Polyelectrolyte Multilayers: Towards Single Cell Studies
Abstract
:1. Introduction
2. LbL Films as Carriers for Bioactive Substances
2.1. Control of the Structure and Dynamics of LbL Films
2.2. Loading of Biomolecules
2.2.1. Loading with Small Molecules
2.2.2. Loading with Nanocontainers
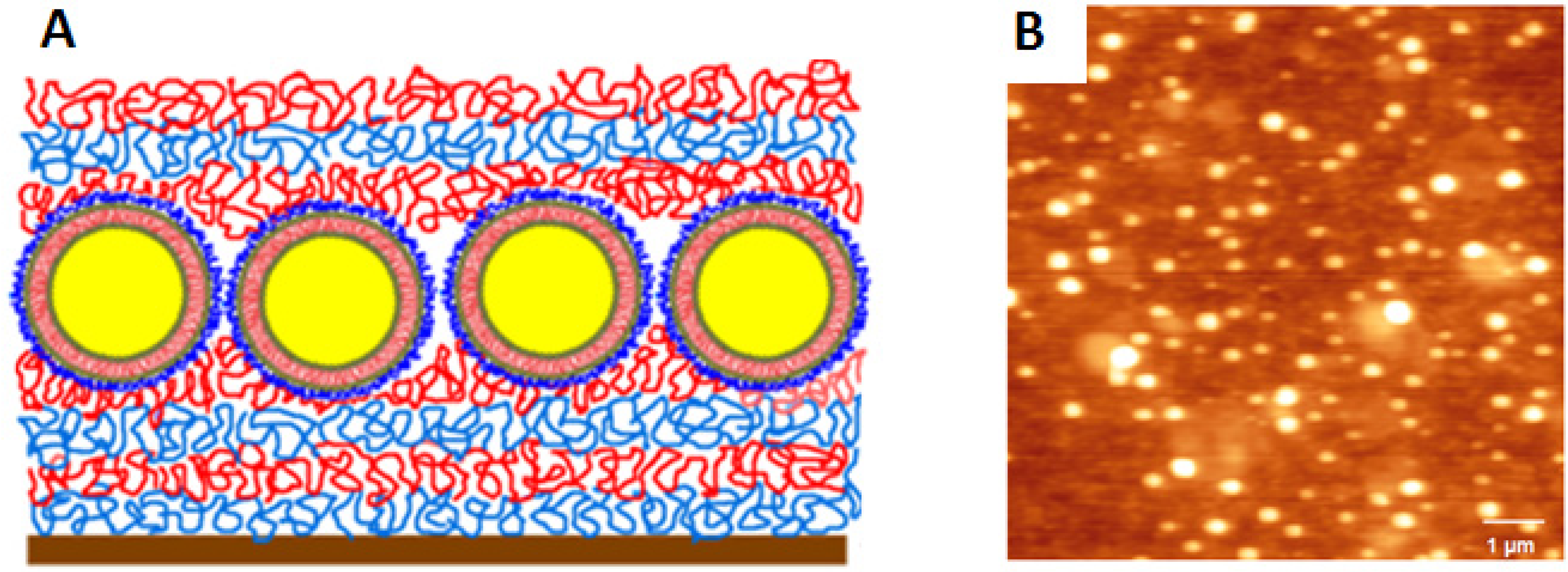
2.2.3. Loading with Proteins and Nucleic Acids
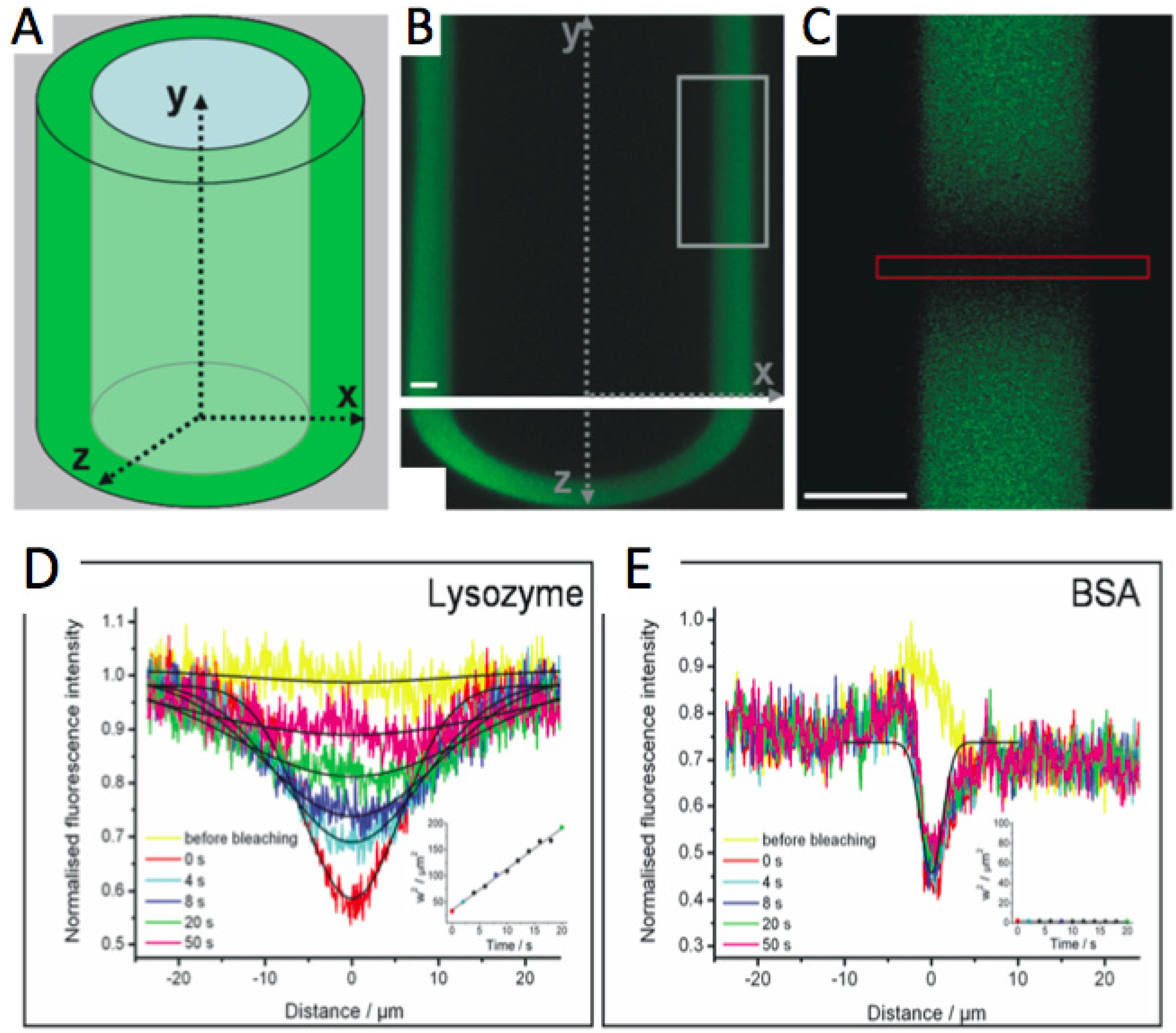
2.2.4. Analysis of (Bio)molecule Diffusion and Distribution in the Film
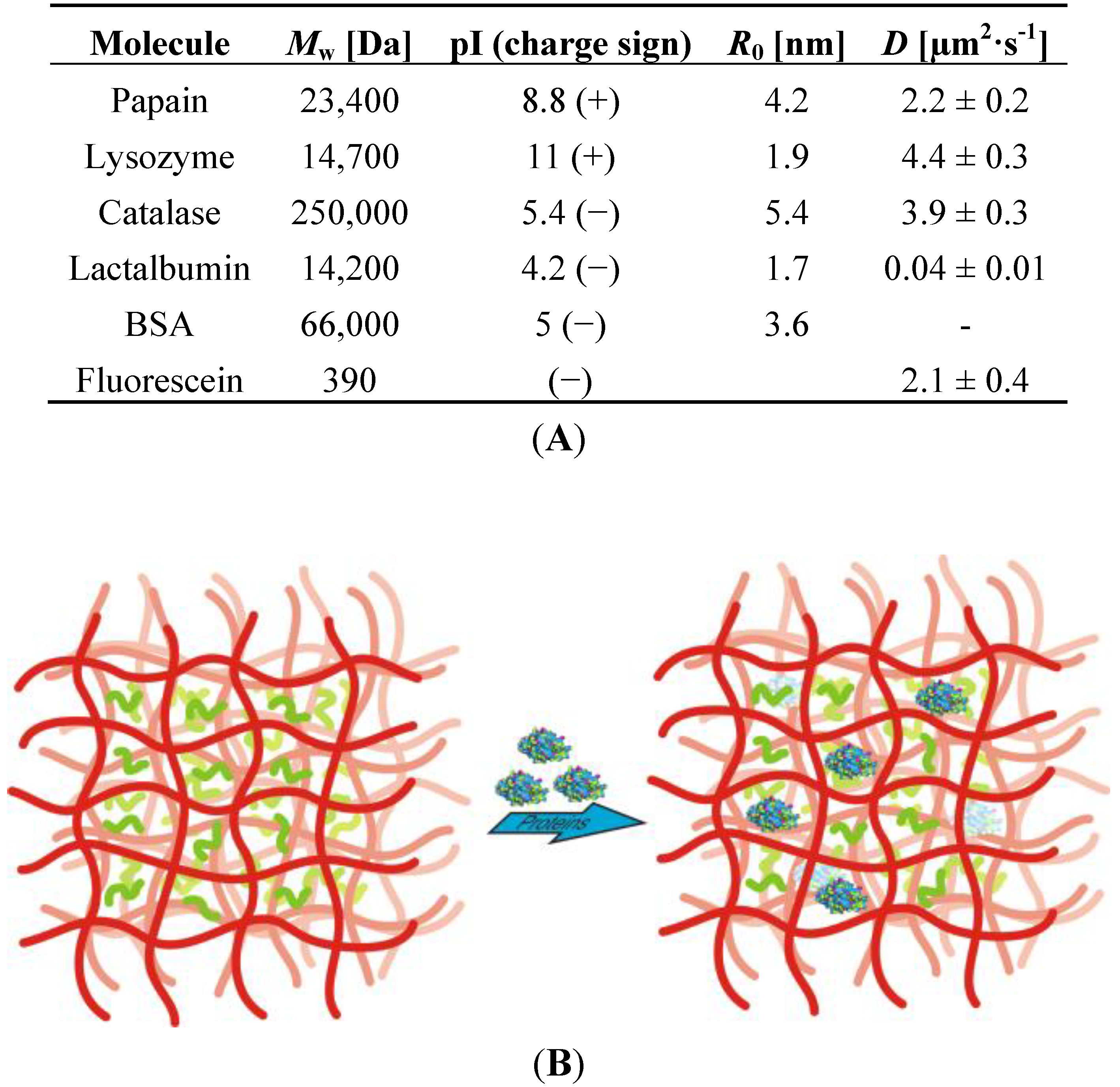
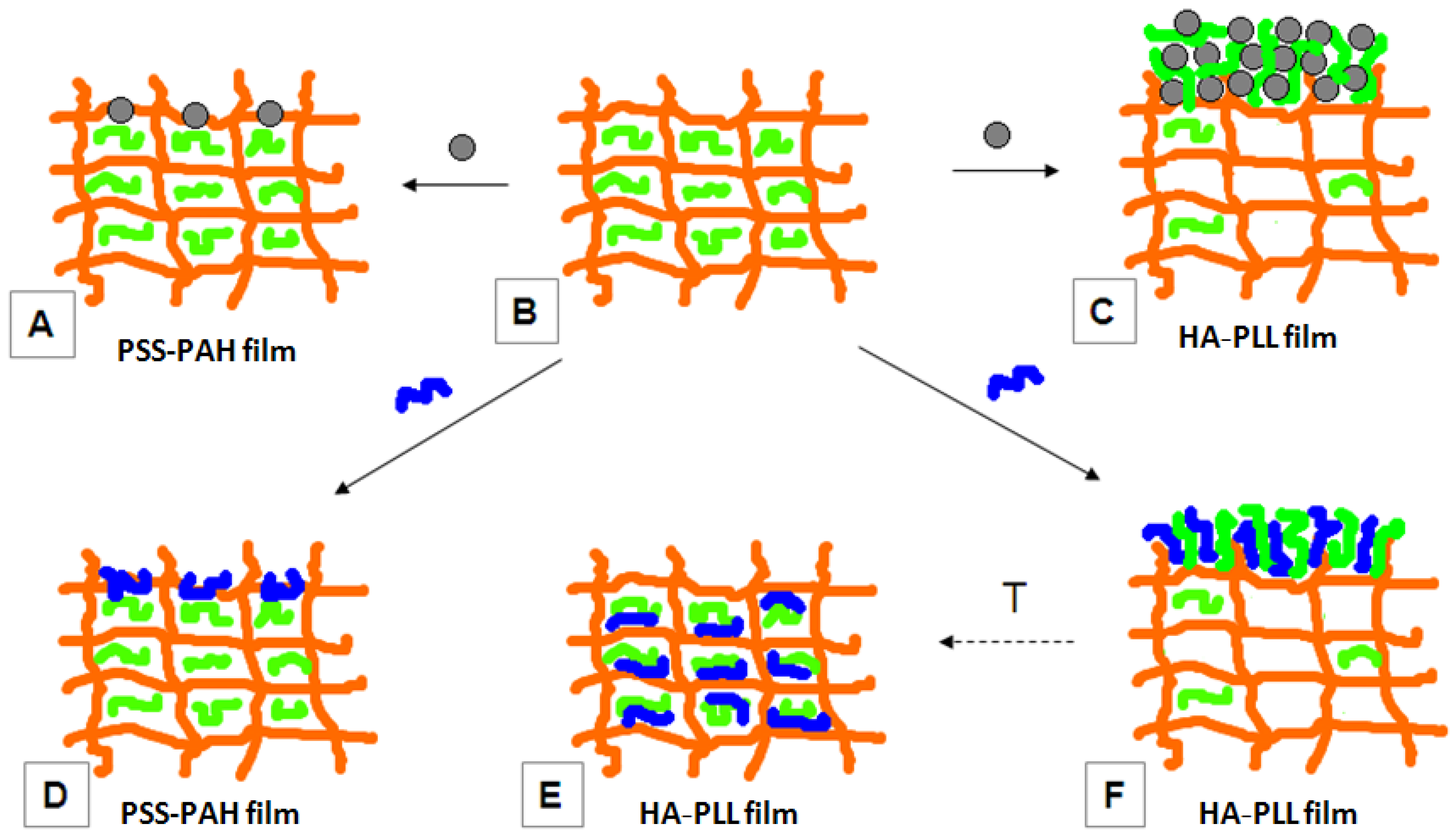
2.2.5. Tailored Polymer Matrices
3. Cell Patterning by LbL Films
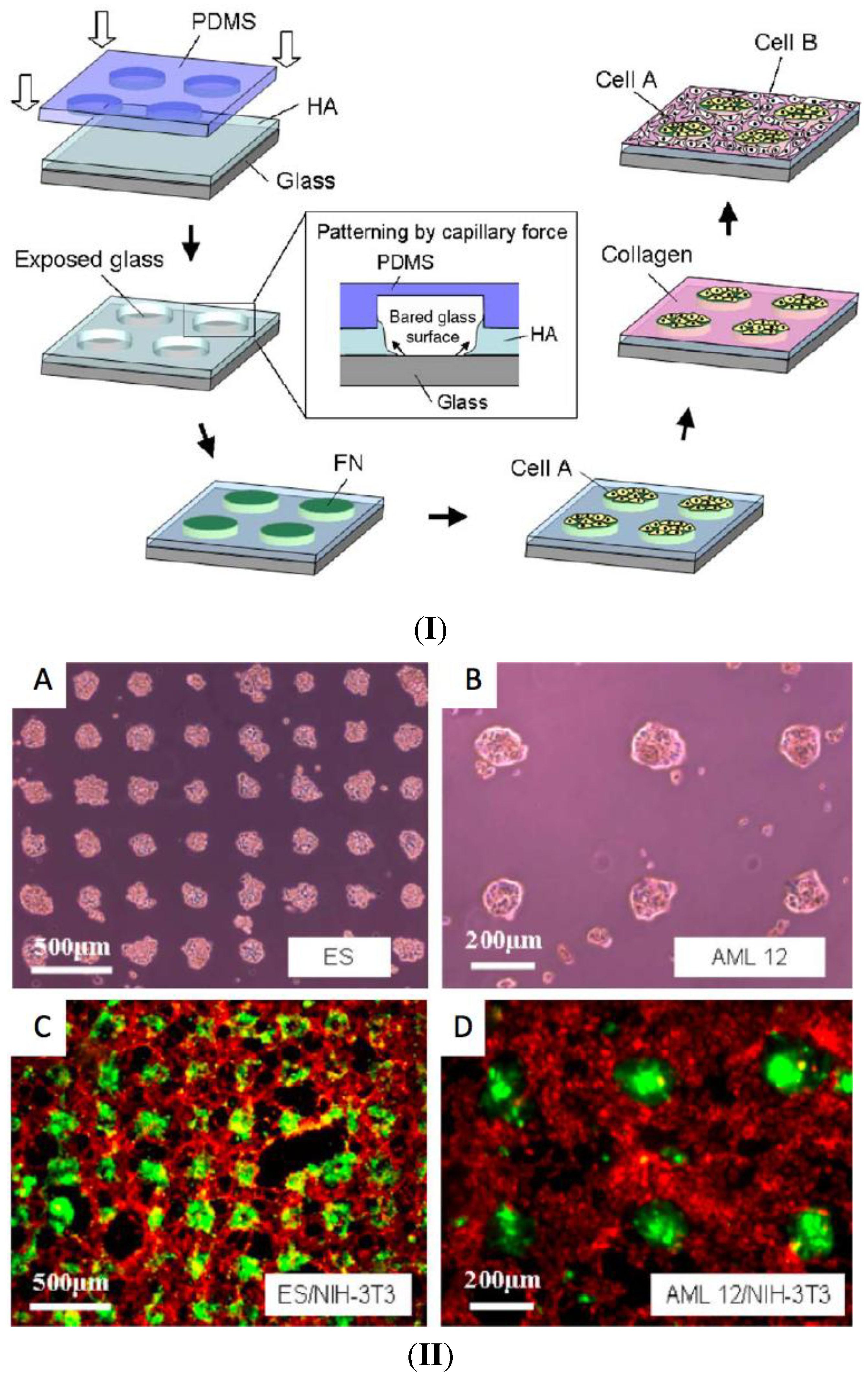
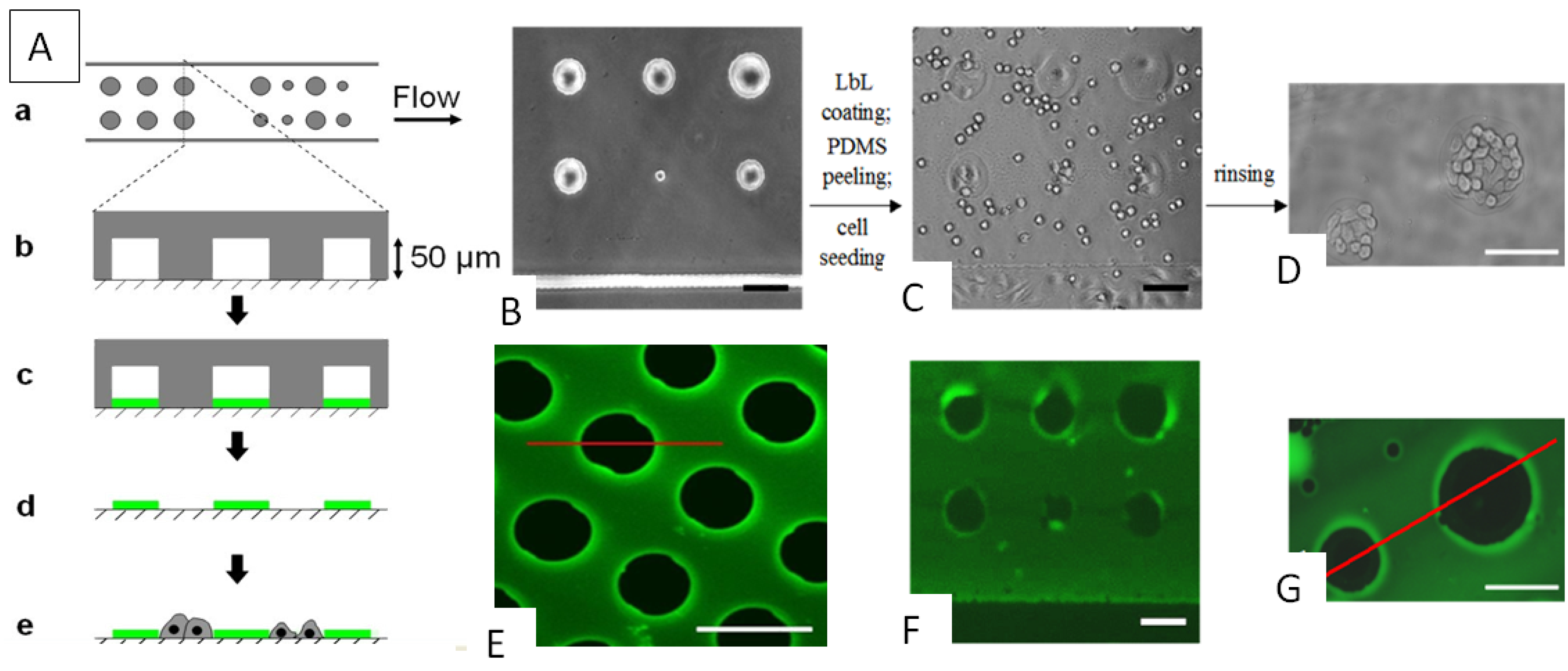
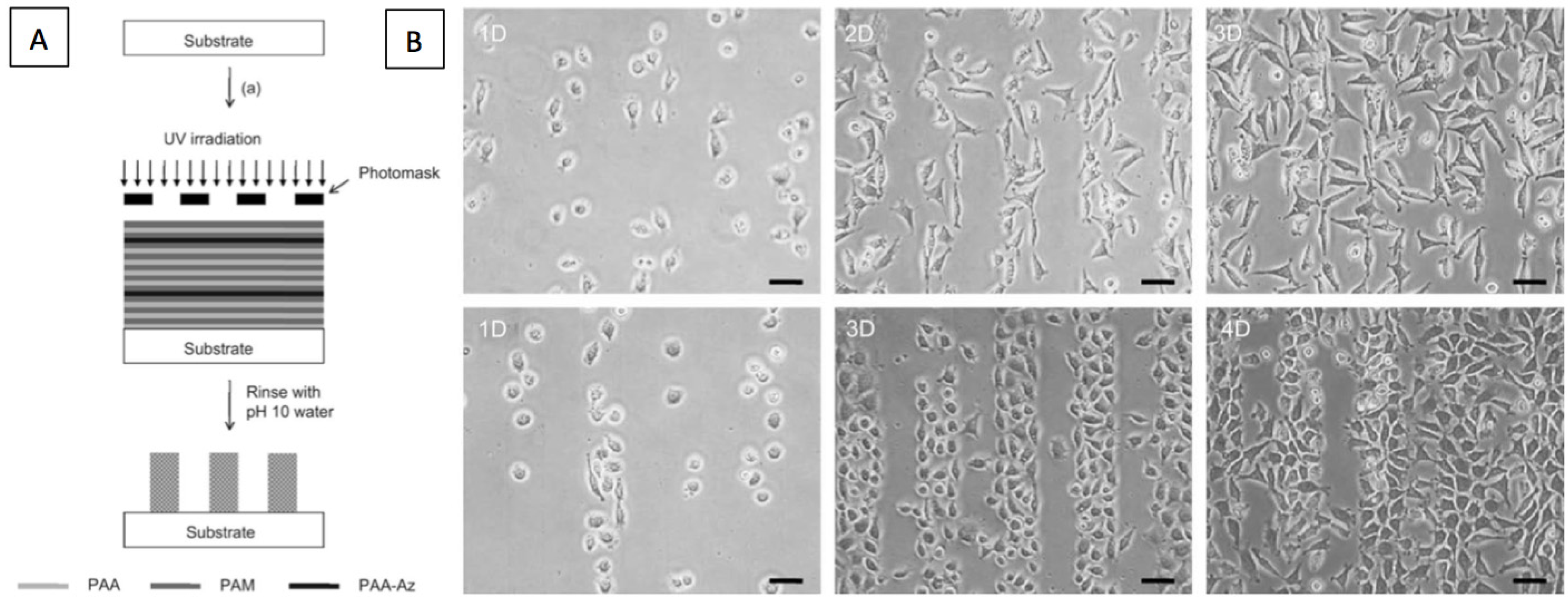
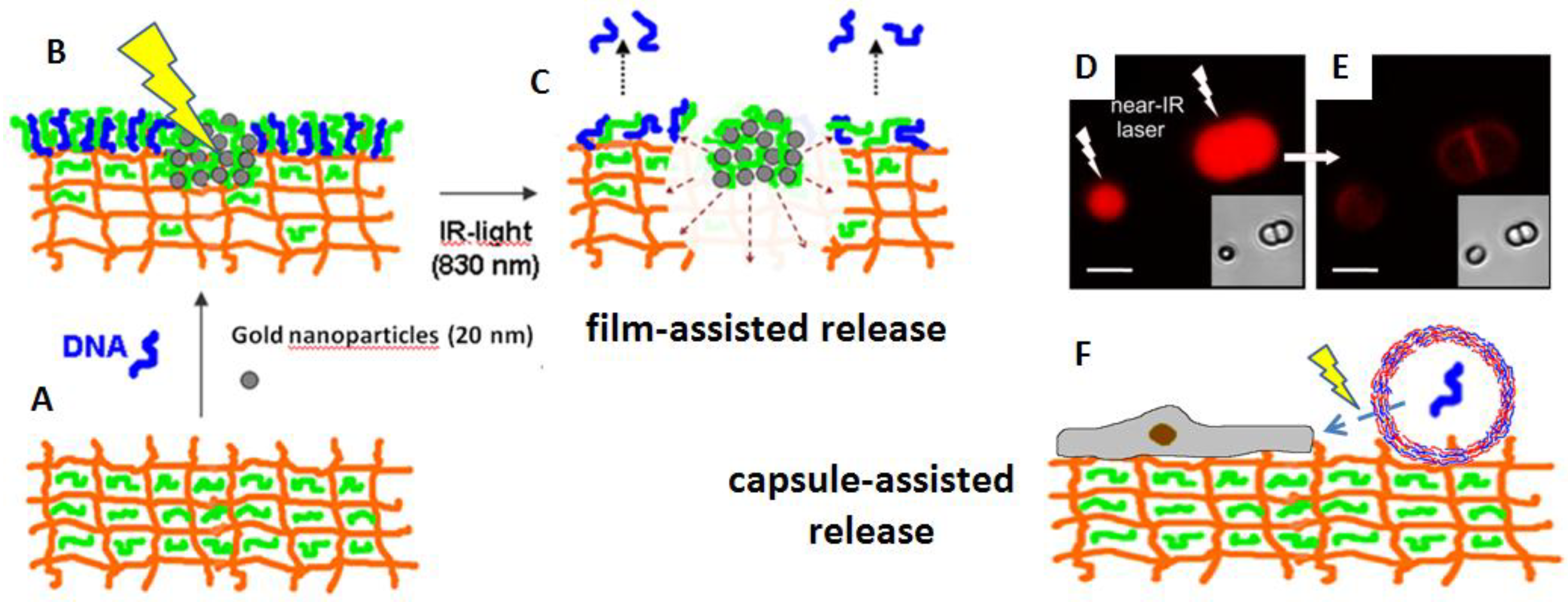
4. Light-Triggered Delivery
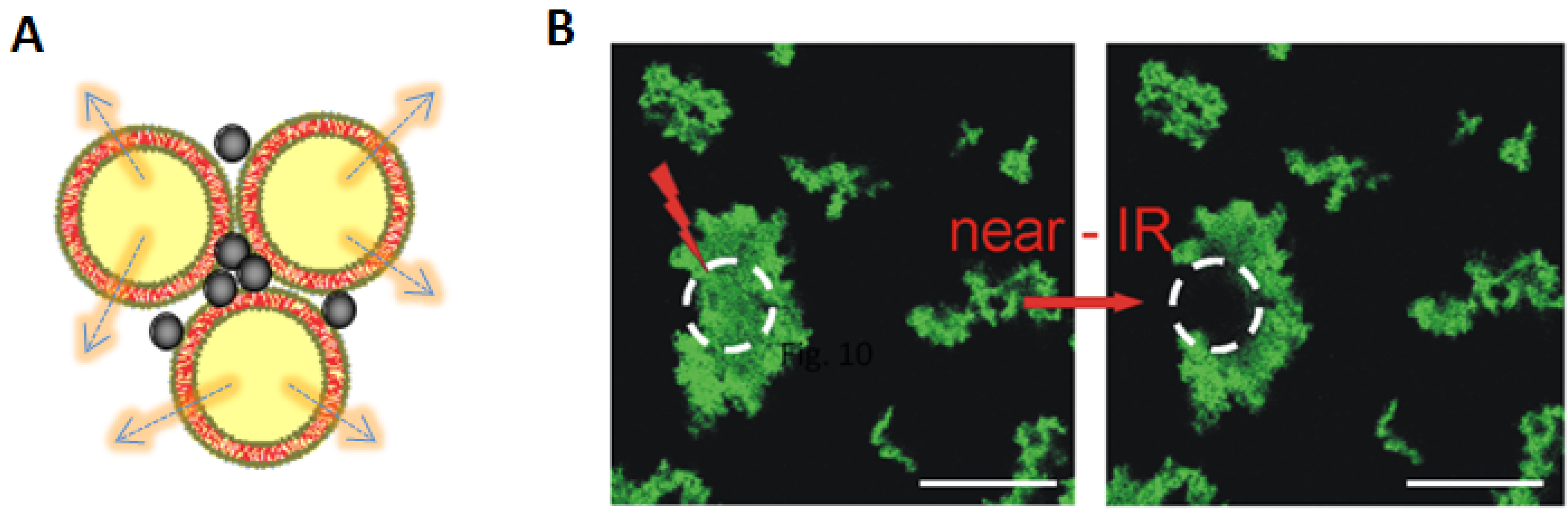
5. Conclusions and Perspectives
Acknowledgment
Conflicts of Interest
References
- Wang, D.; Bodovitz, S. Single cell analysis: The new frontier in “omics”. Trends Biotechnol. 2010, 28, 281–290. [Google Scholar] [CrossRef]
- Fritzsch, F.S.O.; Dusny, C.; Frick, O.; Schmid, A. Single-cell analysis in biotechnology, systems biology, and biocatalysis. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 129–155. [Google Scholar] [CrossRef]
- Graf, T.; Stadtfeld, M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell 2008, 3, 480–483. [Google Scholar] [CrossRef]
- Kleparnik, K.; Foret, F. Recent advances in the development of single cell analysis—A review. Anal. Chim. Acta 2013, 800, 12–21. [Google Scholar] [CrossRef]
- Chao, T.C.; Ros, A. Microfluidic single-cell analysis of intracellular compounds. J. R. Soc. Interface 2008, 5, S139–S150. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.-D. Buildup of ultrathin multilayer films by a self-assembly process: I. consecutive adsorption of anionic and cationic bipolar amphiphiles. Makromol. Chem. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Peyratout, C.S.; Dahne, L. Tailor-made polyelectrolyte microcapsules: From multilayers to smart containers. Angew. Chem. Int. Ed. 2004, 43, 3762–3783. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Li, Q. Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. Phys. Chem. Chem. Phys. 2007, 9, 2319–2340. [Google Scholar] [CrossRef]
- Wang, Y.J.; Hosta-Rigau, L.; Lomas, H.; Caruso, F. Nanostructured polymer assemblies formed at interfaces: Applications from immobilization and encapsulation to stimuli-responsive release. Phys. Chem. Chem. Phys. 2011, 13, 4782–4801. [Google Scholar]
- Gero Decher, J.B.S. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef]
- Lavalle, P.; Voegel, J.C.; Vautier, D.; Senger, B.; Schaaf, P.; Ball, V. Dynamic aspects of films prepared by a sequential deposition of species: Perspectives for smart and responsive materials. Adv. Mater. 2011, 23, 1191–1221. [Google Scholar] [CrossRef]
- Sukhishvili, S.A. Responsive polymer films and capsules via layer-by-layer assembly. Curr. Opin. Colloid Interface Sci. 2005, 10, 37–44. [Google Scholar] [CrossRef]
