Polymeric Slippery Coatings: Nature and Applications
Abstract
:1. Introduction
2. Slippery Surfaces in Nature
2.1. Hydrophobic Coatings
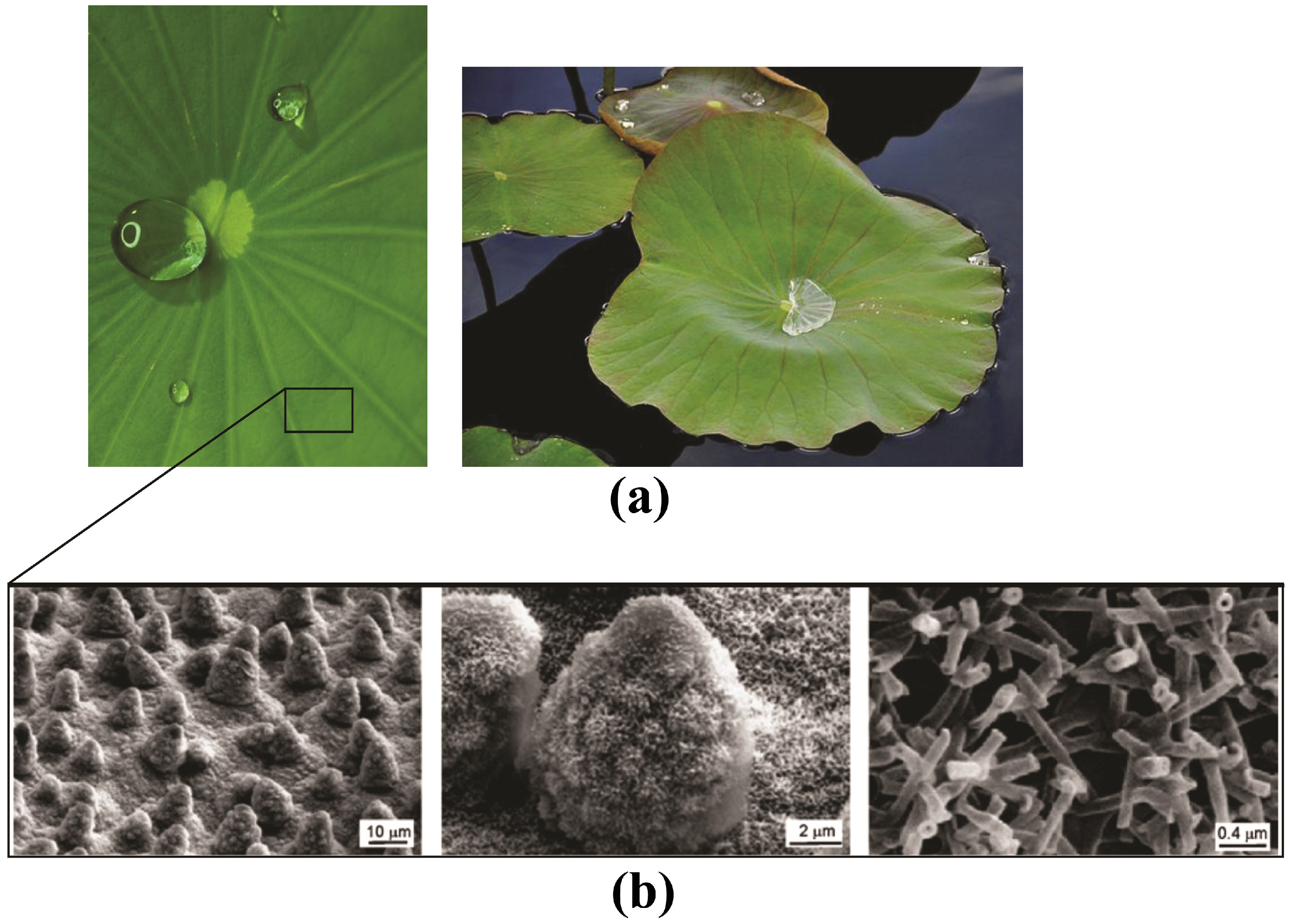
2.1.1. Lotus Leaves

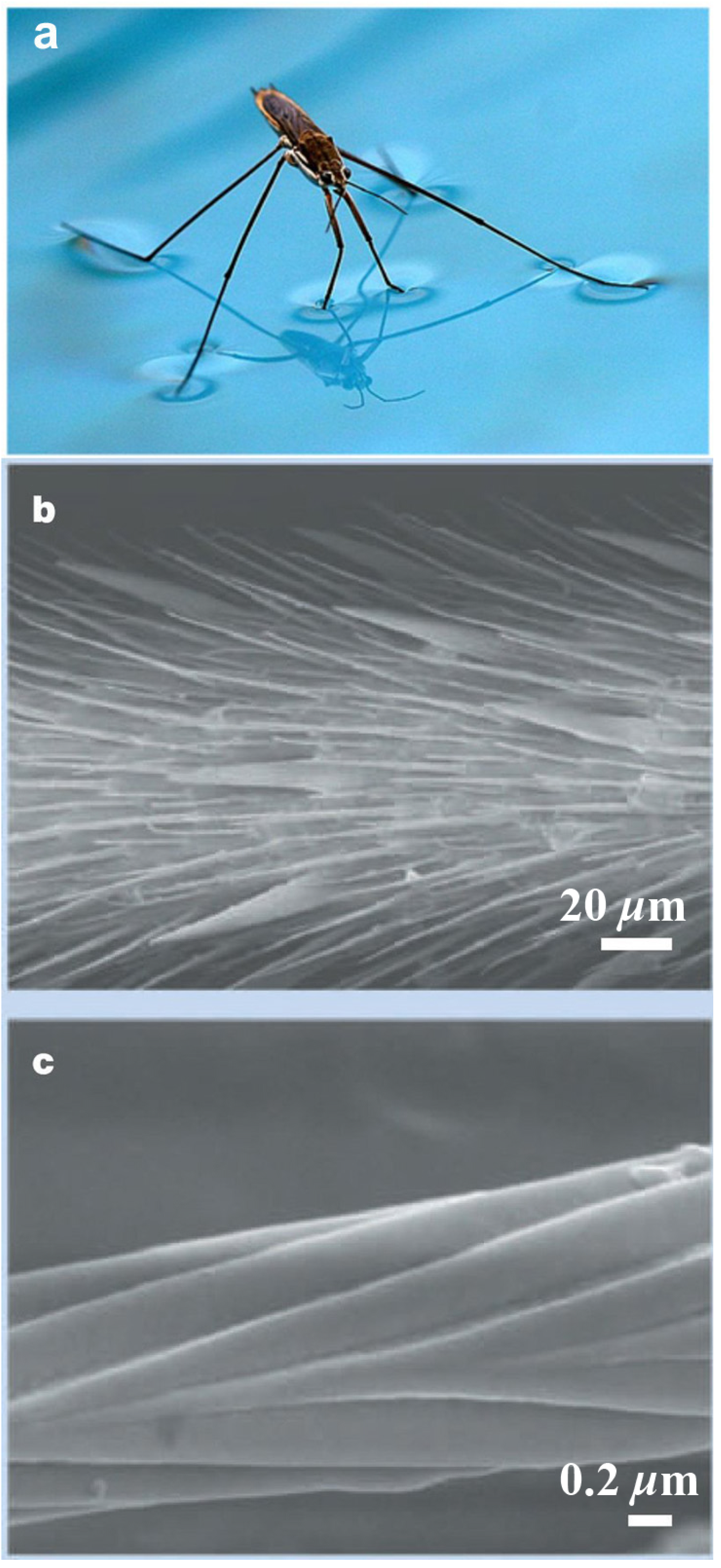
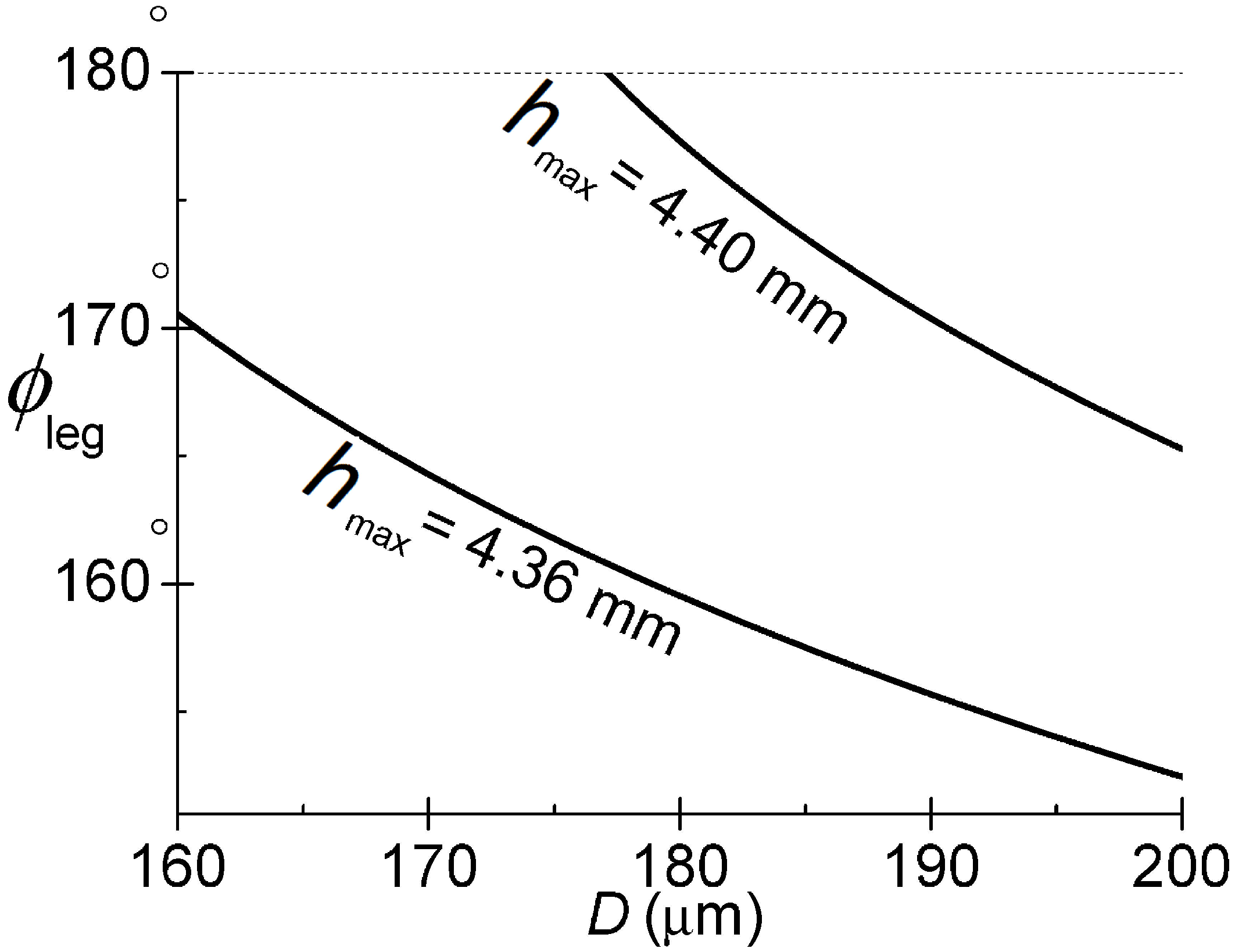
2.1.2. Water Striders

2.1.3. Butterfly Wings

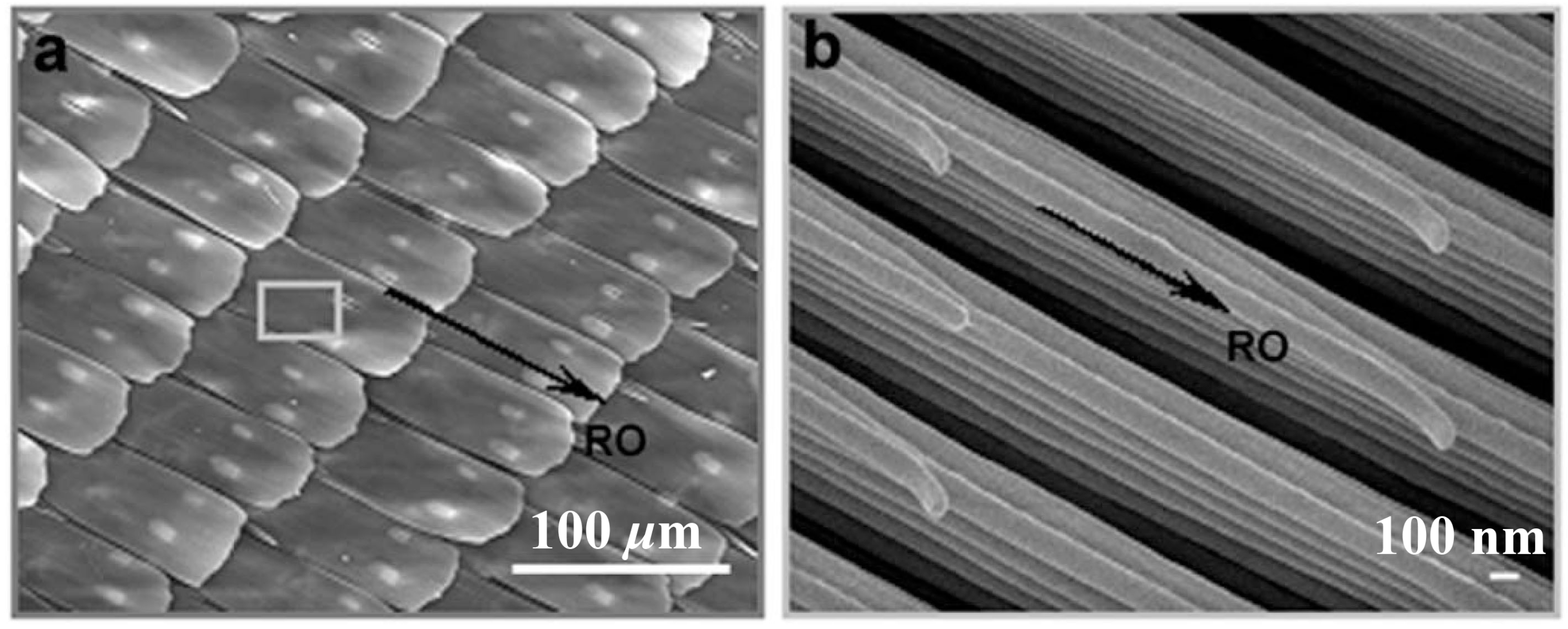
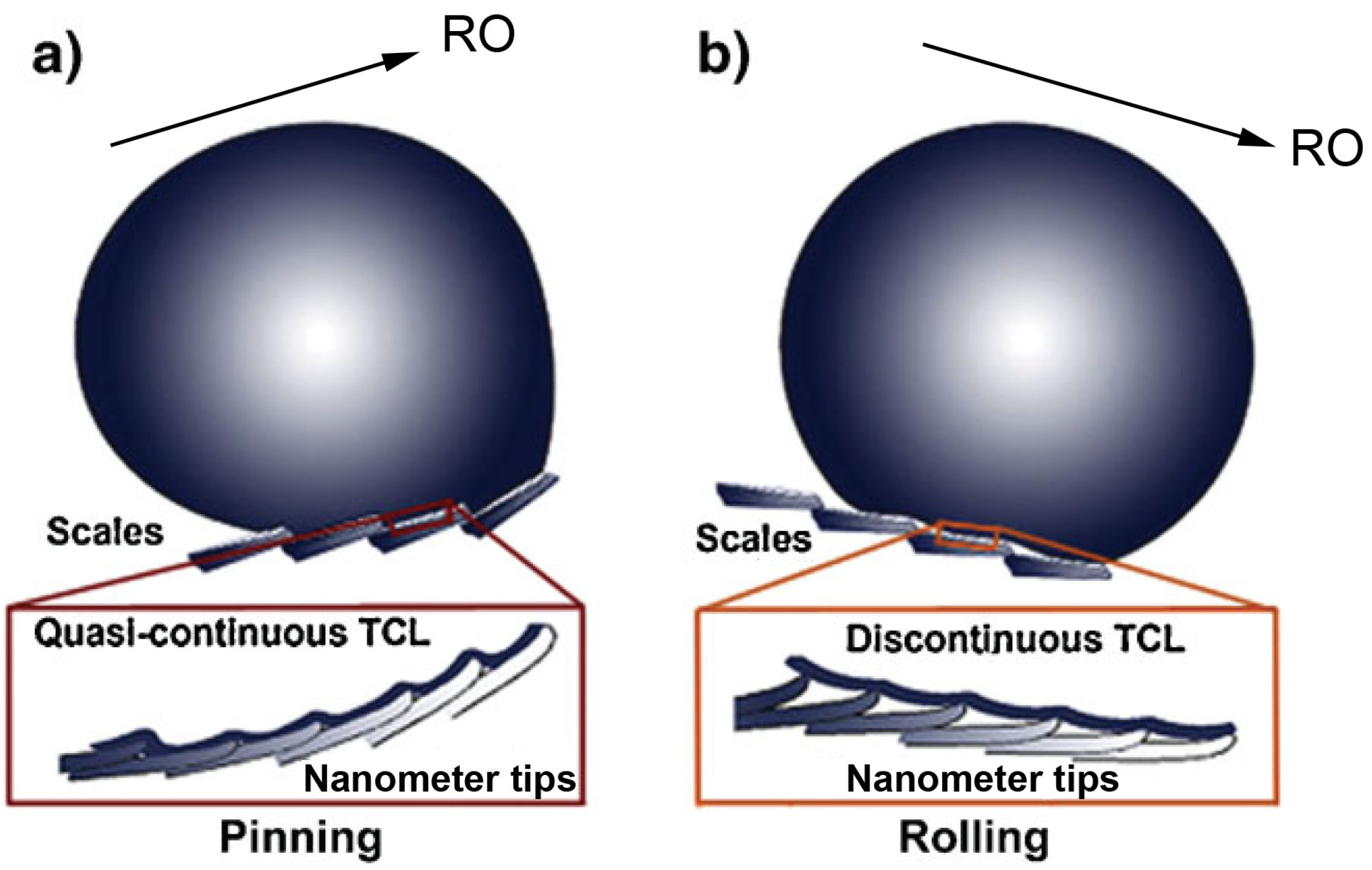
2.2. Omniphobic Coatings in Nature
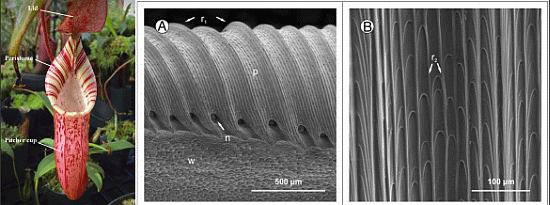
2.2.1. Nepenthes Pitcher Plants

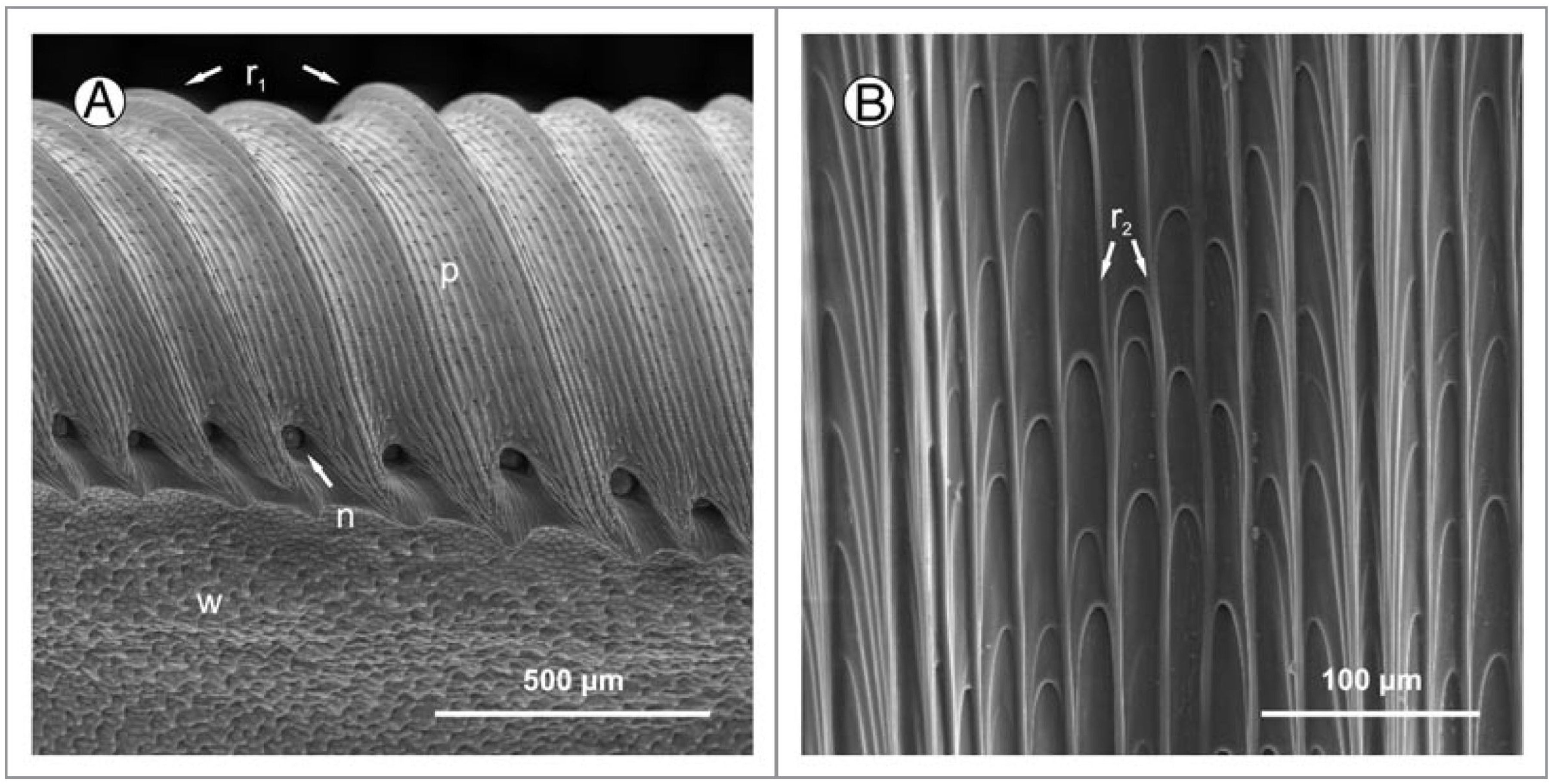
3. Fabrication of Slippery Surfaces
3.1. Superhydrophobic Surfaces
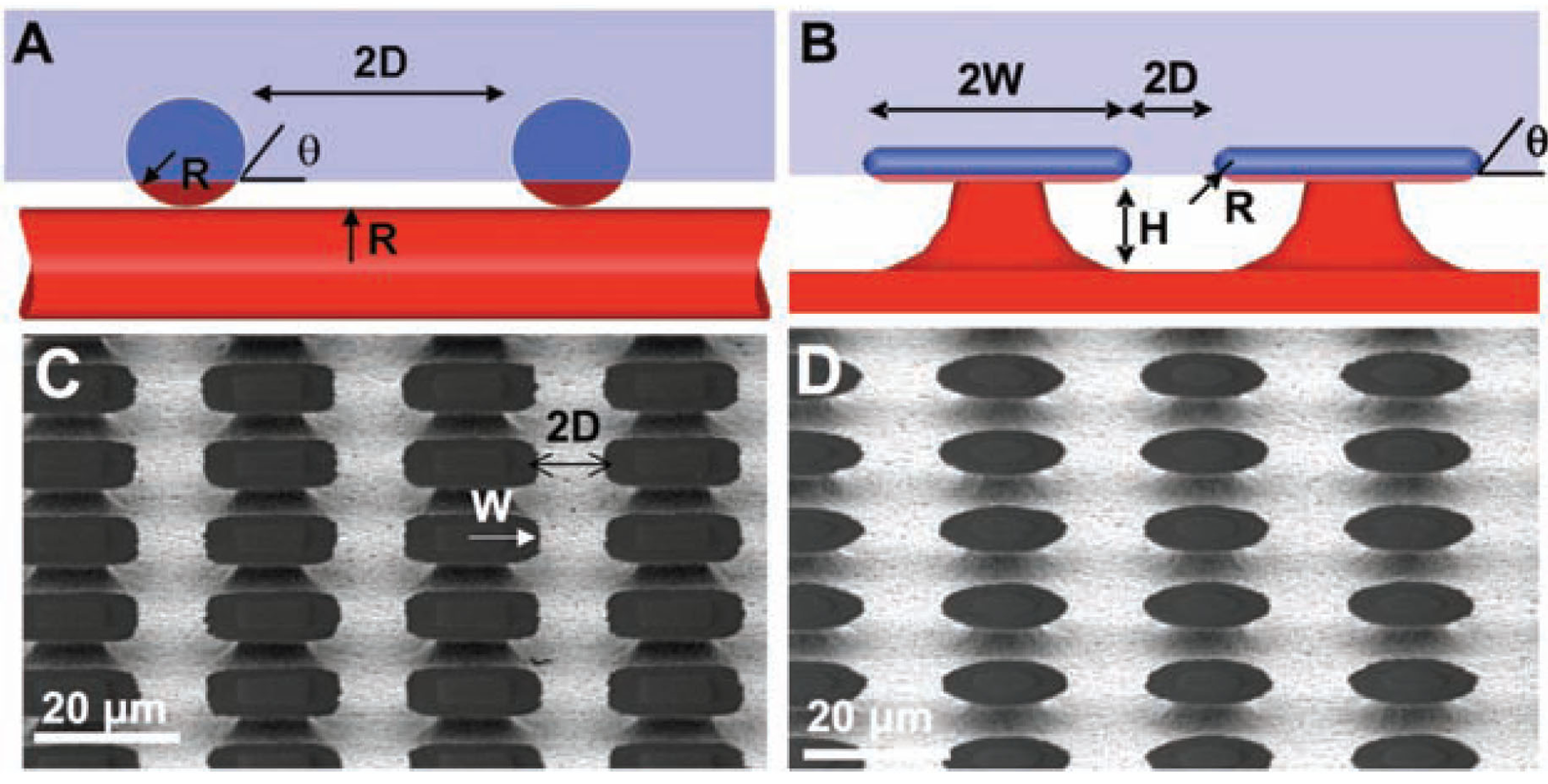
3.1.1. Ordered Microtextures

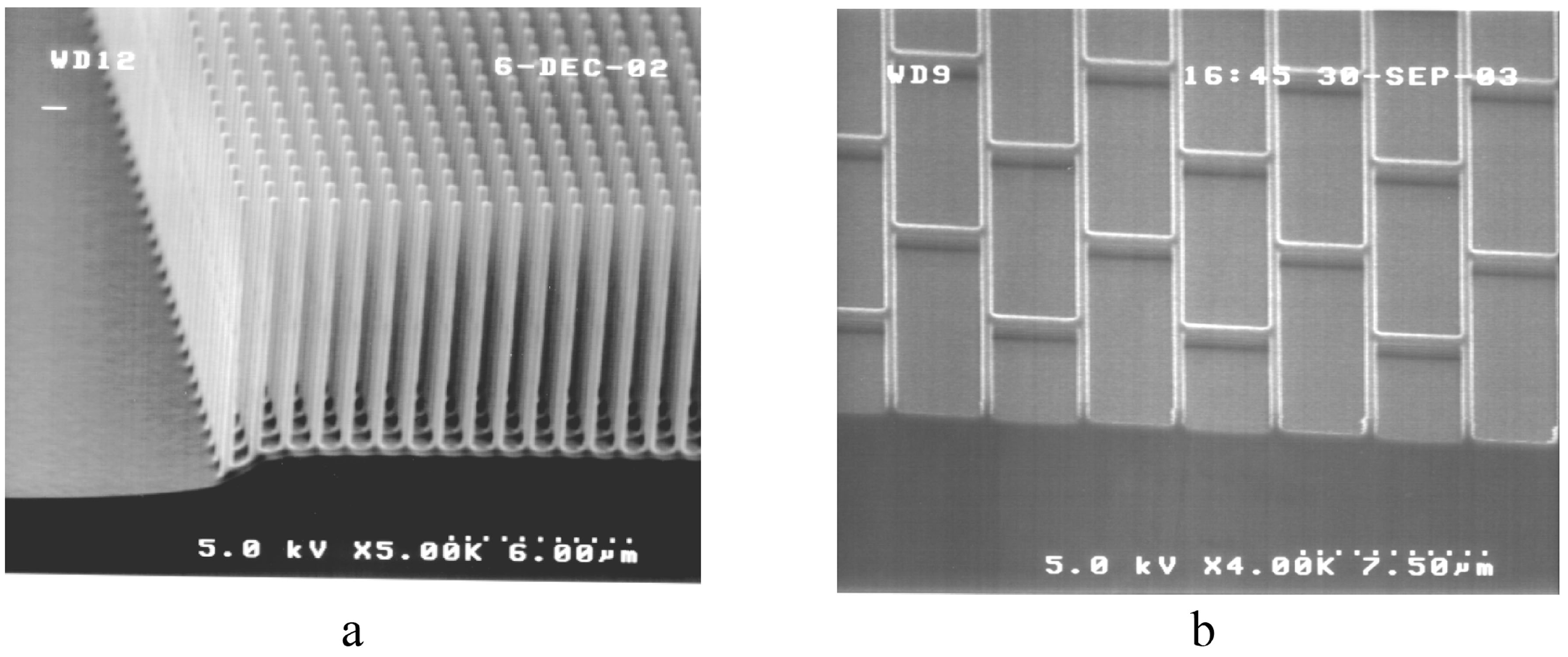
