-jet technology for a biochip system. Fresen. J. Anal. Chem. 2001, 371, 146–150. [Google Scholar] [CrossRef]
- Binder, K.W.; Allen, A.J.; Yoo, J.J.; Atala, A. Drop-on-demand inkjet bioprinting: A primer. Gene. Ther. Regul. 2011, 11, 1–19. [Google Scholar] [CrossRef]
- CBC Canada news technology and science. Available online: http://www.cbc.ca/news/technology/printalive-3d-skin-tissue-printer-wins-canadian-dyson-award-1.2770667 (accessed on 25 November 2015).
- Gardien, K.L.M.; Middelkoop, E.; Ulrich, M.M.W. Progress towards cell-based wound treatments. Regen. Med. 2014, 9, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Atala, A. Engineering organs. Curr. Opin. Biotech. 2009, 20, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Goretsky, M.J.; Greenhalgh, D.G.; Kagan, R.J.; Rieman, M.T.; Warden, G.D. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann. Surg. 1995, 222, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Mironov, V.; Gutowska, A.; Roth, E.A.; Markwald, R.R. Cell and organ printing 2: Fusion of cell aggregates in three-dimensional gels. Anat. Rec. Discov. Mol. Cell. Evol. Biol. 2003, 272, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Sachlos, E.; Czernuszka, J.T. Making tissue engineering scaffolds work. Review: The application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell. Mater. 2003, 5, 29–39. [Google Scholar] [PubMed]
- Burg, K.J.L.; Boland, T. Minimally invasive tissue engineering composites and cell printing. IEEE Eng. Med. Biol. 2003, 22, 84–91. [Google Scholar] [CrossRef]
- Gangatirkar, P.; Paquet-Fifield, S.; Li, A.; Rossi, R.; Kaur, P. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat. Protoc. 2007, 2, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Pamuditha, N.S.; Green, B.J.; Altamentova, S.M.; Rocheleau, J.V. A microfluidic device designed to induce media flow throughout pancreatic islets while limiting shear-induced damage. Lab A Chip 2013, 13, 4374–4384. [Google Scholar]
- Reed, E.J.; Klumb, L.; Koobatian, M.; Viney, C. Biomimicry as a route to new materials: What kinds of lessons are useful? Philos. Trans. R. Soc. A 2009, 367, 1571–1585. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Torisawa, Y.S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered physiological biomimicry: Organs-on-chips. Lab A Chip 2012, 12, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Marga, F.; Neagu, A.; Kosztion, I.; Forgacs, G. Developmental biology and tissue engineering. Birth. Defects. Res. C 2007, 81, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Steer, D.L.; Nigam, S.K. Developmental approaches to kidney tissue engineering. Am. J. Physiol. Renal. 2004, 286, F1–F7. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Kasza, K.E.; Rowat, A.C.; Liu, J.Y.; Angelini, T.E.; Brangwynne, C.P.; Koenderink, G.H.; Weitz, D.A. The cell as a material. Curr. Opin. Cell Biol. 2007, 19, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Lorber, V.; Snedeker, J.G.; Schmidt, D.; Tenzer, A.B.; Weisstanner, M.; Odermatt, B.; Mol, A.; Zund, G.; Hoerstrup, S.P. A novel concept for scaffold-free vessel tissue engineering: Self-assembly of microtissue building blocks. J. Biotechnol. 2010, 148, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kamei, M.; Saunders, W.B.; Bayless, K.J.; Dye, L.; Davis, G.E.; Weinstein, B.M. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006, 442, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Alajati, A.; Laib, A.M.; Weber, H.; Boos, A.M.; Bartol, A.; Ikenberg, K.; Korff, T.; Zentgraf, H.; Obodozie, C.; Graeser, R.; et al. Spheroid-based engineering of a human vasculature in mice. Nat. Methods 2008, 5, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Zavala, M.M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, F.; Schilling, N.; Mader, K.; Gruchow, M.; Klotzbach, U.; Lindner, G.; Horland, R.; Wagner, I.; Lauster, R.; Howitz, S.; et al. Design and prototyping of a chip-based multi-micro-organoid culture system for substance testing, predictive to human (substance) exposure. J. Biotechnol. 2010, 148, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Gunther, A.; Yasotharan, S.; Vagaon, A.; Lochovsy, C.; Pinto, S.; Yang, J.; Lau, C.; Bolz, J.V.; Bolz, S.S. A microfluidic platform for probing small artery structure and function. Lab A Chip 2010, 10, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Mankovich, N.J.; Samson, D.; Pratt, W.; Lew, D.; Beumer, J., 3rd. Surgical planning using three-dimensional imaging and computer modeling. Otolaryngol. Clin. North. Am. 1994, 27, 875–889. [Google Scholar] [PubMed]