- Volodkin, D.; Skirtach, A.; Mohwald, H. LbL films as reservoirs for bioactive molecules. In Bioactive Surfaces; Borner, H.G., Lutz, J.F., Eds.; Springer-Verlag Berlin: Berlin, Germany, 2011; Volume 240, pp. 135–161. [Google Scholar]
- Volodkin, D.V.; Mohwald, H. Polyelectrolyte multilayers for drug delivery. In Encyclopedia of Surface and Colloid Science; Somasundaran, P., Ed.; Taylor & Francis Group, LLC: Abingdon, UK, 2009; Volume 1, p. 14. [Google Scholar]
- Delcea, M.; Mohwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef]
- De Geest, B.G.; Sukhorukov, G.B.; Mohwald, H. The pros and cons of polyelectrolyte capsules in drug delivery. Exp. Opin. Drug Deliv. 2009, 6, 613–624. [Google Scholar] [CrossRef]
- De Geest, B.G.; Sanders, N.N.; Sukhorukov, G.B.; Demeester, J.; de Smedt, S.C. Release mechanisms for polyelectrolyte capsules. Chem. Soc. Rev. 2007, 36, 636–649. [Google Scholar] [CrossRef]
- Hirano, Y.; Mooney, D.J. Peptide and protein presenting materials for tissue engineering. Adv. Mater. 2004, 16, 17–25. [Google Scholar] [CrossRef]
- Crouzier, T.; Ren, K.; Nicolas, C.; Roy, C.; Picart, C. Layer-by-layer films as a biomimetic reservoir for rhBMP-2 delivery: Controlled differentiation of myoblasts to osteoblasts. Small 2009, 5, 598–608. [Google Scholar] [CrossRef]
- Boudou, T.; Crouzier, T.; Ren, K.; Blin, G.; Picart, C. Multiple functionalities of polyelectrolyte multilayer films: new biomedical applications. Adv. Mater. 2010, 22, 441–467. [Google Scholar] [CrossRef]
- Jewell, C.M.; Lynn, D.M. Multilayered polyelectrolyte assemblies as platforms for the delivery of DNA and other nucleic acid-based therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 979–999. [Google Scholar] [CrossRef]
- Jewell, C.M.; Zhang, J.; Fredin, N.J.; Lynn, D.M. Multilayered polyelectrolyte films promote the direct and localized delivery of DNA to cells. J. Control. Release 2005, 106, 214–223. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Madaboosi, N.; Blacklock, J.; Skirtach, A.G.; Mohwald, H. Surface-supported multilayers decorated with bio-active material aimed at light-triggered drug delivery. Langmuir 2009, 25, 14037–14043. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotech. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Guillame-Gentil, O.; Semenov, O.; Roca, A.S.; Groth, T.; Zahn, R.; Voros, J.; Zenobi-Wong, M. Engineering the extracellular environment: Strategies for building 2D and 3D cellular structures. Adv. Mater. 2010, 22, 5443–5462. [Google Scholar] [CrossRef]
- züm, C.; Hellweg, J.; Madaboosi, N.; Volodkin, D.V.; von Klitzing, R. Growth behavior and mechanical properties of PLL/HA multilayer films studied by AFM. Beilstein J. Nanotechnol. 2012, 3, 778–788. [Google Scholar] [CrossRef]
- Picart, C.; Lavalle, P.; Hubert, P.; Cuisinier, F.J.G.; Decher, G.; Schaaf, P.; Voegel, J.-C. Buildup mechanism for poly(l-lysine)/hyaluronic acid films onto a solid surface. Langmuir 2001, 17, 7414–7424. [Google Scholar] [CrossRef]
- Semenov, O.V.; Malek, A.; Bittermann, A.G.; Voros, J.; Zisch, A.H. Engineered polyelectrolyte multilayer substrates for adhesion, proliferation, and differentiation of human mesenchymal stem cells. Tissue Eng. A 2009, 15, 2977–2990. [Google Scholar] [CrossRef]