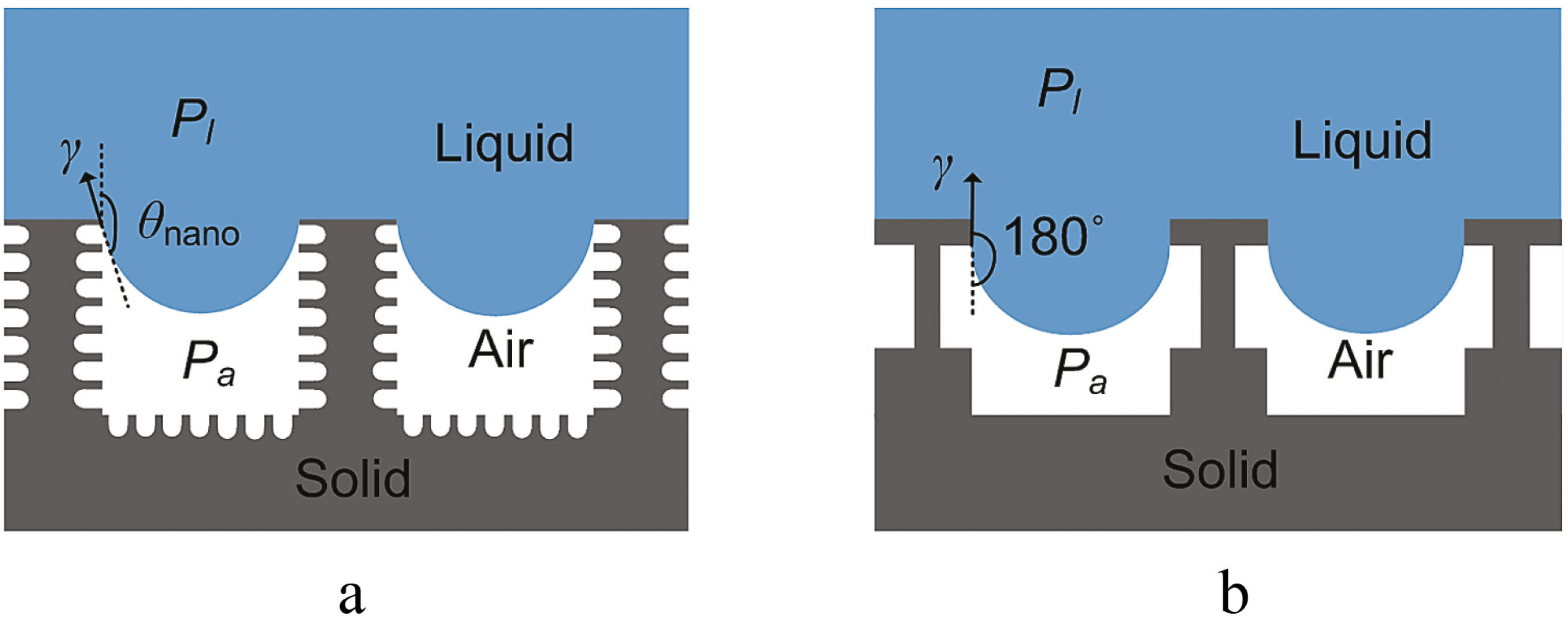
3.1.2. Engineered Cost-Effective Surfaces

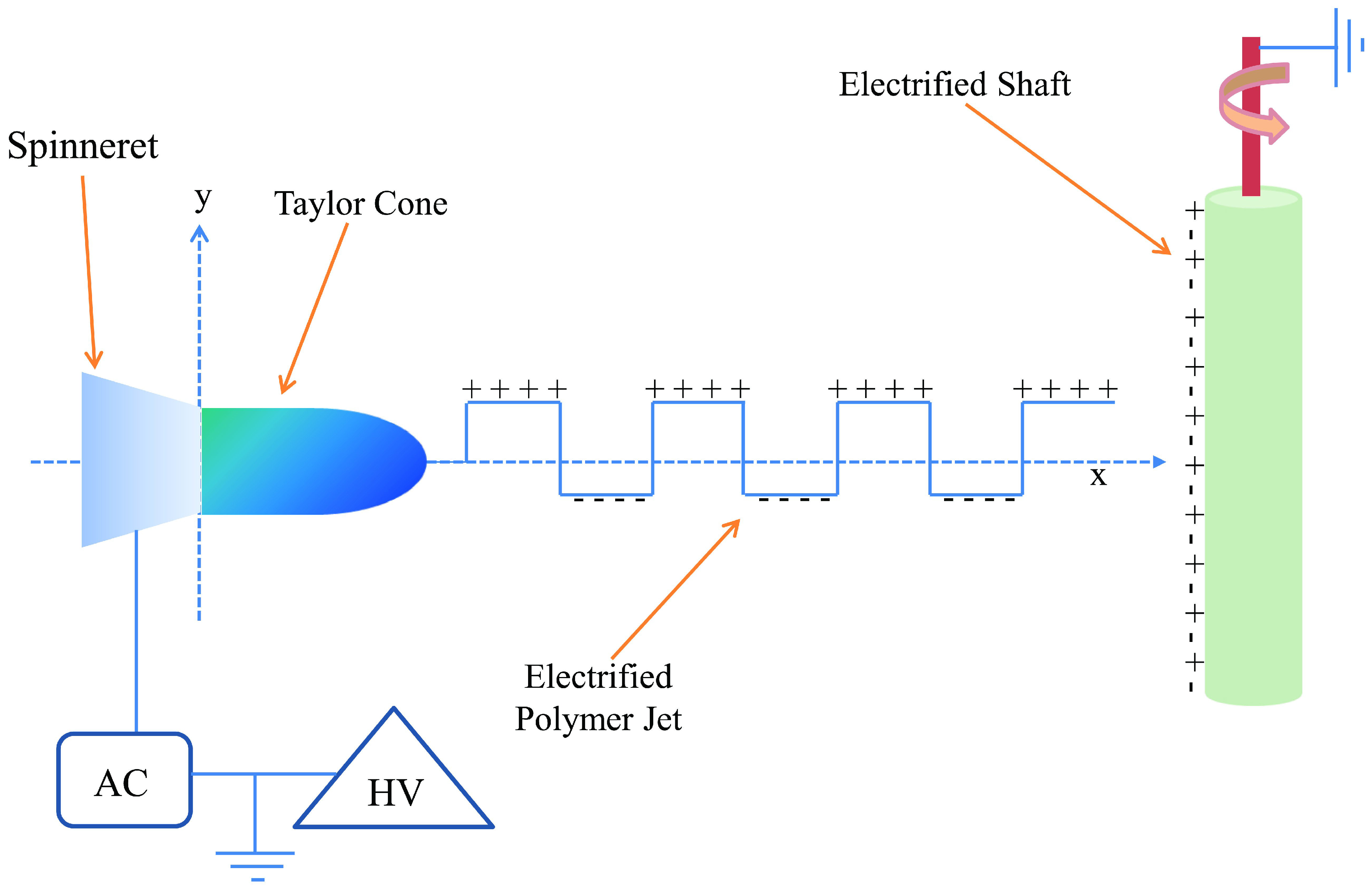
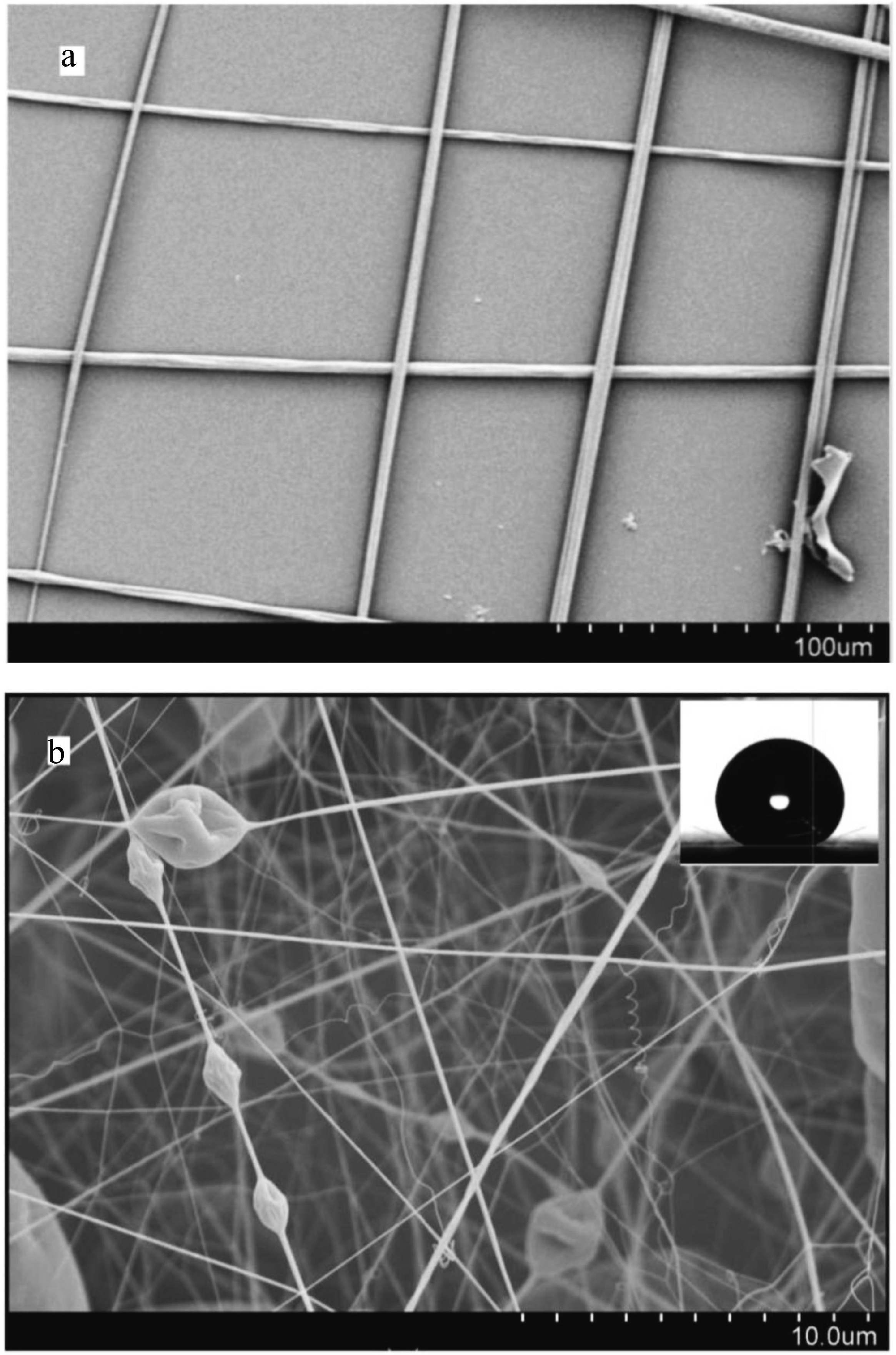

3.2. Fabrication of Omniphobic Surfaces
3.2.1. Superoleophobic Surfaces
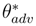 and receding
and receding 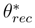 contact angles. From Srinivasan et al. [71].
contact angles. From Srinivasan et al. [71].
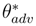 and receding
and receding 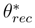 contact angles. From Srinivasan et al. [71].
contact angles. From Srinivasan et al. [71].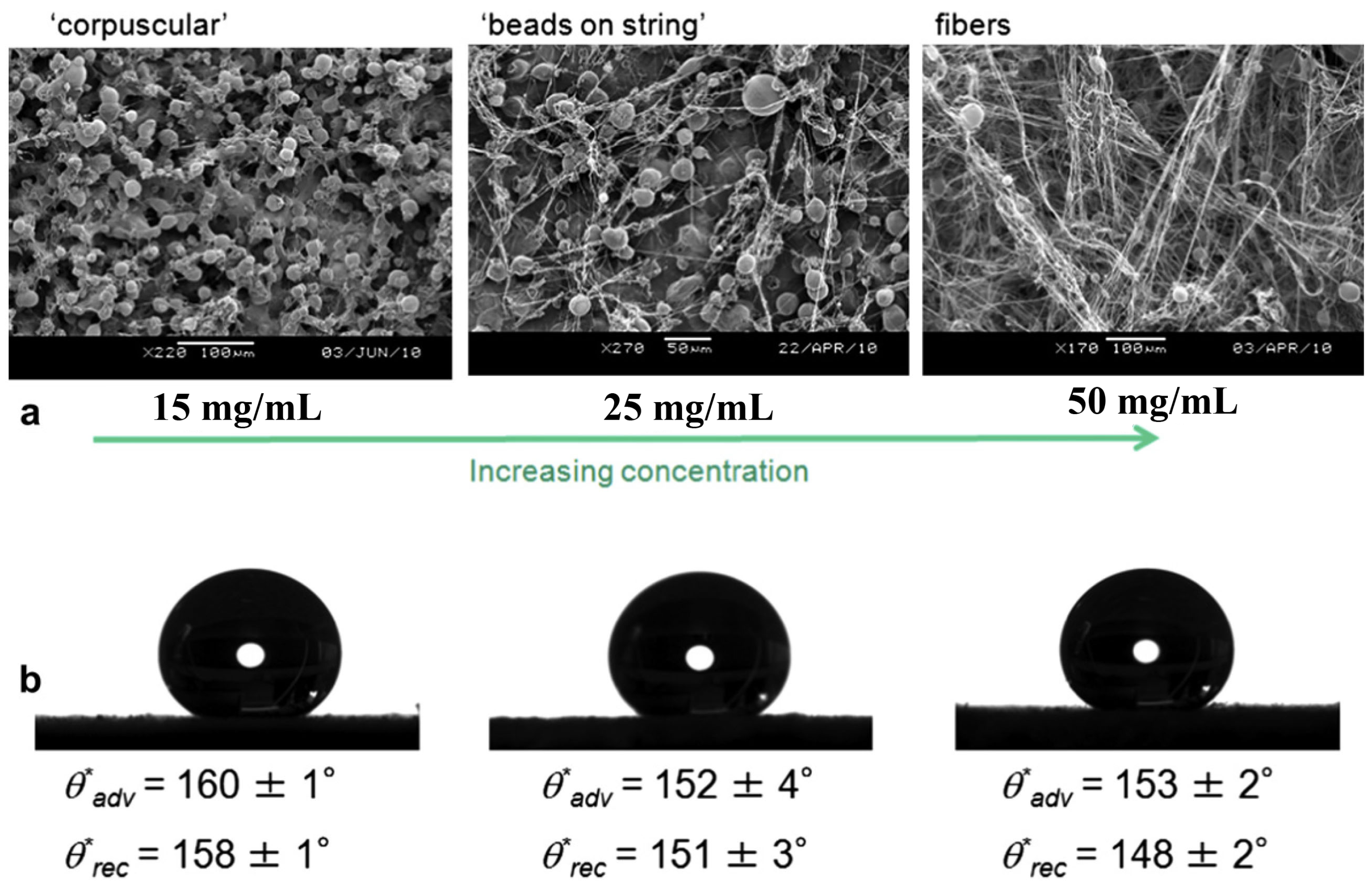
3.2.2. Slippery Liquid-Infused Porous Surfaces (SLIPS)

4. Characterization of Slippery Surfaces