- Pykett, I.L.; Newhouse, J.H.; Buonanno, F.S.; Brady, T.J.; Goldman, M.R.; Kistler, J.P.; Pohost, G.M. Principles of nuclear magnetic resonance imaging. Radiology 1982, 143, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.H.; Fischer, K.; Edmister, K.; Park, J.K.; Yoo, S.S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.; Milleret, V.; Nötzli, S.; Madduri, S.; Sulser, T.; Gobet, R.; Eberli, D. Increased porosity of electrospun hybrid scaffolds improved bladder tissue regeneration. J. Biomed. Mater. Res. A 2014, 102, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluations of hydrogels for bio-printing application. J. Biomed. Mater. Res. A 2012, 101, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Sorg, H.; Peck, C.T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; et al. Laser printing of skin cells and human stem cells. Tissue Eng. Methods 2010, 16, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Okano, H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cell. Cell. Res. 2013, 23, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J. Regenerative medicine for skin disease: iPS cells to the rescue. J. Investig. Dermatol. 2011, 131, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.; Roberts, R.M.; Mirochnitchenko, O. Large animal models for stem cell therapy. Stem Cell Res. Ther. 2013, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Okano, H. Promise of human induced pluripotent stem cells in skin regeneration and investigation. J. Investig. Dermatol. 2014, 134, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Nauta, A.; Wong, V.; Glotzbach, J.; Gurtner, G.C.; Longaker, M.T. The role of stem cells in cutaneous wound healing: What do we really know? Plast. Reconstr. Surg. 2011, 127, 10S–20S. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Nakamura, M.; Yoshida, K.; Okada, Y.; Tsuji, O.; Nore, S.; Ikeda, E.; Yamanaka, S.; Miura, K. Steps toward safe cell therapy using induced pluripotent stem cells. Circ. Res. 2013, 112, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Katsetos, C.D.; Legido, A.; Perentes, E.; Mörk, S.J. Class III β-tubulin isotype: A key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J. Child Neurol. 2003, 18, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Raya, A.; Barrero, M.J.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilic, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Utikal, J.; Maherali, N.; Kulalert, W.; Hochedlinger, K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J. Cell. Sci. 2009, 122, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Bouwman, B.A.; Ang, Y.S.; Kim, S.J.; Lee, D.F.; Lemischka, I.R.; Rendl, M. Single transcription factor reprogramming of hair follicle dermal papilla cells to induced pluripotent stem cells. Stem Cells 2011, 29, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Kiuru, M.; Cairo, M.S.; Christiano, A.M. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8797–8802. [Google Scholar] [CrossRef] [PubMed]
- Veraitch, O.; Kobayashi, T.; Imaizumi, Y.; Akamatsu, W.; Sasaki, T.; Yamanaka, S.; Amagai, M.; Okano, H.; Ohyama, M. Human induced pluripotent stem cell-derived ectodermal precursor cells contribute to hair follicle morphogenesis in vivo. J. Investig. Dermatol. 2009, 133, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jiang, M.; Kumar, S.; Xu, T.; Wang, F.; Xiang, L.; Xu, X. Generation of melanocytes from induced pluripotent stem cells. J. Investig. Dermatol. 2011, 131, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.E.; Bentz, M.L.; Hematti, P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast. Reconstr. Surg. 2010, 125, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, T.S.; Machado, R.G.; Silvia, J.P.; Leonardi, D.F.; Trentin, A.G. Dermal substitutes support the growth of human skin-derived mesenchymal stromal cells: Potential tool for skin regeneration. PLoS ONE 2014, 9, e89542. [Google Scholar] [CrossRef] [PubMed]
- Perng, C.K.; Kao, C.I.; Yang, Y.P.; Lin, H.T.; Lin, W.B.; Chu, Y.R.; Wang, H.J.; Ma, H.; Ku, H.H.; Chiou, S.H. Culturing adult human bone marrow stem cells on gelatin scaffold with pNIPAAm as transplanted grafts for skin regeneration. J. Biomed. Mater. Res. A 2008, 84, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Fu, X.; Cai, S.; Lei, Y.; Sun, T.; Bai, T.; Chen, M. Regeneration of functional sweat gland-like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Repair Regen. 2009, 17, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.B.; Ramirez, R.D.; Andrews, C.M.; Wright, W.E.; Shay, J.W. H-Ras Expression in immortalized keratinocytes produces an invasive epithelium in cultured skin equivalents. PLoS ONE 2009, 4, e7908. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.P.; Huang, C.H.; Shyu, J.F.; Lee, H.S.; Chen, S.G.; Chan, J.Y.; Huang, S.M. Promotion of wound healing using adipose-derived stem cells in radiation ulcer of a rat model. J. Biomed. Sci. 2013, 20, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Assis, A.M.; Moura, D.J.; Halmenschlager, G.; Jenifer, S.; Xavier, L.L.; Fernandes, M.C.; Wink, M.R. New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS ONE 2013, 9, e96241. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.; Singh, D.; Han, S.S. 3D Printing of Scaffold for Cells Delivery: Advances in Skin Tissue Engineering. Polymers 2016, 8, 19. https://doi.org/10.3390/polym8010019
Singh D, Singh D, Han SS. 3D Printing of Scaffold for Cells Delivery: Advances in Skin Tissue Engineering. Polymers. 2016; 8(1):19. https://doi.org/10.3390/polym8010019
Chicago/Turabian StyleSingh, Deepti, Dolly Singh, and Sung Soo Han. 2016. "3D Printing of Scaffold for Cells Delivery: Advances in Skin Tissue Engineering" Polymers 8, no. 1: 19. https://doi.org/10.3390/polym8010019
APA StyleSingh, D., Singh, D., & Han, S. S. (2016). 3D Printing of Scaffold for Cells Delivery: Advances in Skin Tissue Engineering. Polymers, 8(1), 19. https://doi.org/10.3390/polym8010019