- Richert, L.; Lavalle, P.; Payan, E.; Shu, X.Z.; Prestwich, G.D.; Stoltz, J.-F.; Schaaf, P.; Voegel, J.-C.; Picart, C. Layer by layer buildup of polysaccharide films: Physical chemistry and cellular adhesion aspects. Langmuir 2004, 20, 448–458. [Google Scholar] [CrossRef]
- Francius, G.; Hemmerlé, J.; Ball, V.; Lavalle, P.; Picart, C.; Voegel, J.-C.; Schaaf, P.; Senger, B. Stiffening of soft polyelectrolyte architectures by multilayer capping evidenced by viscoelastic analysis of AFM indentation measurements. J. Phys. Chem. C 2007, 111, 8299–8306. [Google Scholar] [CrossRef]
- Schneider, A.; Francius, G.; Obeid, R.; Schwinté, P.; Hemmerlé, J.; Frisch, B.; Schaaf, P.; Voegel, J.-C.; Senger, B.; Picart, C. Polyelectrolyte multilayers with a tunable young’s modulus: Influence of film stiffness on cell adhesion. Langmuir 2005, 22, 1193–1200. [Google Scholar]
- Ren, K.; Crouzier, T.; Roy, C.; Picart, C. Polyelectrolyte multilayer films of controlled stiffness modulate myoblast cell differentiation. Adv. Funct. Mater. 2008, 18, 1–12. [Google Scholar]
- Schmidt, S.; Madaboosi, N.; Uhlig, K.; Köhler, D.; Skirtach, A.; Duschl, C.; Möhwald, H.; Volodkin, D.V. Control of cell adhesion by mechanical reinforcement of soft polyelectrolyte films with nanoparticles. Langmuir 2012, 28, 7249–7257. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Tang, Z.; Shim, B.S.; Kotov, N.A. Counterintuitive effect of molecular strength and role of molecular rigidity on mechanical properties of layer-by-layer assembled nanocomposites. Nano Lett. 2007, 7, 1224–1231. [Google Scholar] [CrossRef]
- Vodouhê, C.; le Guen, E.; Mendez Garza, J.; Francius, G.; Déjugnat, C.; Ogier, J.; Schaaf, P.; Voegel, J.-C.; Lavalle, P. Control of drug accessibility on functional polyelectrolyte multilayer films. Biomaterials 2006, 27, 4149–4156. [Google Scholar] [CrossRef]
- Madaboosi, N.; Uhlig, K.; Schmidt, S.; Jager, M.S.; Mohwald, H.; Duschl, C.; Volodkin, D.V. Microfluidics meets soft layer-by-layer films: selective cell growth in 3D polymer architectures. Lab Chip 2012, 12, 1434–1436. [Google Scholar] [CrossRef]
- Ariga, K.; McShane, M.; Lvov, Y.M.; Ji, Q.M.; Hill, J.P. Layer-by-layer assembly for drug delivery and related applications. Exp. Opin. Drug Deliv. 2011, 8, 633–644. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotech. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef]
- Takayama, S.; Ostuni, E.; LeDuc, P.; Naruse, K.; Ingber, D.E.; Whitesides, G.M. Laminar flows-Subcellular positioning of small molecules. Nature 2001, 411, 1016. [Google Scholar] [CrossRef]
- Schonhoff, M. Self-assembled polyelectrolyte multilayers. Curr. Opin. Colloid Interface Sci. 2003, 8, 86–95. [Google Scholar] [CrossRef]
- Von Klitzing, R. Internal structure of polyelectrolyte multilayer assemblies. Phys. Chem. Chem. Phys. 2006, 8, 5012–5033. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R. Competing mechanisms in polyelectrolyte multilayer formation and swelling: Polycation–polyanion pairing vs. polyelectrolyte–ion pairing. Curr. Opin. Colloid Interface Sci. 2014, 19, 25–31. [Google Scholar] [CrossRef]