4.1. Superhydrophobic Surfaces
4.1.1. Slip Flow and Drag Reduction
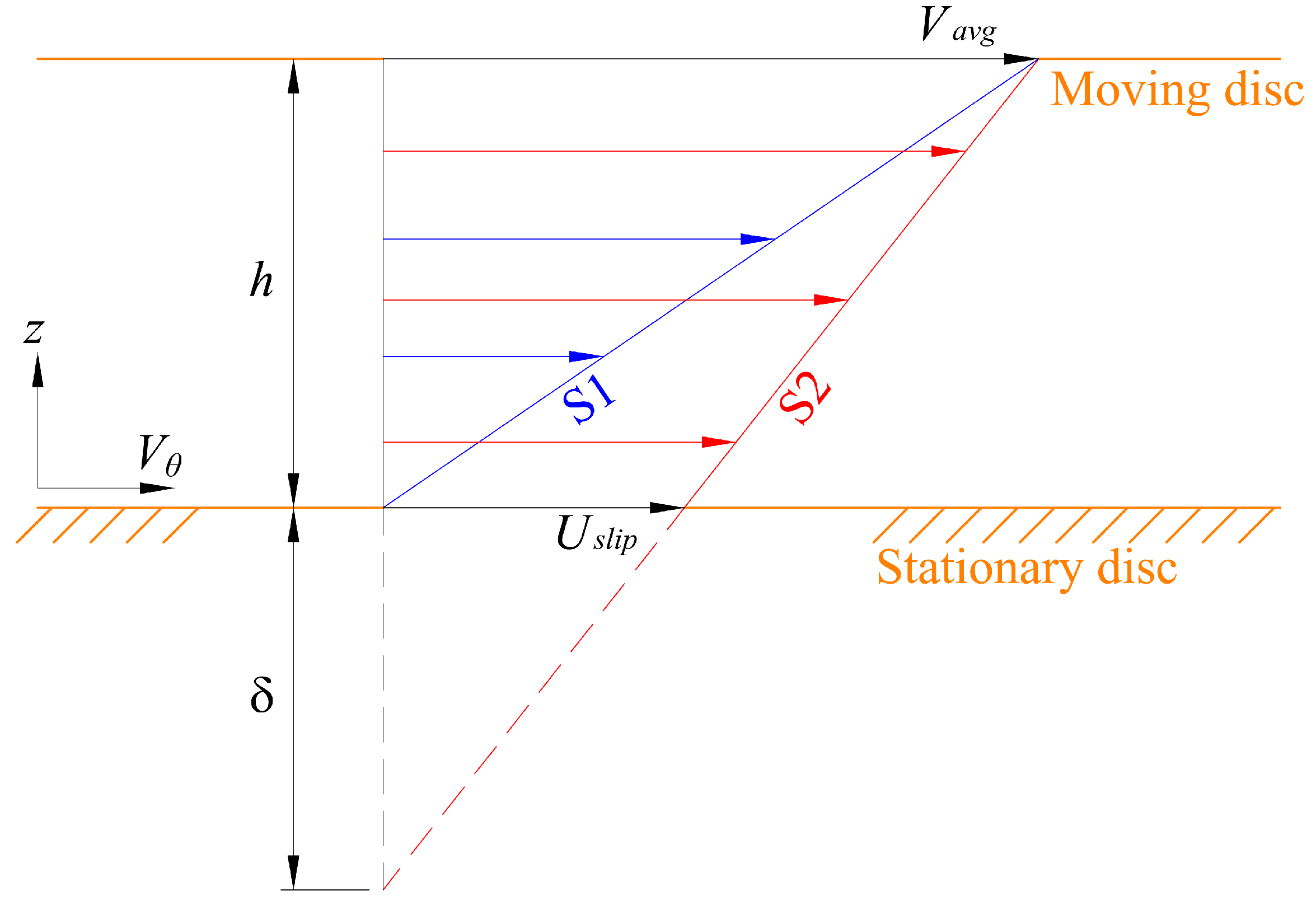
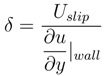
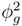 . Meanwhile, if the gas fraction is higher than 0.7, the slip length is proportional to
. Meanwhile, if the gas fraction is higher than 0.7, the slip length is proportional to 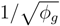 . Within the range 0.4 < ϕg < 0.7, interpolation is utilized. The above mathematical proportionalities were derived and validated by Ybert et al. [83]. The drag reduction also increases as gas fraction increases, as shown in Figure 23. The figure includes both experimental and numerical results for a microchannel with a microridged superhydrophobic wall.