- Nazaran, P.; Bosio, V.; Jaeger, W.; Anghel, D.F.; von Klitzing, R. Lateral mobility of polyelectrolyte chains in multilayers. J. Phys. Chem. B 2007, 111, 8572–8581. [Google Scholar] [CrossRef]
- Lavalle, P.; Picart, C.; Mutterer, J.; Gergely, C.; Reiss, H.; Voegel, J.-C.; Senger, B.; Schaaf, P. Modeling the buildup of polyelectrolyte multilayer films having exponential growth. J. Phys. Chem. B 2004, 108, 635–648. [Google Scholar] [CrossRef]
- Ghostine, R.A.; Markarian, M.Z.; Schlenoff, J.B. Asymmetric growth in polyelectrolyte multilayers. J. Am. Chem. Soc. 2013, 135, 7636–7646. [Google Scholar] [CrossRef]
- Wong, J.E.; Zastrow, H.; Jaeger, W.; von Klitzing, R. Specific ion vs. electrostatic effects on the construction of polyelectrolyte multilayers. Langmuir 2009, 25, 14061–14070. [Google Scholar] [CrossRef]
- Laugel, N.; Betscha, C.; Winterhalter, M.; Voegel, J.-C.; Schaaf, P.; Ball, V. Relationship between the growth regime of polyelectrolyte multilayers and the polyanion/polycation complexation enthalpy. J. Phys. Chem. B 2006, 110, 19443–19449. [Google Scholar]
- Dodoo, S.; Steitz, R.; Laschewsky, A.; von Klitzing, R. Effect of ionic strength and type of ions on the structure of water swollen polyelectrolyte multilayers. Phys. Chem. Chem. Phys. 2011, 13, 10318–10325. [Google Scholar]
- Kovacevic, D.; van der Burgh, S.; de Keizer, A.; Stuart, M.A.C. Kinetics of formation and dissolution of weak polyelectrolyte multilayers: Role of salt and free polyions. Langmuir 2002, 18, 5607–5612. [Google Scholar] [CrossRef]
- Tedeschi, C.; Mohwald, H.; Kirstein, S. Polarity of layer-by-layer deposited polyelectrolyte films as determined by pyrene fluorescence. J. Am. Chem. Soc. 2001, 123, 954–960. [Google Scholar] [CrossRef]
- Schneider, A.; Vodouhê, C.; Richert, L.; Francius, G.; le Guen, E.; Schaaf, P.; Voegel, J.-C.; Frisch, B.; Picart, C. Multifunctional polyelectrolyte multilayer films: Combining mechanical resistance, biodegradability, and bioactivity. Biomacromolecules 2007, 8, 139–145. [Google Scholar] [CrossRef]
- Wang, X.F.; Ji, J. Postdiffusion of oligo-peptide within exponential growth multilayer films for localized peptide delivery. Langmuir 2009, 25, 11664–11671. [Google Scholar] [CrossRef]
- Riva, E.R.; Desii, A.; Sartini, S.; la Motta, C.; Mazzolai, B.; Mattoli, V. PMMA/polysaccharides nanofilm loaded with adenosine deaminase inhibitor for targeted anti-inflammatory drug delivery. Langmuir 2013, 29, 13190–13197. [Google Scholar] [CrossRef]
- Chuang, H.F.; Smith, R.C.; Hammond, P.T. Polyelectrolyte multilayers for tunable release of antibiotics. Biomacromolecules 2008, 9, 1660–1668. [Google Scholar] [CrossRef]
- Ball, V. Organic and inorganic dyes in polyelectrolyte multilayer films. Materials 2012, 5, 2681–2704. [Google Scholar] [CrossRef]
- Pavlukhina, S.; Sukhishvili, S. Polymer assemblies for controlled delivery of bioactive molecules from surfaces. Adv. Drug Deliv. Rev. 2011, 63, 822–836. [Google Scholar] [CrossRef]
- Lasic, D.D. Liposomes: From Physics to Applications; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Lasic, D.D.; Papahadjopoulos, D. Medical Applications of Liposomes; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Graff, A.; Winterhalter, M.; Meier, W. Nanoreactors from polymer-stabilized liposomes. Langmuir 2001, 17, 919–923. [Google Scholar] [CrossRef]