. Within the range 0.4 < ϕg < 0.7, interpolation is utilized. The above mathematical proportionalities were derived and validated by Ybert et al. [83]. The drag reduction also increases as gas fraction increases, as shown in Figure 23. The figure includes both experimental and numerical results for a microchannel with a microridged superhydrophobic wall.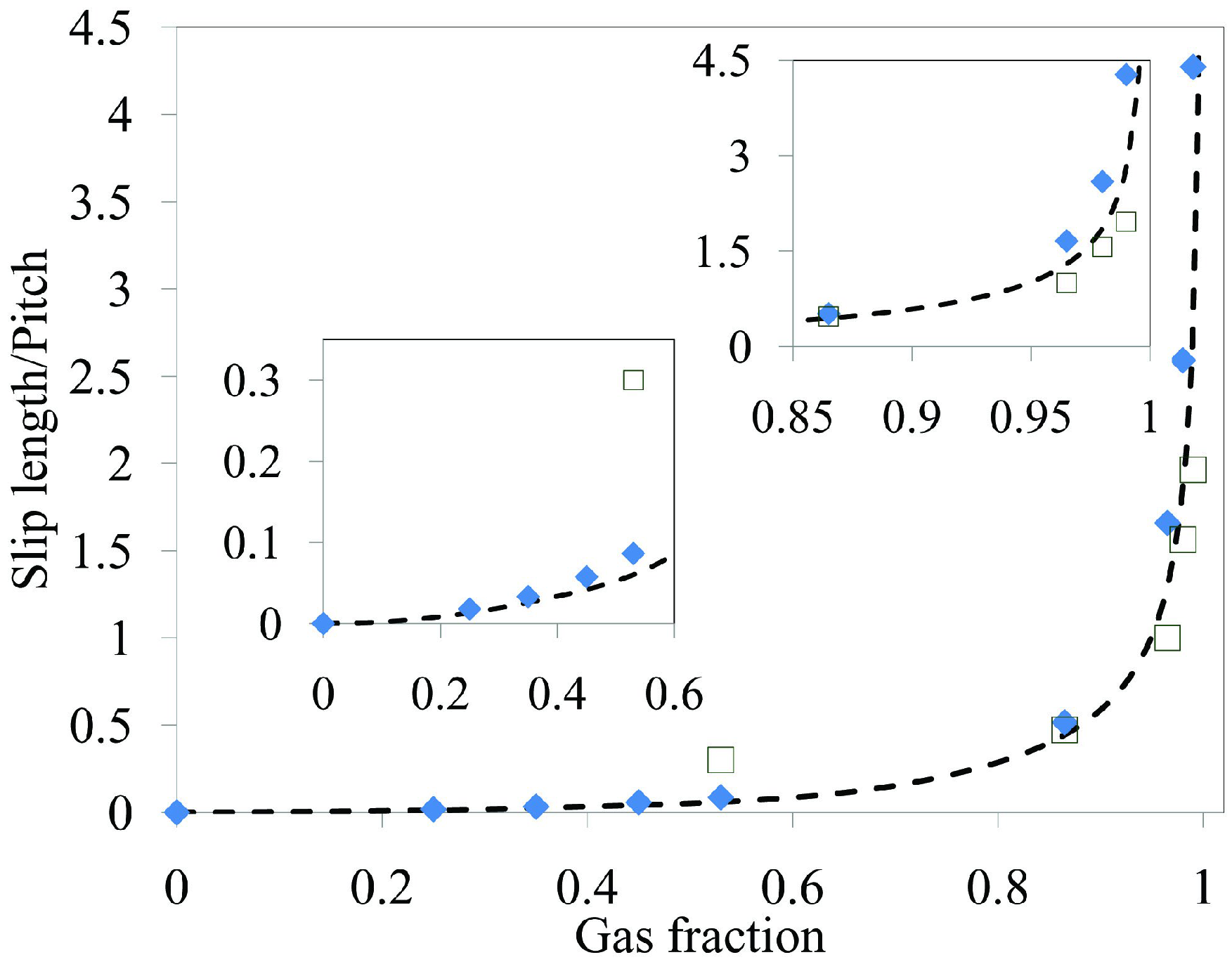

4.1.2. Air–Water Meniscus Stability Under Hydrostatic Pressure
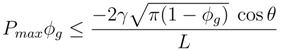
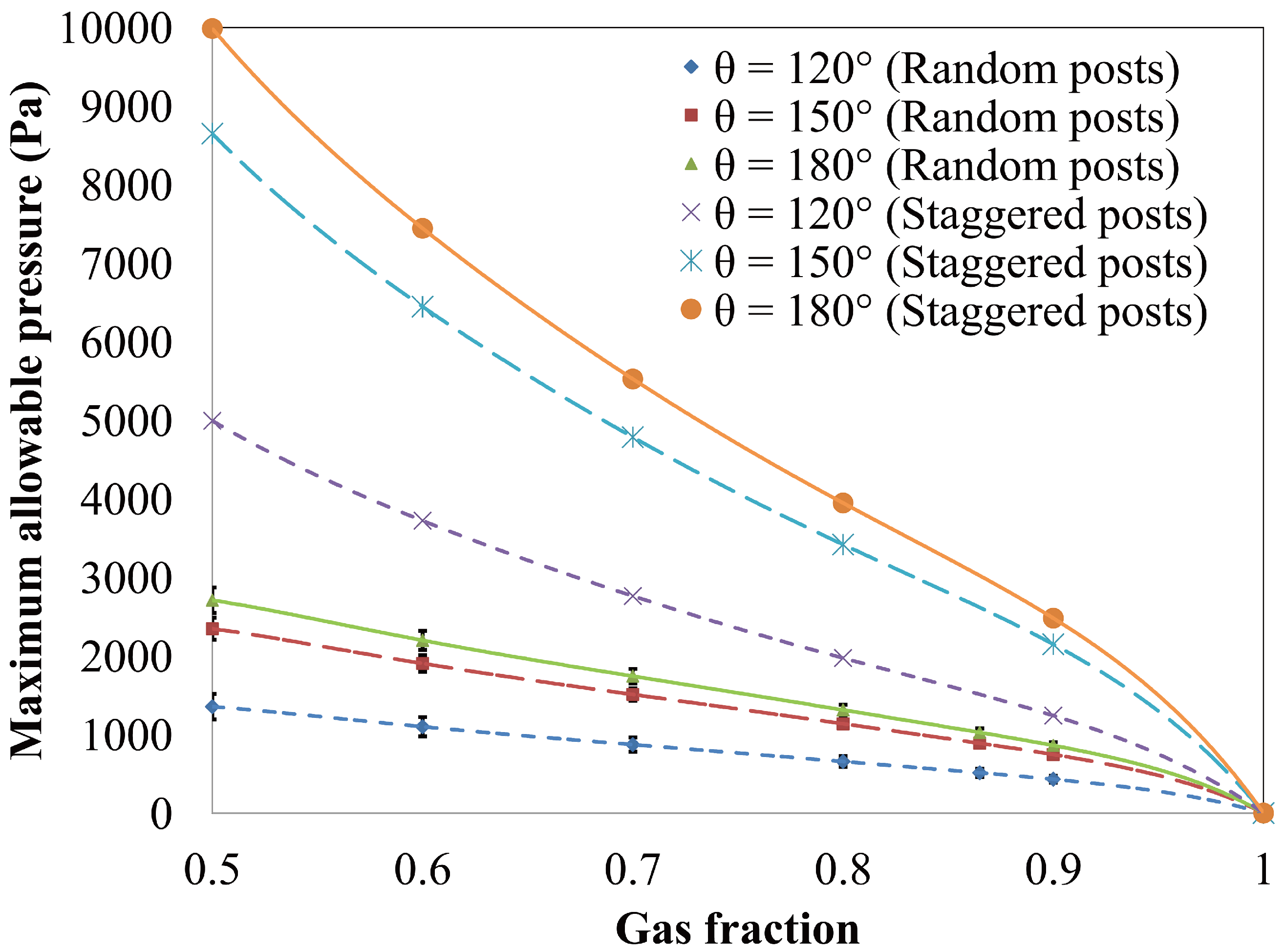
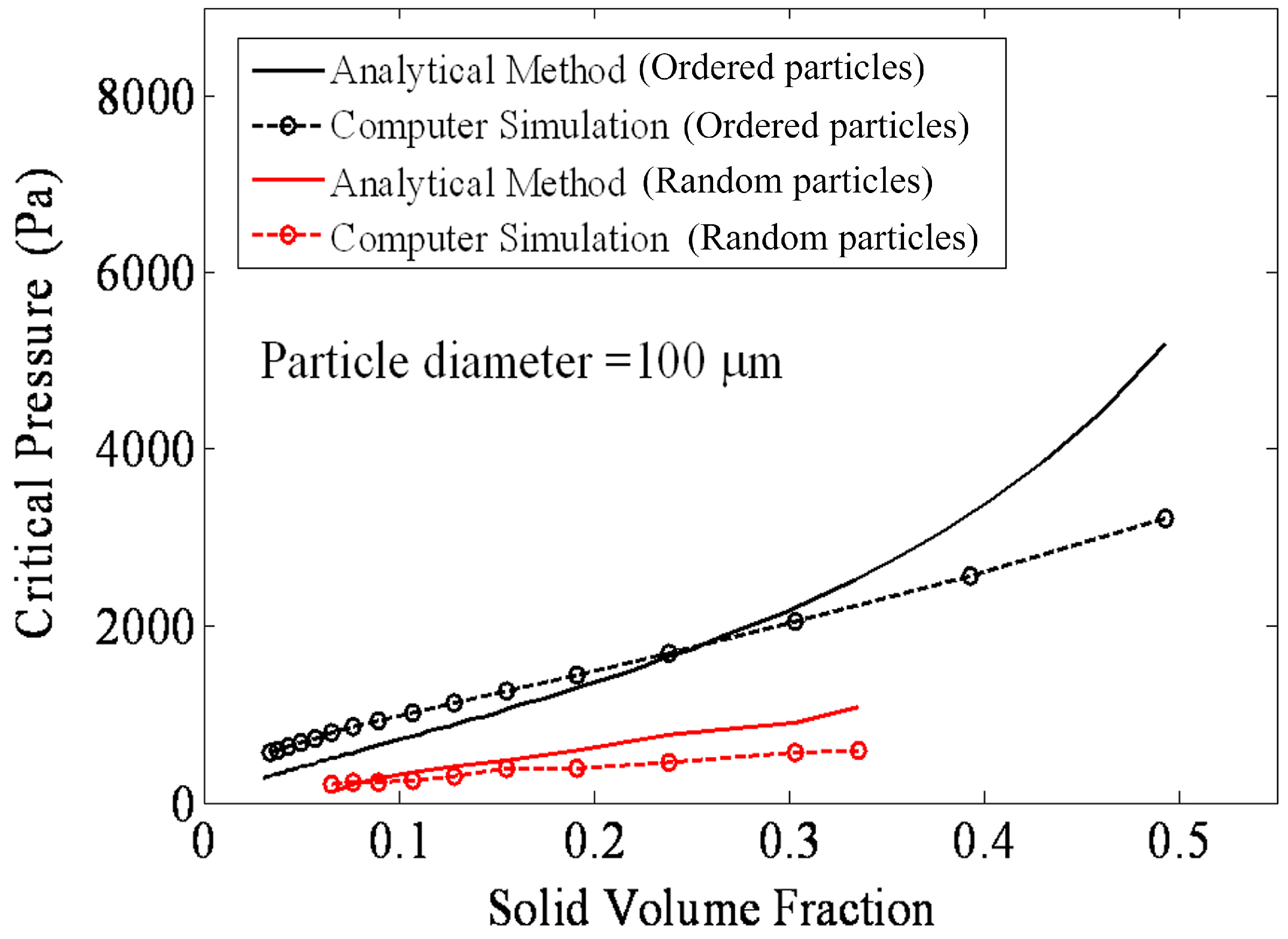
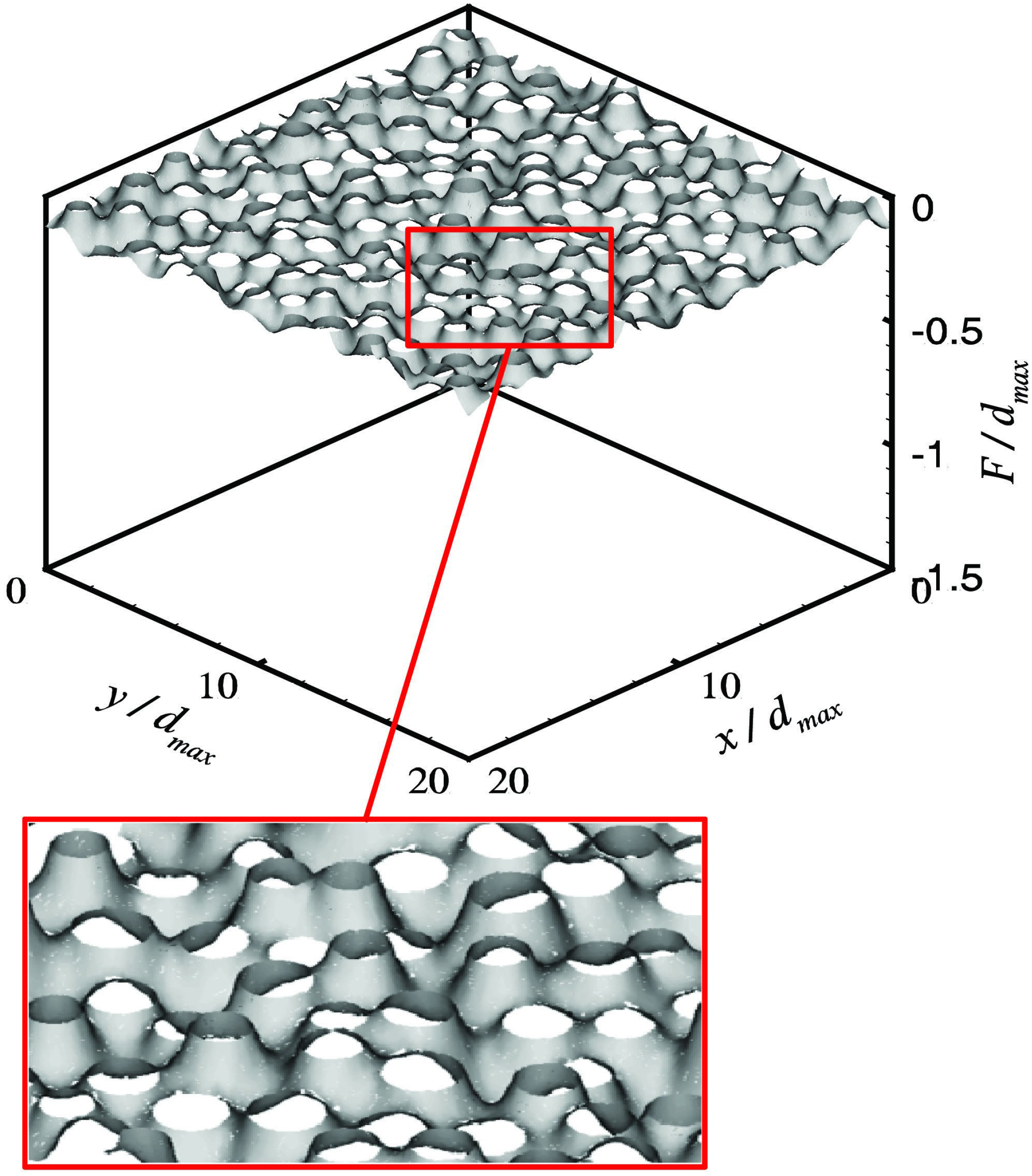
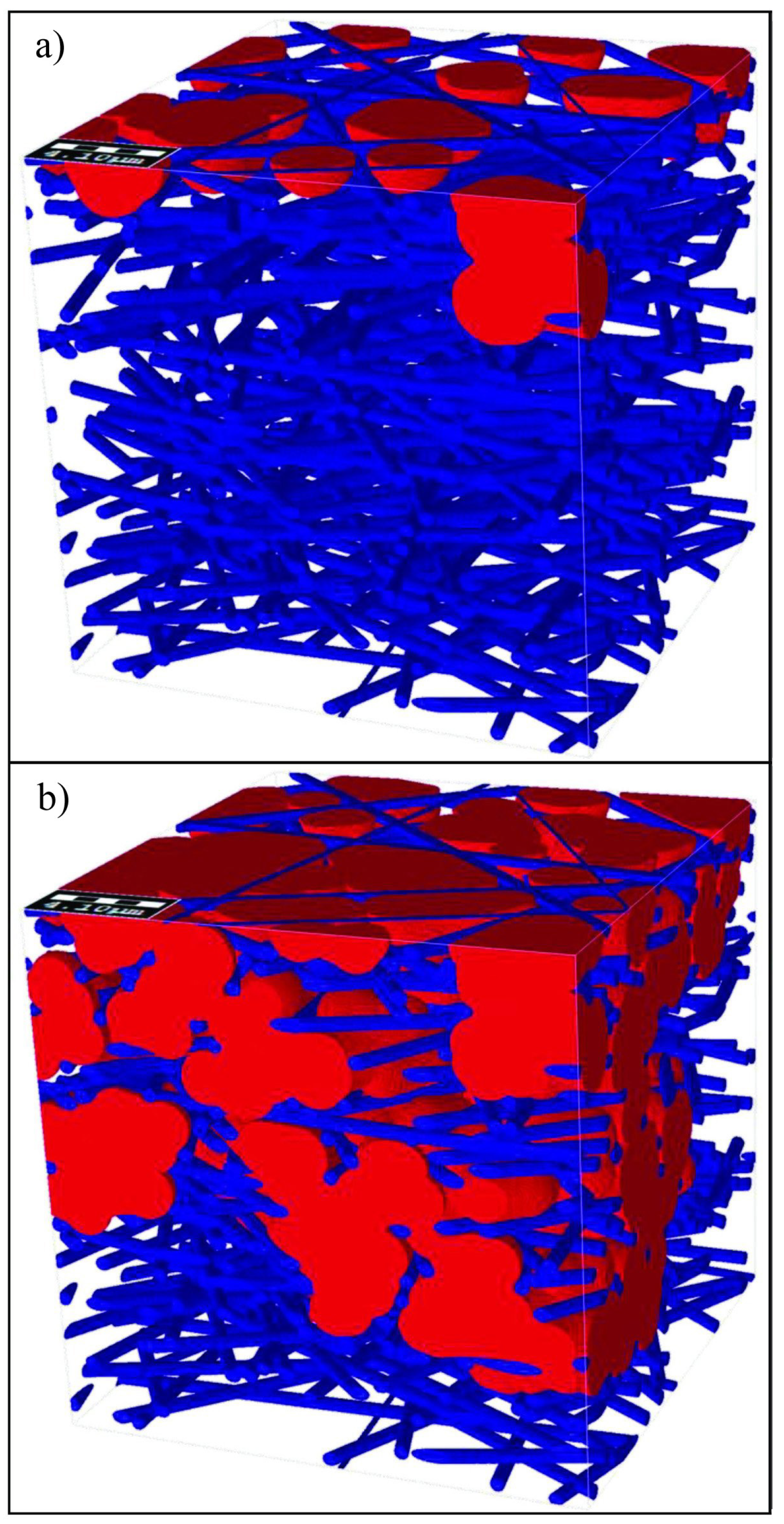
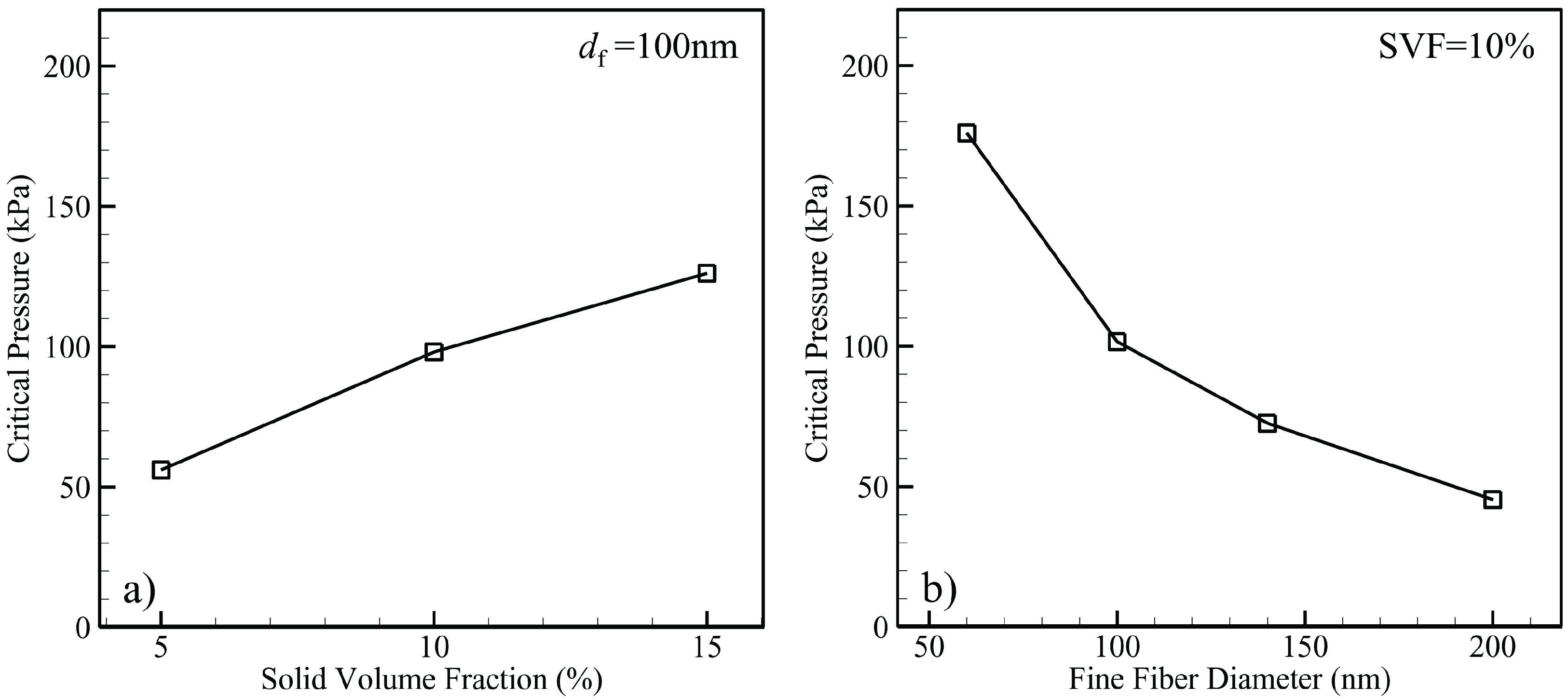
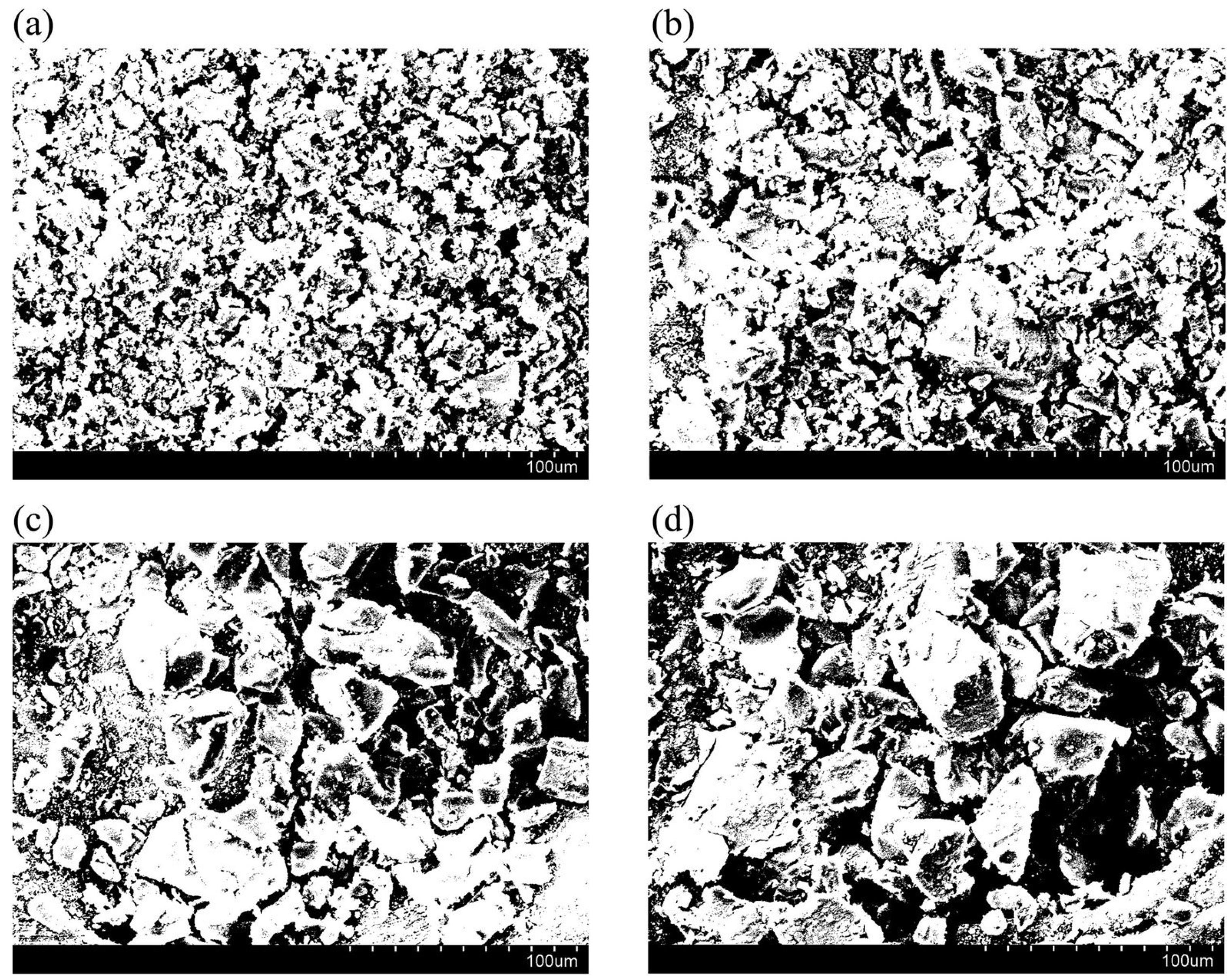

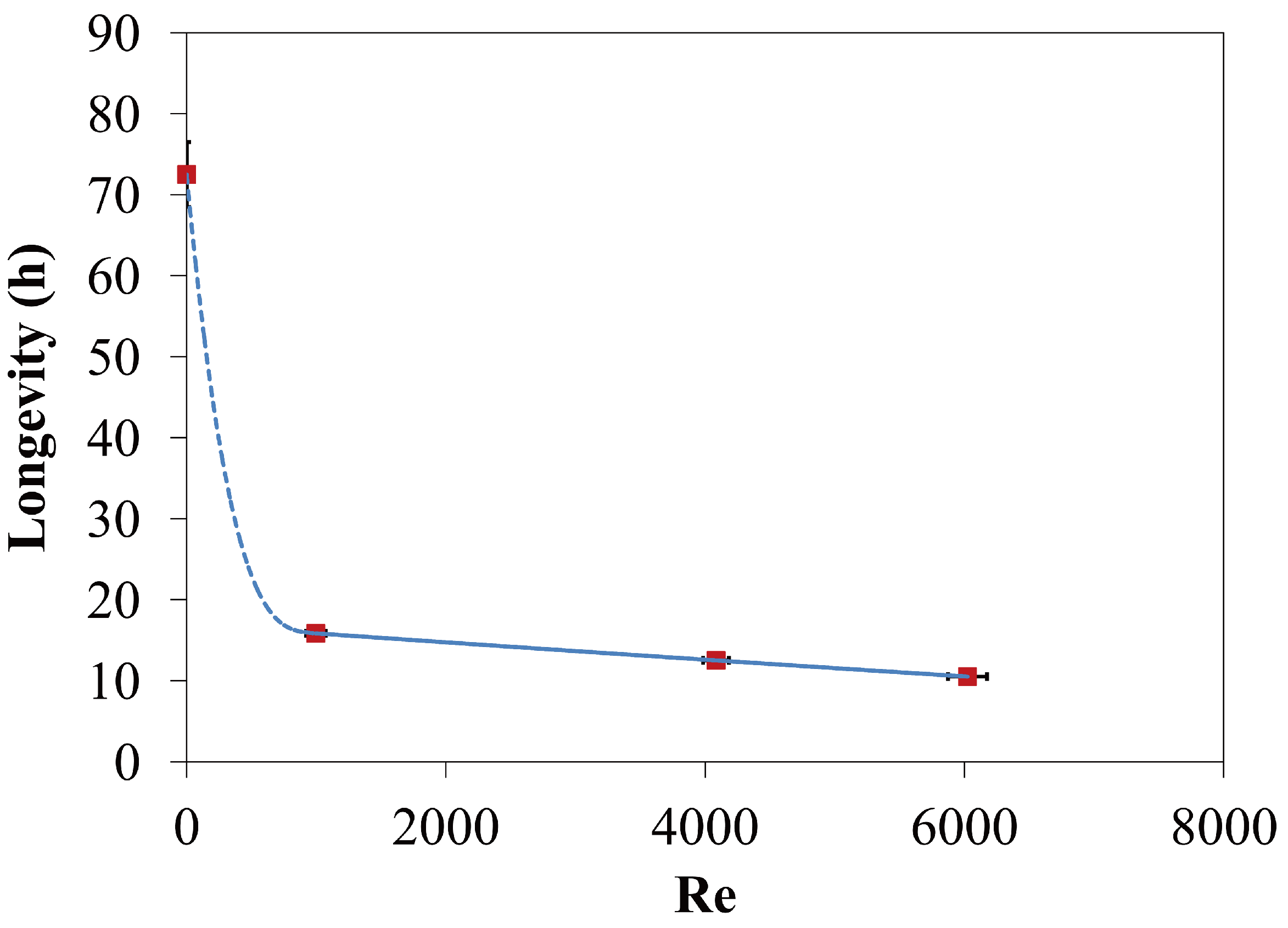
4.1.3. Longevity of Superhydrophobic Coatings
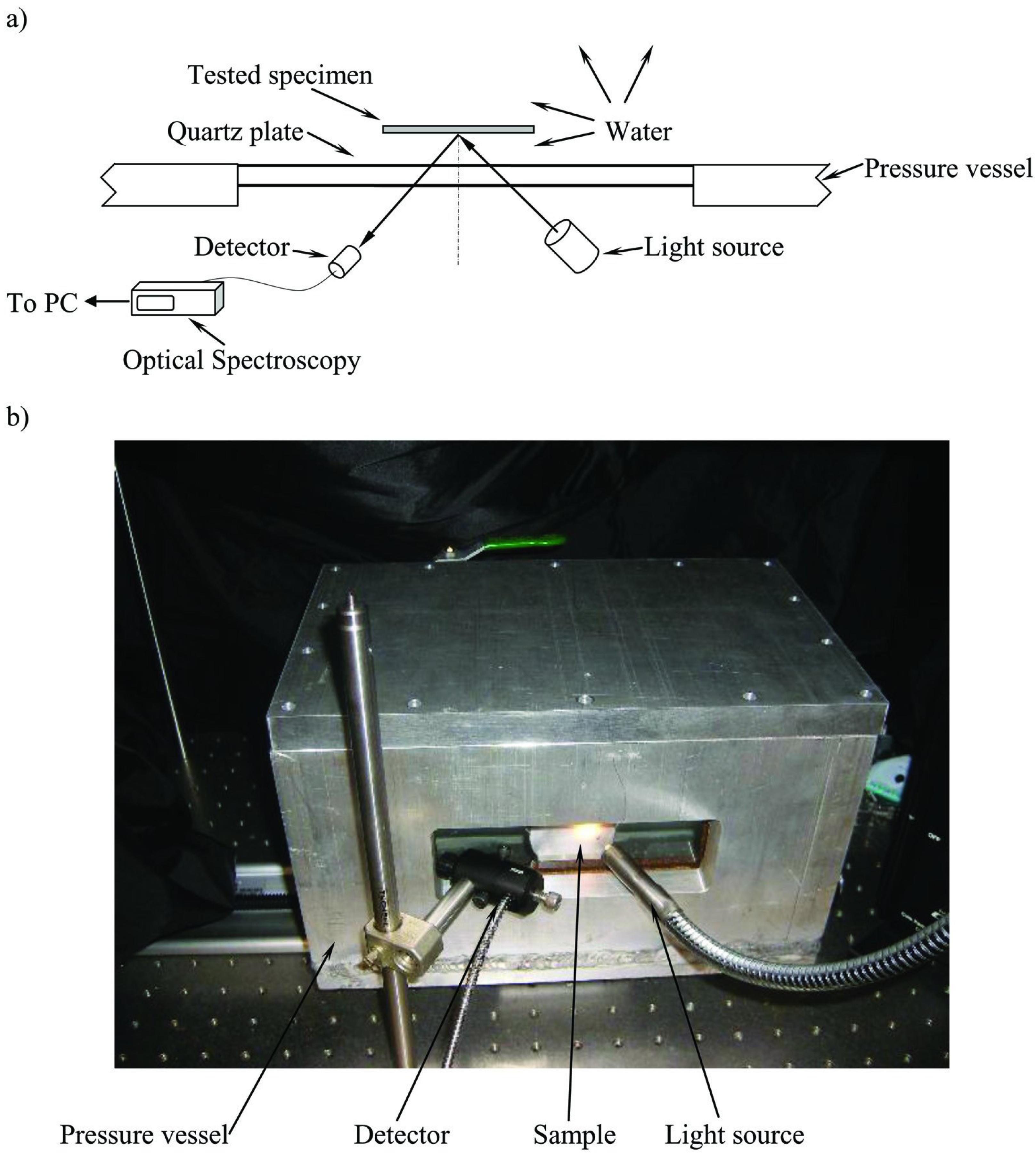
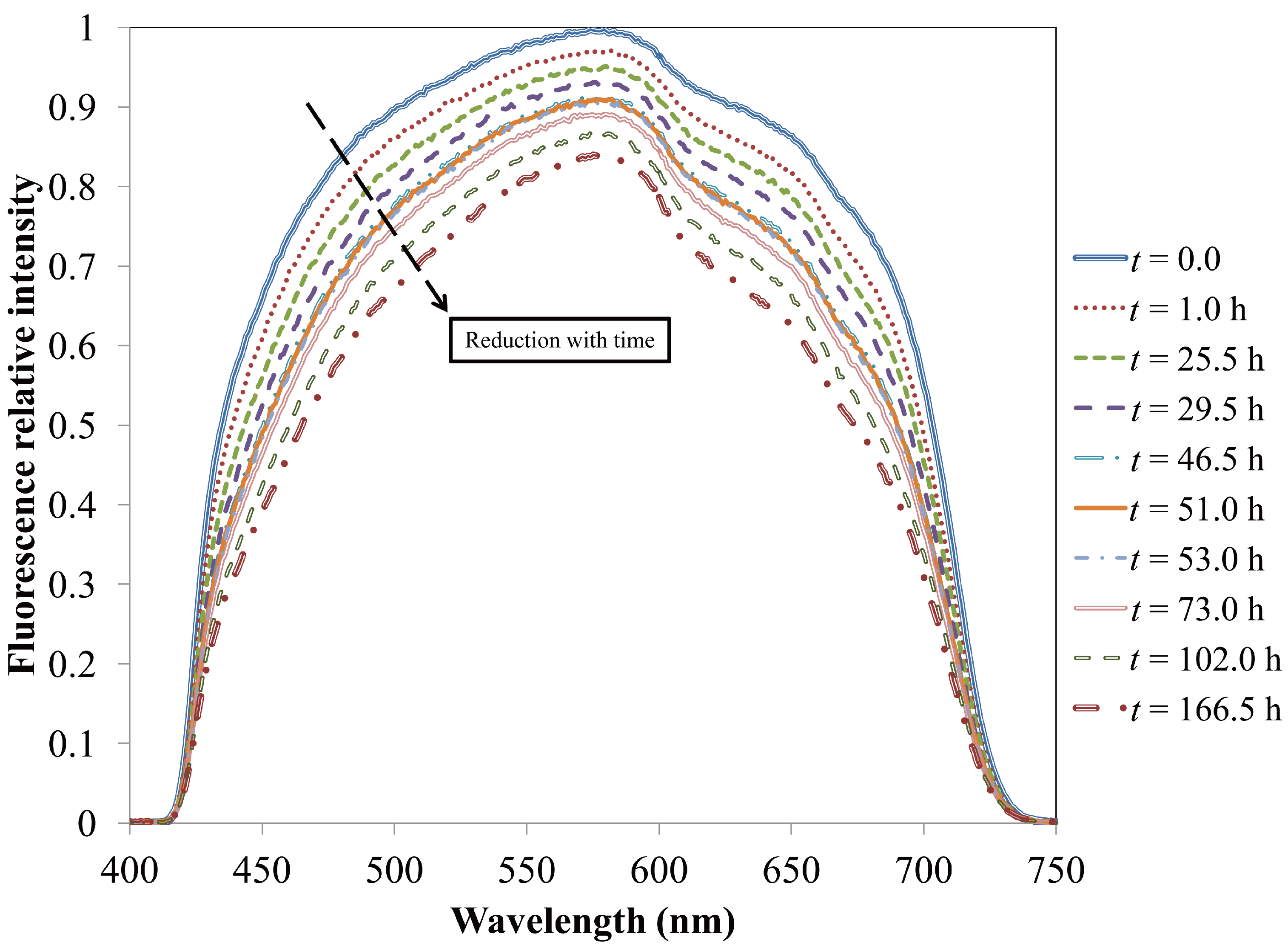
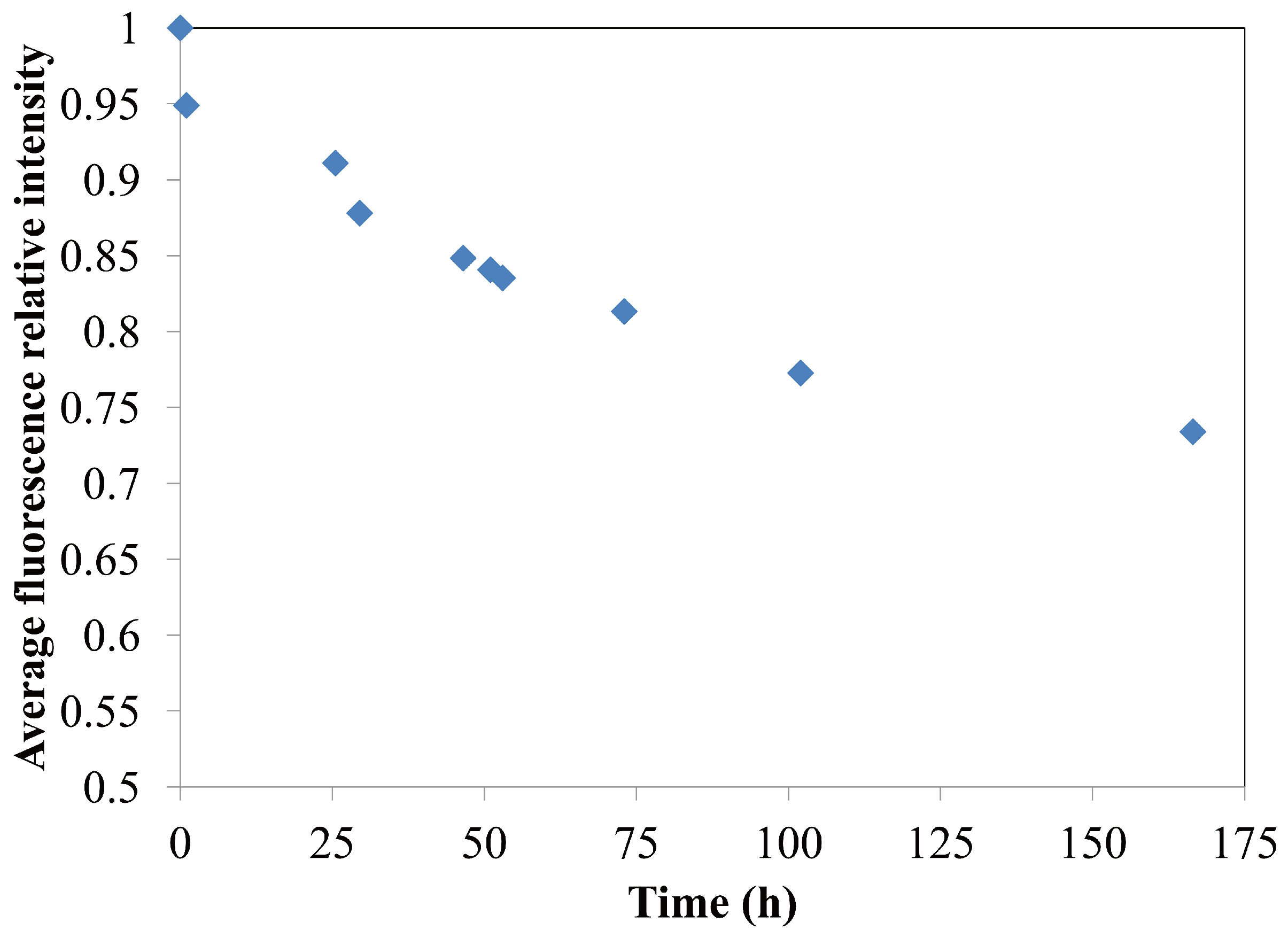
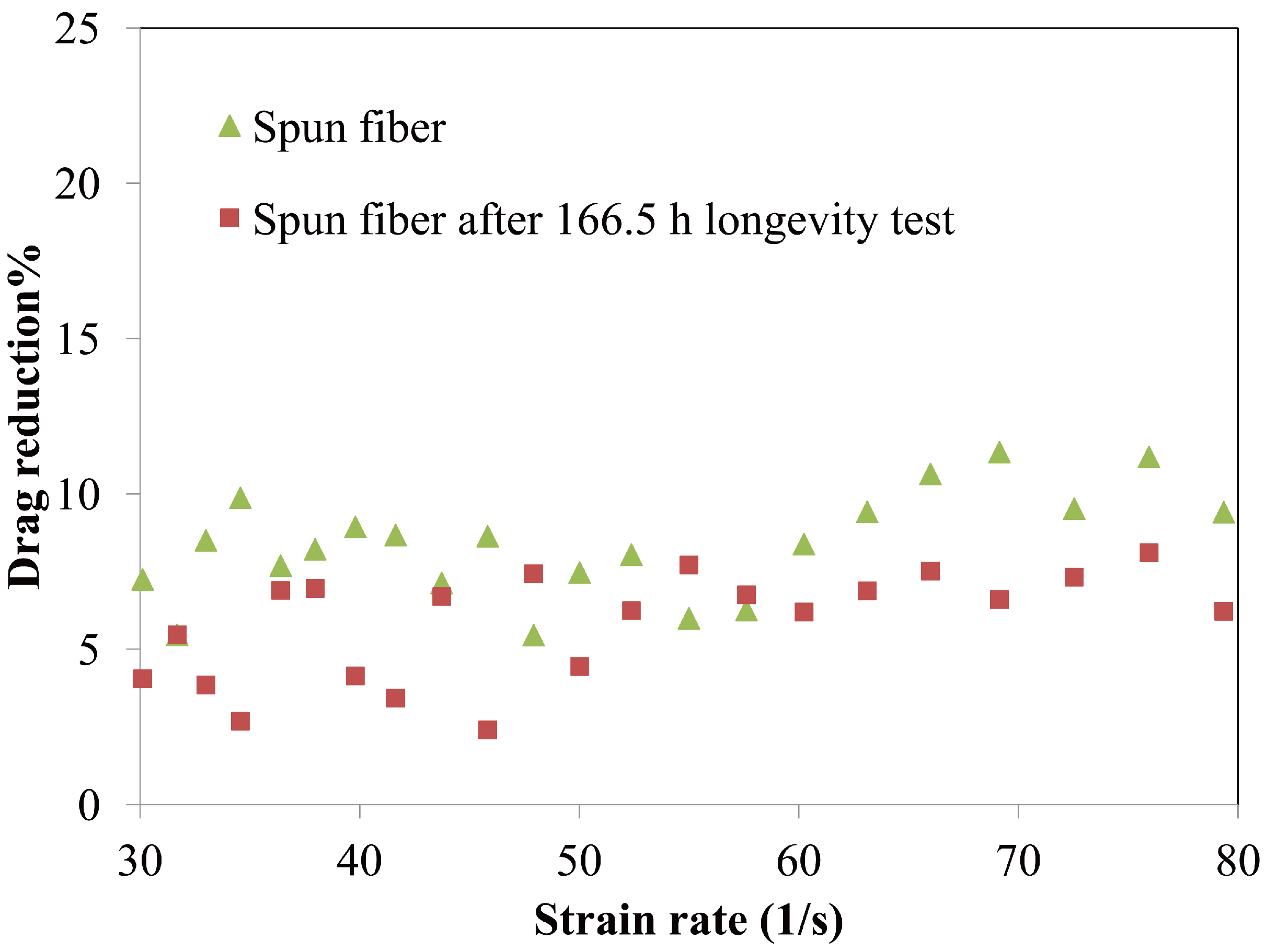
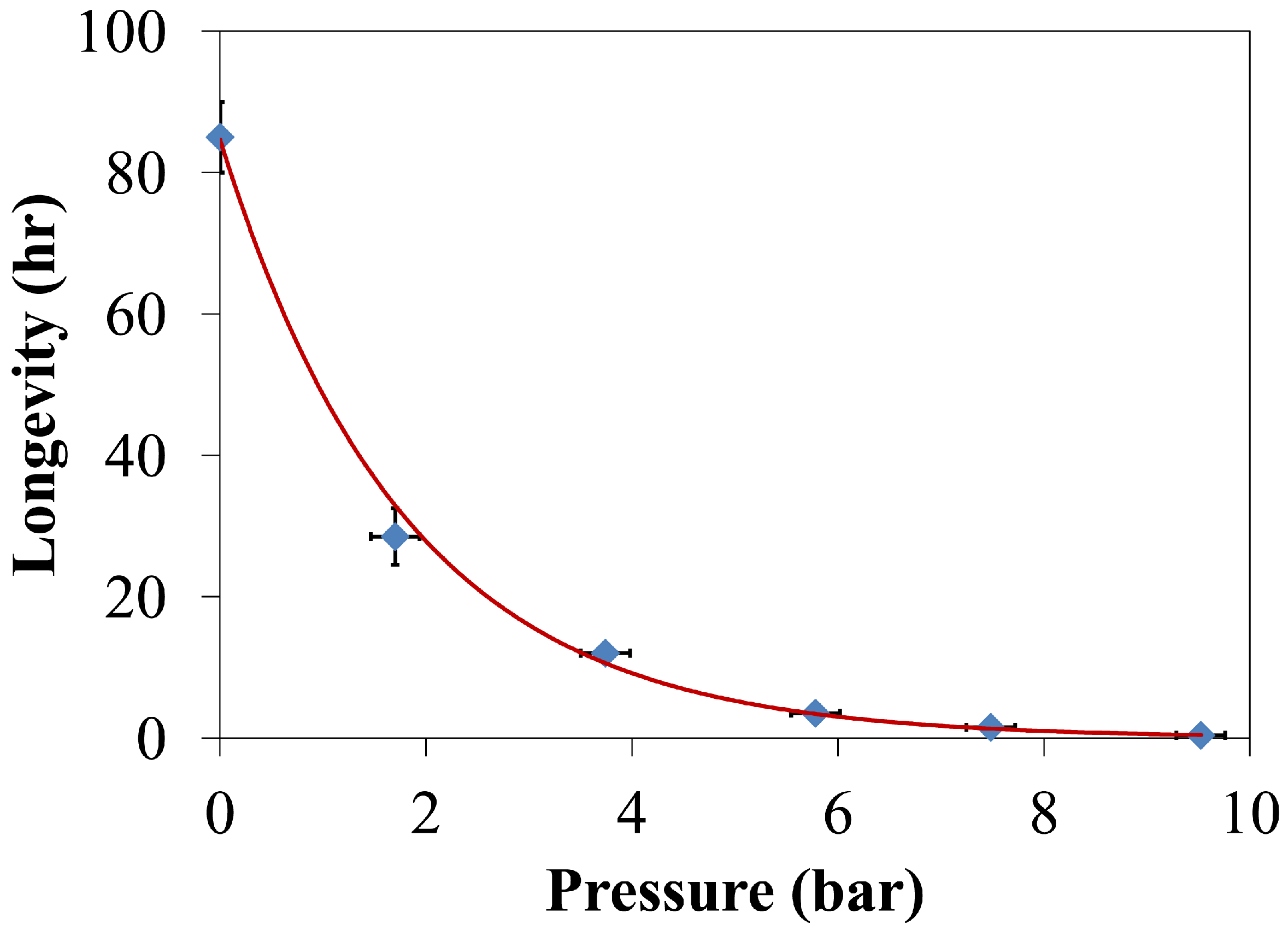
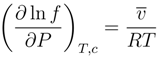
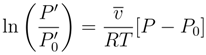
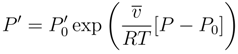
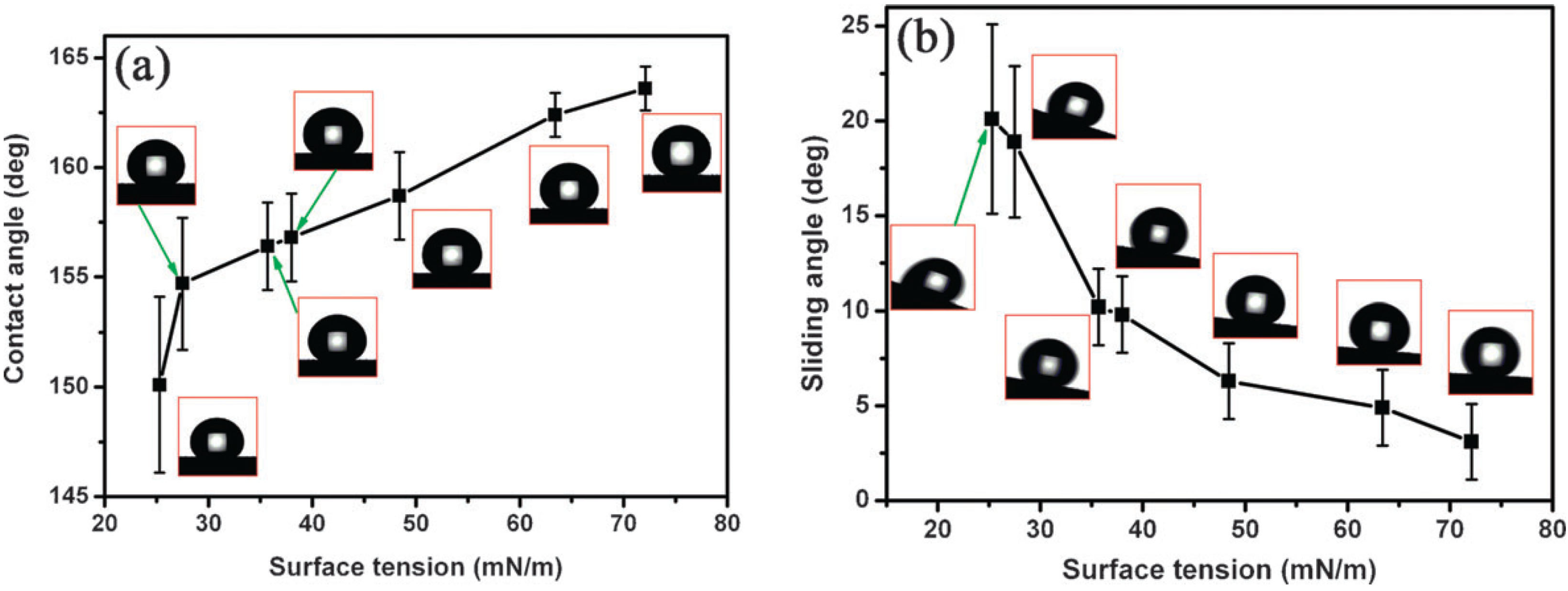
4.2. Characterization of Omniphobic Surfaces

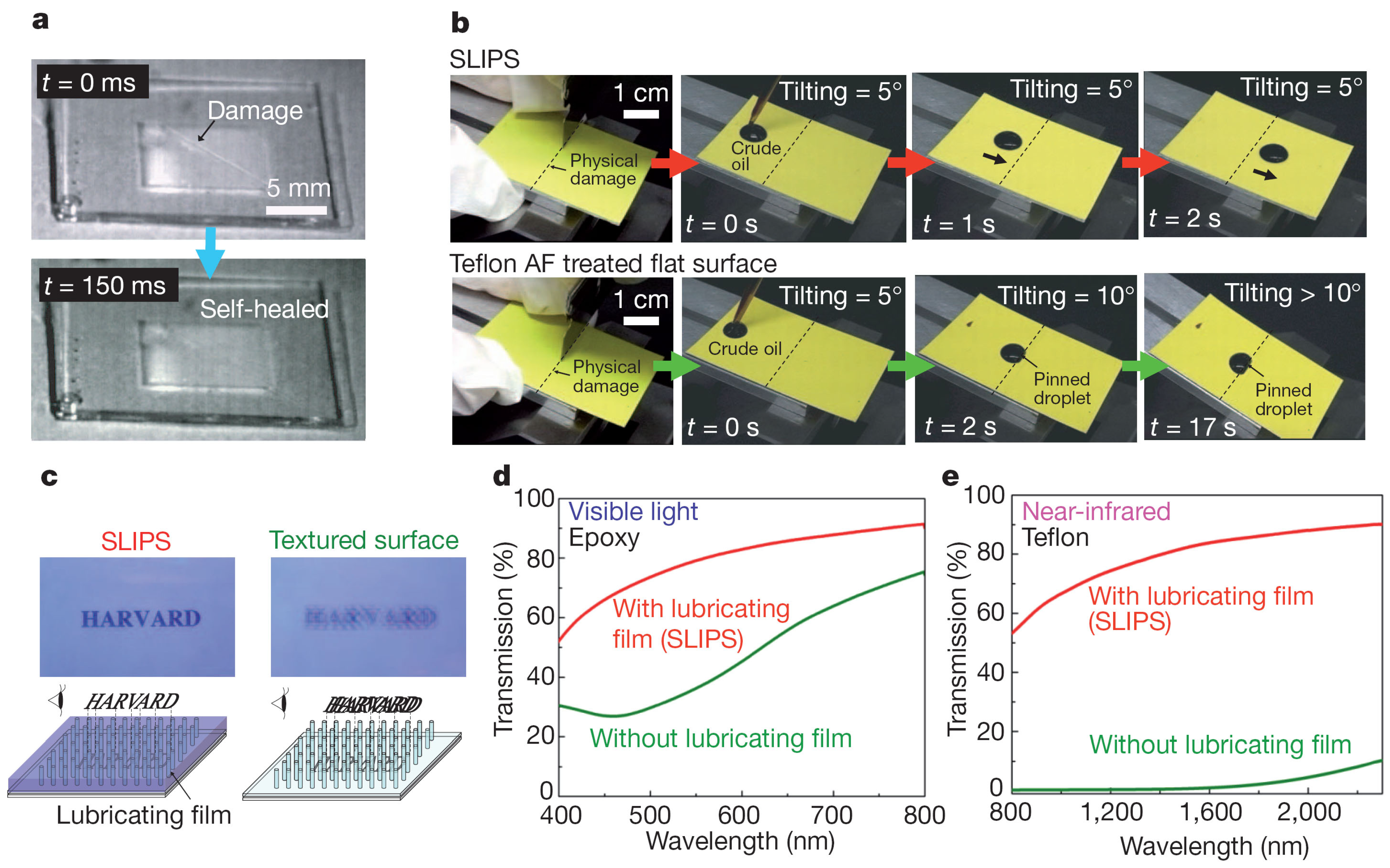
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bico, J.; Marzolin, C.; Quéré, D. Pearl drops. Europhys. Lett. 1999, 47, 220–226. [Google Scholar] [CrossRef]
- Quéré, D. Surface chemistry: Fakir droplets. Nat. Mater. 2002, 1, 14–15. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Callies, M.; Chen, Y.; Marty, F.; Pépin, A.; Quéré, D. Microfabricated textured surfaces for super-hydrophobicity investigations. Microelectron. Eng. 2005, 78–79, 100–105. [Google Scholar] [CrossRef]
- Davies, J.; Maynes, D.; Webb, B.W.; Woolford, B. Laminar flow in a microchannel with superhydrophobic walls exhibiting transverse ribs. Phys. Fluids 2006, 18. [Google Scholar] [CrossRef]
- Lee, C.; Choi, C.-H.; Kim, C.-J. Structured surfaces for giant liquid slip. Phys. Rev. Lett. 2008, 101. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Bobji, M.S.; Kumar, S.V.; Asthana, A.; Govardhan, R.N. Underwater sustainability of the “Cassie” state of wetting. Langmuir 2009, 25, 12120–12126. [Google Scholar] [CrossRef]
- Sakai, M.; Yanagisawa, T.; Nakajima, A.; Kameshima, Y.; Okada, K. Effect of surface structure on the sustainability of an air Layer on superhydrophobic coatings in a water–ethanol mixture. Langmuir 2009, 25, 13–16. [Google Scholar] [CrossRef]
- Rothstein, J.P. Slip on superhydrophobic surfaces. Annu. Rev. Fluid Mech. 2010, 42, 89–109. [Google Scholar] [CrossRef]
- Poetes, R.; Holtzmann, K.; Franze, K.; Steiner, U. Metastable underwater superhydrophobicity. Phys. Rev. Lett. 2010, 105. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef]
- Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Superhydrophobic surfaces: From the lotus leaf to the submarine. C. R. Mec. 2012, 340, 18–34. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Navier, C.L.M.H. Memoire sur les lois du mouvement des fluides. Mem. Acad. R. Sci. Inst. France 1823, 6, 389–440. [Google Scholar]
- Samaha, M.A.; Ochanda, F.O.; Tafreshi, H.V.; Tepper, G.C.; Gad-el-Hak, M. In Situ, non-invasive characterization of superhydrophobic coatings. Rev. Sci. Instrum. 2011, 82, 045109. [Google Scholar]
- Pennisi, E. Water’s tough skin. Science 2014, 343, 1194–1197. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; McKinley, G.H.; Cohen, R.E.; Rubner, M.F. Design parameters for superhydrophobicity and superoleophobicity. MRS Bull. 2008, 33, 752–758. [Google Scholar] [CrossRef]