- Barenholz, Y. Liposome application: problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Christensen, S.M.; Stamou, D. Surface-based lipid vesicle reactor systems: Fabrication and applications. Soft Matter 2007, 3, 828–836. [Google Scholar] [CrossRef]
- Yoshina-Ishii, C.; Miller, G.P.; Kraft, M.L.; Kool, E.T.; Boxer, S.G. General method for modification of liposomes for encoded assembly on supported bilayers. J. Am. Chem. Soc. 2005, 127, 1356–1357. [Google Scholar] [CrossRef]
- Chifen, A.N.; Forch, R.; Knoll, W.; Cameron, P.J.; Khor, H.L.; Williams, T.L.; Jenkins, A.T.A. Attachment and phospholipase A2-induced lysis of phospholipid bilayer vesicles to plasmapolymerized maleic anhydride/SiO2 multilayers. Langmuir 2007, 23, 6294–6298. [Google Scholar]
- Richter, R.P.; Berat, R.; Brisson, A.R. Formation of solid-supported lipid bilayers: An integrated view. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Reviakine, I.; Brisson, A. Formation of supported phospholipid bilayers from unilamellar vesicles investigated by atomic force microscopy. Langmuir 2000, 16, 1806–1815. [Google Scholar] [CrossRef]
- Volodkin, D.; Ball, V.; Schaaf, P.; Voegel, J.-C.; Mohwald, H. Complexation of phosphocholine liposomes with polylysine: Stabilization by surface coverage vs. aggregation. Biochim. Biophys. Acta 2007, 1768, 280–290. [Google Scholar] [CrossRef]
- Volodkin, D.; Mohwald, H.; Voegel, J.-C.; Ball, V. Stabilization of negatively charged liposomes by polylysine surface coating: Drug release study. J. Control. Release 2007, 117, 111–120. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Ball, V.; Voegel, J.-C.; Möhwald, H.; Dimova, R.; Marchi-Artzner, V. Control of the interaction between membranes and vesicles: Adhesion, fusion and release of dyes. Colloids Surf. A 2007, 303, 89–96. [Google Scholar] [CrossRef]
- Volodkin, D.; Schaaf, P.; Mohwald, H.; Voegel, J.-C.; Ball, V. Effective embedding of liposomes into polyelectrolyte multilayered films: The relative importance of lipid-polyelectrolyte and interpolyelectrolyte interactions. Soft Matter 2009, 5, 1394–1405. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Arntz, Y.; Schaaf, P.; Mohwald, H.; Voegel, J.-C.; Ball, V. Composite multilayered biocompatible polyelectrolyte films with intact liposomes: Stability and triggered dye release. Soft Matter 2008, 4, 122–130. [Google Scholar] [CrossRef]
- Michel, M.; Izquierdo, A.; Decher, G.; Voegel, J.-C.; Schaaf, P.; Ball, V. Layer-by-layer self-assembled polyelectrolyte multilayers with embedded phospholipid vesicles obtained by spraying: Integrity of the vesicles. Langmuir 2005, 21, 7854–7859. [Google Scholar]
- Michel, M.; Vautier, D.; Voegel, J.-C.; Schaaf, P.; Ball, V. Layer-by-layer self-assembled polyelectrolyte multilayers with embedded phospholipid vesicles. Langmuir 2004, 20, 4835–4839. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Michel, M.; Schaaf, P.; Voegel, J.-C.; Mohwald, H.; Ball, V. Liposome embedding into polyelectrolyte multilayers: A new way to create drug reservoirs at solid-liquid interfaces. In Advances in Planar Lipid Bilayers and Liposomes; Liu, A.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 8. [Google Scholar]
- Delcea, M.; Madaboosi, N.; Yashchenok, A.M.; Subedi, P.; Volodkin, D.V.; de Geest, B.G.; Mohwald, H.; Skirtach, A.G. Anisotropic multicompartment micro- and nano-capsules produced via embedding into biocompatible PLL/HA films. Chem. Commum. 2011, 47, 2098–2100. [Google Scholar] [CrossRef]
- Kohler, D.; Madaboosi, N.; Delcea, M.; Schmidt, S.; de Geest, B.G.; Volodkin, D.V.; Möhwald, H.; Skirtach, A.G. Patchiness of embedded particles and film stiffness control through concentration of gold nanoparticles. Adv. Mater. 2012, 24, 1095–1100. [Google Scholar]
- Graf, N.; Albertini, F.; Petit, T.; Reimhult, E.; Voros, J.; Zambelli, T. Electrochemically stimulated release from liposomes embedded in a polyelectrolyte multilayer. Adv. Funct. Mater. 2011, 21, 1666–1672. [Google Scholar] [CrossRef]
- Schwinte, P.; Voegel, J.-C.; Picart, C.; Haikel, Y.; Schaaf, P.; Szalontai, B. Stabilizing effects of various polyelectrolyte multilayer films on the structure of adsorbed/embedded fibrinogen molecules: An ATR-FTIR study. J. Phys. Chem. B 2001, 15, 11906–11916. [Google Scholar]
- Haynie, D.T.; Balkundi, S.; Palath, N.; Chakravarthula, K.; Dave, K. Polypeptide multilayer films: role of molecular structure and charge. Langmuir 2004, 20, 4540–4547. [Google Scholar] [CrossRef]
- Boulmedais, F.; Schwinte, P.; Gergely, C.; Voegel, J.-C.; Schaaf, P. Secondary structure of polypeptide multilayer films: An example of locally ordered polyelectrolyte multilayers. Langmuir 2002, 18, 4523–4525. [Google Scholar]
- Boulmedais, F.; Ball, V.; Schwinte, P.; Frisch, B.; Schaaf, P.; Voegel, J.-C. Buildup of exponentially growing multilayer polypeptide films with internal secondary structure. Langmuir 2003, 19, 440–445. [Google Scholar] [CrossRef]
- Müller, M. Orientation of α-helical poly(l-lysine) in consecutively adsorbed polyelectrolyte multilayers on texturized silicon substrates. Biomacromolecules 2001, 2, 262–269. [Google Scholar] [CrossRef]
- Uhlig, K.; Madaboosi, N.; Schmidt, S.; Jager, M.S.; Rose, J.; Duschl, C.; Volodkin, D.V. 3D localization and diffusion of proteins in polyelectrolyte multilayers. Soft Matter 2012, 8, 11786–11789. [Google Scholar]
- Volodkin, D.V.; Balabushevitch, N.G.; Sukhorukov, G.B.; Larionova, N.I. Model system for controlled protein release: pH-sensitive polyelectrolyte microparticles. STP Pharm. Sci. 2003, 13, 163–170. [Google Scholar]
- Volodkin, D.V.; Balabushevitch, N.G.; Sukhorukov, G.B.; Larionova, N.I. Inclusion of proteins into polyelectrolyte microparticles by alternative adsorption of polyelectrolytes on protein aggregates. Biochemistry (Moscow) 2003, 68, 236–241. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Pechenkin, M.A.; Shibanova, E.D.; Volodkin, D.V.; Mikhalchik, E.V. Multifunctional polyelectrolyte microparticles for oral insulin delivery. Macromol. Biosci. 2013, 13, 1379–1388. [Google Scholar] [CrossRef]
- Krafft, C.; Dietzek, B.; Popp, J. Raman and CARS microspectroscopy of cells and tissues. Analyst 2009, 134, 1046–1057. [Google Scholar] [CrossRef]