- Joly, L.; Biben, T. Wetting and friction on superoleophobic surfaces. Soft Matter 2009, 5, 2549–2557. [Google Scholar]
- Leng, B.; Shao, Z.; de With, G.; Ming, W. Superoleophobic cotton textiles. Langmuir 2009, 25, 2456–2460. [Google Scholar]
- Ramos, S.M.M.; Benyagoub, A.; Canut, B.; Jamois, C. Superoleophobic behavior induced by nanofeatures on oleophilic surfaces. Langmuir 2010, 26, 5141–5146. [Google Scholar]
- Jin, H.; Kettunen, M.; Laiho, A; Pynnönen, J.; Paltakari, J.; Marmur, A.; Ikkala, O.; Ras, R.H.A. Superhydrophobic and superoleophobic nanocellulose aerogel membranes as bioinspired cargo carriers on water and oil. Langmuir 2011, 27, 1930–1934. [Google Scholar]
- Yang, J.; Zhang, Z.; Men, X.; Xu, X.; Zhu, X. A simple approach to fabricate superoleophobic coatings. New J. Chem. 2011, 35, 576–580. [Google Scholar] [CrossRef]
- Zhao, H.; Law, K.-Y. Directional self-cleaning superoleophobic surface. Langmuir 2012, 28, 11812–11818. [Google Scholar] [CrossRef]
- Jin, H.; Tian, X.; Ikkala, O.; Ras, R.H.A. Preservation of superhydrophobic and superoleophobic properties upon wear damage. ACS Appl. Mater. Interfaces 2013, 5, 485–488. [Google Scholar]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Rodak, D.E.; Hayden, C.A. Effects of micro- and nano-structures on the self-cleaning behaviour of lotus leaves. Nanotechnology 2006, 17, 1359–1362. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W. Fabrication of artificial Lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 2006, 5, 1386–1393. [Google Scholar]
- WordPress. Available online: http://biodsign.files.wordpress.com/2008/08/lotusan.jpg (accessed on 5 May 2011).
- Flickr. Available online: http://www.flickr.com/photos/leonefabre/3922996122/ (accessed on 5 May 2011).
- Atomic World. Available online: http://www.hk-phy.org/atomic_world/lotus/lotus01_e.html (accessed on 5 May 2011).
- Thenakedscientists. Available online: http://www.thenakedscientists.com/HTML/articles/article/biomimetics borrowingfrombiology/ (accessed on 5 May 2011).
- Cheng, Y.T.; Rodak, D.E. Is the lotus leaf superhydrophobic? Appl. Phys. Lett. 2005, 86. [Google Scholar] [CrossRef]
- Wikimedia Commons. Available online: http://commons.wikimedia.org/wiki/File:Water-strider-1.jpg (accessed on 11 May 2011).
- Feng, X.-Q.; Gao, X.; Wu, Z.; Jiang, L.; Zheng, Q.-S. Superior water repellency of water strider legs with hierarchical structures: Experiments and analysis. Langmuir 2007, 23, 4892–4896. [Google Scholar] [CrossRef]
- Bush, J.W.M.; Hu, D.L. Walking on water: Biolocomotion at the interface. Annu. Rev. Fluid Mech. 2006, 38, 339–369. [Google Scholar] [CrossRef]
- Hu, D.L.; Chan, B.; Bush, J.W.M. The hydrodynamics of water strider locomotion. Nature 2003, 424, 663–666. [Google Scholar] [CrossRef]
- Hu, D.L.; Prakash, M.; Chan, B.; Bush, J.W.M. Water-walking devices. Exp. Fluids 2007, 43, 769–778. [Google Scholar] [CrossRef]
- Stonedahl, G.M.; Lattin, J.D. The Gerridae or Water striders of oregon and Washington (Hemiptera:Heteroptera). In Technical Bulletin 144; Oregon State University: Corvallis, OR, USA, 1982; pp. 1–36. [Google Scholar]
- Flynn, M.R.; Bush, J.W.M. Underwater breathing: The mechanics of plastron respiration. J. Fluid Mech. 2008, 608, 275–296. [Google Scholar]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar]
- Xia, F.; Jiang, L. Bio-inspired, smart, multiscale interfacial materials. Adv. Mater. 2008, 20, 2842–2858. [Google Scholar] [CrossRef]
- Nitrogenseekers. Available online: http://nitrogenseekers.wordpress.com/page/36/ (accessed on 20 November 2013).