- Krafft, C.; Dietzek, B.; Schmitt, M.; Popp, J. Raman and coherent anti-Stokes Raman scattering microspectroscopy for biomedical applications. J. Biomed. Opt. 2012, 17, 040801. [Google Scholar] [CrossRef]
- Wang, M.; Benford, M.; Jing, N.; Cote, G.; Kameoka, J. Optofluidic device for ultra-sensitive detection of proteins using surface-enhanced Raman spectroscopy. Microfluidics Nanofluidics 2009, 6, 411–417. [Google Scholar] [CrossRef]
- Han, W.P.; Chuang, K.H.; Chang, Y.T.; Olivo, M.; Velan, S.S.; Bhakoo, K.; Townsend, D.; Radda, G.K. Imaging metabolic syndrome. EMBO Mol. Med. 2010, 2, 196–210. [Google Scholar] [CrossRef]
- Stetciura, I.Y.; Markin, A.V.; Ponomarev, A.N.; Yakimansky, A.V.; Demina, T.S.; Grandfils, C.; Volodkin, D.V.; Gorin, D.A. New surface-enhanced Raman scattering platforms: Composite calcium carbonate microspheres coated with astralen and silver nanoparticles. Langmuir 2013, 29, 4140–4147. [Google Scholar] [CrossRef]
- Crouzier, T.; Picart, C. Ion pairing and hydration in polyelectrolyte multilayer films containing polysaccharides. Biomacromolecules 2009, 10, 433–442. [Google Scholar] [CrossRef]
- Srivastava, S.; Ball, V.; Podsiadlo, P.; Lee, J.; Ho, P.; Kotov, N.A. Reversible loading and unloading of nanoparticles in “exponentially” growing polyelectrolyte LBL films. J. Am. Chem. Soc. 2008, 130, 3748–3749. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Volodkin, D.V.; Mohwald, H. Bio-interfaces-interaction of PLL/HA thick films with nanoparticles and microcapsules. ChemPhysChem 2010, 11, 822–829. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.Y.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar]
- Fukuda, J.; Khademhosseini, A.; Yeh, J.; Eng, G.; Cheng, J.J.; Farokhzad, O.C.; Langer, R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials 2006, 27, 1479–1486. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Suh, K.Y.; Yang, J.M.; Eng, G.; Yeh, J.; Levenberg, S.; Langer, R. Layer-by-layer deposition of hyaluronic acid and poly-l-lysine for patterned cell co-cultures. Biomaterials 2004, 25, 3583–3592. [Google Scholar] [CrossRef]
- Richert, L.; Boulmedais, F.; Lavalle, P.; Mutterer, J.; Ferreux, E.; Decher, G.; Schaaf, P.; Voegel, J.C.; Picart, C. Improvement of stability and cell adhesion properties of polyelectrolyte multilayer films by chemical cross-linking. Biomacromolecules 2004, 5, 284–294. [Google Scholar] [CrossRef]
- Ricotti, L.; Taccola, S.; Bernardeschi, I.; Pensabene, V.; Dario, P.; Menciassi, A. Quantification of growth and differentiation of C2C12 skeletal muscle cells on PSS–PAH-based polyelectrolyte layer-by-layer nanofilms. Biomed. Mater. 2011, 6, 031001. [Google Scholar] [CrossRef]
- Ren, K.F.; Crouzier, T.; Roy, C.; Picart, C. Polyelectrolyte multilayer films of controlled stiffness modulate myoblast cell differentiation. Adv. Funct. Mater. 2008, 18, 1378–1389. [Google Scholar] [CrossRef]
- Madaboosi, N.; Uhlig, K.; Jäger, M.S.; Möhwald, H.; Duschl, C.; Volodkin, D.V. Microfluidics as a tool to understand the build-up mechanism of exponential-like growing films. Macromol. Rapid Commun. 2012, 33, 1775–1779. [Google Scholar] [CrossRef]