- Bauer, U.; Federle, W. The insect-trapping rim of Nepenthes pitchers surface structure and function. Plant Signal. Behav. 2009, 4, 1019–1023. [Google Scholar] [CrossRef]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef]
- Barthlott, W.; Porembski, S.; Seine, R.; Theisen, I. The Curious World of Carnivorous Plants; Timber Press: Portland, ON, USA, 2007. [Google Scholar]
- Maynes, D.; Jeffs, K.; Woolford, B.; Webb, B.W. Laminar flow in a microchannel with hydrophobic surface patterned microribs oriented parallel to the flow direction. Phys. Fluids 2007, 19. [Google Scholar] [CrossRef]
- Bico, J.; Thiele, U.; Quéré, D. Wetting of textured surfaces. Colloids Surf. A 2002, 206, 41–46. [Google Scholar] [CrossRef]
- Henoch, C.; Krupenkin, T.N.; Kolodner, P.; Taylor, J.A.; Hodes, M.S.; Lyons, A.M.; Peguero, C.; Breuer, K. Turbulent drag reduction using superhydrophobic surfaces. In Proceedings of 3rd AIAA Flow Control Conference, San Francisco, CA, USA, 5–8 June 2006.
- Choi, C.-H.; Kim, C.-J. Large slip of aqueous liquid flow over a nanoengineered superhydrophobic surface. Phys. Rev. Lett. 2006, 96. [Google Scholar] [CrossRef]
- Lee, C.; Kim, C-J. Maximizing the giant slip on superhydrophobic microstructures by nanostructuring their sidewalls. Langmuir 2009, 25, 12812–12818. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Mechanically durable carbon nanotube–composite hierarchical structures with superhydrophobicity, self-cleaning, and low-drag. ACS Nano 2009, 3, 4155–4163. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Z.; Men, X.; Yang, J.; Wang, K.; Xu, X.; Zhou, X.; Xue, Q. Robust superhydrophobic surfaces with mechanical durability and easy repairability. J. Mater. Chem. 2011, 21, 15793–15797. [Google Scholar]
- Emami, B.; Bucher, T.M.; Tafreshi, H.V.; Pestov, D.; Gad-el-Hak, M.; Tepper, G.C. Simulation of meniscus stability in superhydrophobic granular surfaces under hydrostatic pressures. Colloids Surf. A 2011, 385, 95–103. [Google Scholar]
- Yang, H.; Deng, Y. Preparation and physical properties of superhydrophobic papers. J. Colloid Interface Sci. 2008, 325, 588–593. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Kim, Y.-H.; Suh, K.-H.; Ahn, Y.-S.; Yeo, J.-G.; Han, J.-H. Super hydrophobic silica aerogel powders with simultaneous surface modification, solvent exchange and sodium ion removal from hydrogels. Microporous Mesoporous Mater. 2008, 112, 504–509. [Google Scholar] [CrossRef]
- Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Effects of hydrostatic pressure on the drag reduction of submerged aerogel-particle coatings. Colloids Surf. A 2012, 399, 62–70. [Google Scholar]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.K.; Rutledge, G.C. Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Singh, A.; Steely, L.; Allcock, H.R. Poly[bis(2,2,2-trifluoroethoxy)phosphazene] superhydrophobic nanofibers. Langmuir 2005, 21, 11604–11607. [Google Scholar] [CrossRef]
- Zhu, M.; Zuo, W.; Yu, H.; Yang, W.; Chen, Y. Superhydrophobic surface directly created by electrospinning based on hydrophilic material. J. Mater. Sci. 2006, 41, 3793–3797. [Google Scholar]
- Ochanda, F.O.; Samaha, M.A.; Tafreshi, H.V.; Tepper, G.C.; Gad-el-Hak, M. Fabrication of superhydrophobic fiber coatings by DC-biased AC-electrospinning. J. Appl. Polym. Sci. 2012, 123, 1112–1119. [Google Scholar] [CrossRef]
- Sarkar, S.; Deevi, S.; Tepper, G. Biased AC electrospinning of aligned polymer nanofibers. Macromol. Rapid Commun. 2007, 28, 1034–1039. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering biomimetic superhydrophobic surfaces of electrospun nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Ding, B.; Yu, J.; Sun, G.; Wang, M. Biomimicry via electrospinning. Crit. Rev. Solid State Mater. Sci. 2012, 37, 94–114. [Google Scholar] [CrossRef]
- Laird, E.D.; Bose, R.K.; Qi, H.; Lau, K.K.S.; Li, C.Y. Electric field-induced, reversible lotus-to-rose transition in nanohybrid shish kebab paper with hierarchical roughness. ACS Appl. Mater. Interfaces 2013, 5, 12089–12098. [Google Scholar]
- Srinivasan, S.; Chhatre, S.S.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. Solution spraying of poly(methyl methacrylate) blends to fabricate microtextured, superoleophobic surfaces. Polymer 2011, 52, 3209–3218. [Google Scholar] [CrossRef]
- Srinivasan, S.; Choi, W.; Park, K.-C.; Chhatre, S.S.; Cohen, R.E.; McKinley, G.H. Drag reduction for viscous laminar flow on spray-coated non-wetting surfaces. Soft Matter 2013, 9, 5691–5702. [Google Scholar]
- Huang, X.; Chrisman, J.D.; Zacharia, N.S. Omniphobic slippery coatings based on lubricant-infused porous polyelectrolyte multilayers. ACS Macro Lett. 2013, 2, 826–829. [Google Scholar] [CrossRef]
- Shillingford, C.; MacCallum, N.; Wong, T-S.; Kim, P.; Aizenberg, J. Fabrics coated with lubricated nanostructures display robust omniphobicity. Nanotechnology 2014, 25. [Google Scholar] [CrossRef]
- Gad-el-Hak, M. Flow Control: Passive, Active, and Reactive Flow Management; Cambridge University Press: London, UK, 2000. [Google Scholar]
- Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Sustainability of superhydrophobicity under pressure. Phys. Fluids 2012, 24. [Google Scholar] [CrossRef]
- Gad-el-Hak, M. The fluid mechanics of microdevices—The freeman scholar lecture. J. Fluids Eng. 1999, 121, 5–33. [Google Scholar] [CrossRef]
- Lauga, E.; Brenner, M.P.; Stone, H.A. Microfluidics: The no-slip boundary condition. In Handbook of Experimental Fluid Dynamics; Tropea, C., Yarin, A., Foss, J., Eds.; Springer: New York, NY, USA, 2007; pp. 1219–1240. [Google Scholar]
- Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Modeling drag reduction and meniscus stability of superhydrophobic surfaces comprised of random roughness. Phys. Fluids 2011, 23. [Google Scholar] [CrossRef]
- Lauga, E.; Stone, H.A. Effective slip in pressure-driven Stokes flow. J. Fluid Mech. 2003, 489, 55–77. [Google Scholar] [CrossRef]
- Ou, J.; Perot, J.B.; Rothstein, J.P. Laminar drag reduction in microchannels using ultrahydrophobic surfaces. Phys. Fluids 2004, 16, 4635–4643. [Google Scholar] [CrossRef]
- Ou, J.; Rothstein, J.P. Direct velocity measurements of the flow past drag-reducing ultrahydrophobic surfaces. Phys. Fluids 2005, 17. [Google Scholar] [CrossRef]
- Ybert, C.; Barentin, C.; Cécile, C.-B.; Jospeh, P.; Bocquet, L. Achieving large slip with superhydrophobic surfaces: Scaling laws for generic geometries. Phys. Fluids 2007, 19. [Google Scholar] [CrossRef]
- Cheng, Y.P.; Teo, C.J.; Khoo, B.C. Microchannel flow with superhydrophobic surfaces: Effects of Reynolds number and pattern width to channel height ratio. Phys. Fluids 2009, 21. [Google Scholar] [CrossRef]
- Daniello, R.; Waterhouse, N.E.; Rothstein, J.P. Drag reduction in turbulent flows over superhydrophobic surfaces. Phys. Fluids 2009, 21. [Google Scholar] [CrossRef]
- Martell, M.; Perot, J.B.; Rothstein, J.P. Direct numerical simulations of turbulent flows over superhydrophobic surfaces. J. Fluid Mech. 2009, 620, 31–41. [Google Scholar]
- Davis, A.M.J.; Lauga, E. The friction of a mesh-like super-hydrophobic surface. Phys. Fluids 2009, 21. [Google Scholar] [CrossRef]
- Woolford, B.; Prince, J.; Maynes, D.; Webb, B.W. Particle image velocimetry characterization of turbulent channel flow with rib patterned superhydrophobic walls. Phys. Fluids 2009, 21. [Google Scholar] [CrossRef]
- Patankar, N.A. Transition between superhydrophobic states on rough surfaces. Langmuir 2004, 20, 7097–7102. [Google Scholar] [CrossRef]
- Barbieri, L.; Wagner, E.; Hoffmann, P. Water wetting transition parameters of perfluorinated substrates with periodically distributed flat-top microscale obstacles. Langmuir 2007, 23, 1723–1734. [Google Scholar] [CrossRef]
- Extrand, C.W. Criteria for ultralyophobic surfaces. Langmuir 2004, 20, 5013–5018. [Google Scholar] [CrossRef]
- Extrand, C.W. Designing for optimum liquid repellency. Langmuir 2006, 22, 1711–1714. [Google Scholar]
- Zheng, Q.S.; Yu, Y.; Zhao, Z.H. Effects of hydraulic pressure on the stability and transition of wetting modes of superhydrophobic surfaces. Langmuir 2005, 21, 12207–12212. [Google Scholar] [CrossRef]
- Okabe, A.; Boots, B.; Sugihara, K.; Chiu, S.N.; Kendall, D.G. Spatial Tessellations: Concepts andApplications of Voronoi Diagrams, 2nd ed.; John Wiley & Sons Ltd: Chichester, England, 2000. [Google Scholar]
- Emami, B.; Tafreshi, H.V.; Gad-el-Hak, M.; Tepper, G.C. Predicting shape and stability of air–water interface on superhydrophobic surfaces with randomly distributed, dissimilar posts. Appl. Phys. Lett. 2011, 98, 203106:1–203106:3. [Google Scholar]
- Bucher, T.M.; Emami, B.; Tafreshi, H.V.; Gad-el-Hak, M.; Tepper, G.C. Modeling resistance of nanofibrous superhydrophobic coatings to hydrostatic pressures: The role of microstructure. Phys. Fluids 2012, 24. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, M.; Mukherjee, P.P.; Lichtner, P.C. Mesoscopic modeling of multiphysicochemical transport phenomena in porous media. Adv. Mech. Eng. 2010, 2010, 142879:1–142879:11. [Google Scholar]
- Mukherjee, P.P.; Kang, Q.; Wang, C-Y. Pore-scale modeling of two-phase transport in polymer electrolyte fuel cells—Progress and perspective. Energy Environ. Sci. 2011, 4, 346–369. [Google Scholar] [CrossRef]
- Emami, B.; Tafreshi, H.V.; Gad-el-Hak, M.; Tepper, G.C. Predicting shape and stability of air–water interface on superhydrophobic surfaces comprised of pores with arbitrary shapes and depths. Appl. Phys. Lett. 2012, 100, 013104:1–013104:4. [Google Scholar]
- Emami, B.; Tafreshi, H.V.; Gad-el-Hak, M.; Tepper, G.C. Effect of fiber orientation on shape and stability of air–water interface on submerged superhydrophobic electrospun thin coatings. J. Appl. Phys. 2012, 111. [Google Scholar] [CrossRef]
- Extrand, C.W. Repellency of the Lotus Leaf: Resistance to Water Intrusion under Hydrostatic Pressure. Langmuir 2011, 27, 6920–6925. [Google Scholar] [CrossRef]
- Sheng, X.; Zhang, J. Air layer on superhydrophobic surface underwater. Colloids Surf. A 2011, 377, 374–378. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Novel method to characterize superhydrophobic coatings. J. Colloid Interface Sci. 2013, 395, 315–321. [Google Scholar] [CrossRef]
- Lei, L.; Li, H.; Shi, J.; Chen, Y. Diffraction patterns of a water-submerged superhydrophobic grating under pressure. Langmuir 2010, 26, 3666–3669. [Google Scholar] [CrossRef]
- Rathgen, H.; Mugele, F. Microscopic shape and contact angle measurement at a superhydrophobic surface. Faraday Discuss. 2010, 146, 49–56. [Google Scholar] [CrossRef]
- Drelich, J. Guidelines to measurements of reproducible contact angles using a sessile-drop technique. Surface Innovations 2013, 1, 248–254. [Google Scholar] [CrossRef]
- Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Influence of flow on longevity of superhydrophobic coatings. Langmuir 2012, 28, 9759–9766. [Google Scholar] [CrossRef]
- Ochanda, F.O.; Samaha, M.A.; Tafreshi, H.V.; Tepper, G.C.; Gad-el-Hak, M. Salinity effects on the degree of hydrophobicity and longevity for superhydrophobic fibrous coatings. J. Appl. Polym. Sci. 2012, 124, 5021–5026. [Google Scholar]
- Emami, B.; Hemeda, A.A.; Amrei, M.M.; Luzar, A.; Gad-el-Hak, M.; Tafreshi, H.V. Predicting longevity of submerged superhydrophobic surfaces with parallel grooves. Phys. Fluids 2013, 25. [Google Scholar] [CrossRef]
- Barth, C.A.; Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Convective mass transfer from submerged superhydrophobic surfaces. Int. J. Flow Contr. 2013, 5, 79–88. [Google Scholar] [CrossRef]
- Barth, C.A.; Samaha, M.A.; Tafreshi, H.V.; Gad-el-Hak, M. Convective mass transfer from submerged superhydrophobic surfaces: Turbulent flow. Int. J. Flow Contr. 2013, 5, 143–152. [Google Scholar] [CrossRef]
- Stephani, K.A.; Goldstein, D.B. An Examination of trapped bubbles for viscous drag reduction on submerged surfaces. J. Fluids Eng. 2010, 132. [Google Scholar] [CrossRef]
- Lee, C.; Kim, C.-J. Underwater restoration and retention of gases on superhydrophobic surfaces for drag reduction. Phys. Rev. Lett. 2011, 106. [Google Scholar] [CrossRef]
- Smith, J.D.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2013, 9, 1772–1780. [Google Scholar]
- Okada, I.; Shiratori, S. High-transparency, self-standable Gel-SLIPS fabricated by a facile nanoscale phase separation. ACS Appl. Mater. Interfaces 2014, 6, 1502–1508. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.-S.; Belisleb, R.A.; Boggsa, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar]
- Xiao, L.; Li, J.; Mieszkin, S.; Fino, A.D.; Clare, A.S.; Callow, M.E.; Callow, J.A.; Grunze, M.; Rosenhahn, A.; Levkin, P.A. Slippery liquid-infused porous surfaces showing marine antibiofouling properties. ACS Appl. Mater. Interfaces 2013, 5, 10074–10080. [Google Scholar]
- Li, J.; Kleintschek, T.; Rieder, A.; Cheng, Y.; Baumbach, T.; Obst, U.; Schwartz, T.; Levkin, P.A. Hydrophobic liquid-infused porous polymer surfaces for antibacterial applications. ACS Appl. Mater. Interfaces 2013, 5, 6704–6711. [Google Scholar] [CrossRef]
- Daniel, D.; Mankin, M.N.; Belisleb, R.A.; Wong, T.-S.; Aizenberg, J. Lubricant-infused micro/nano-structured surfaces with tunable dynamic omniphobicity at high temperatures. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Samaha, M.A.; Gad-el-Hak, M. Polymeric Slippery Coatings: Nature and Applications. Polymers 2014, 6, 1266-1311. https://doi.org/10.3390/polym6051266
Samaha MA, Gad-el-Hak M. Polymeric Slippery Coatings: Nature and Applications. Polymers. 2014; 6(5):1266-1311. https://doi.org/10.3390/polym6051266
Chicago/Turabian StyleSamaha, Mohamed A., and Mohamed Gad-el-Hak. 2014. "Polymeric Slippery Coatings: Nature and Applications" Polymers 6, no. 5: 1266-1311. https://doi.org/10.3390/polym6051266
APA StyleSamaha, M. A., & Gad-el-Hak, M. (2014). Polymeric Slippery Coatings: Nature and Applications. Polymers, 6(5), 1266-1311. https://doi.org/10.3390/polym6051266