- Chien, H.W.; Chang, T.Y.; Tsai, W.B. Spatial control of cellular adhesion using photo-crosslinked micropatterned polyelectrolyte multilayer films. Biomaterials 2009, 30, 2209–2218. [Google Scholar] [CrossRef]
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely triggerable drug delivery systems. Adv. Mater. 2010, 22, 4925–4943. [Google Scholar] [CrossRef]
- Malcher, M.; Volodkin, D.; Heurtault, B.; Andre, P.; Schaaf, P.; Mohwald, H.; Voegel, J.-C.; Sokolowski, A.; Ball, V.; Boulmedais, F.; et al. Embedded silver ions-containing liposomes in polyelectrolyte multilayers: Cargos films for antibacterial agents. Langmuir 2008, 24, 10209–10215. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Skirtach, A.G.; Mohwald, H. Near-IR remote release from assemblies of liposomes and nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 1807–1809. [Google Scholar] [CrossRef]
- Volodkin, D.; Skirtach, A.; Madaboosi, N.; Blacklock, J.; von Klitzing, R.; Lankenau, A.; Duschl, C.; Mohwald, H. IR-light triggered drug delivery from micron-sized polymer biocoatings. J. Controll. Release 2010, 148, e70–e71. [Google Scholar] [CrossRef]
- Harris, J.J.; DeRose, P.M.; Bruening, M.L. Synthesis of passivating, nylon-like coatings through cross-linking of ultrathin polyelectrolyte films. J. Am. Chem. Soc. 1999, 121, 1978–1979. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, B.-S.; Chen, S.; Shao-Horn, Y.; Hammond, P.T. Layer-by-layer assembly of all carbon nanotube ultrathin films for electrochemical applications. J. Am. Chem. Soc. 2009, 131, 671–679. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Dejugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Sukhorukov, G.B. Nanoparticles distribution control by polymers: Aggregates vs. nonaggregates. J. Phys. Chem. C 2007, 111, 555–564. [Google Scholar]
- Volodkin, D.; Skirtach, A.; Möhwald, H. Bioapplications of light-sensitive polymer films and capsules assembled using the layer-by-layer technique. Polym. Int. 2012, 61, 673–679. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Delcea, M.; Mohwald, H.; Skirtach, A.G. Remote near-IR light activation of a hyaluronic acid/poly(l-lysine) multilayered film and film-entrapped microcapsules. ACS Appl. Mater. Interfaces 2009, 1, 1705–1710. [Google Scholar] [CrossRef]
- Pechenkin, M.A.; Mohwald, H.; Volodkin, D.V. pH- and salt-mediated response of layer-by-layer assembled PSS/PAH microcapsules: Fusion and polymer exchange. Soft Matter 2012, 8, 8659–8665. [Google Scholar] [CrossRef]
- Carregal-Romero, S.; Ochs, M.; Rivera-Gil, P.; Ganas, C.; Pavlov, A.M.; Sukhorukov, G.B.; Parak, W.J. NIR-light triggered delivery of macromolecules into the cytosol. J. Controll. Release 2012, 159, 120–127. [Google Scholar]
- Javier, A.M.; del Pino, P.; Bedard, M.F.; Ho, D.; Skirtach, A.G.; Sukhorukov, G.B.; Plank, C.; Parak, W.J. Photoactivated release of cargo from the cavity of polyelectrolyte capsules to the cytosol of cells. Langmuir 2008, 24, 12517–12520. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Dejugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Parak, W.J.; Möhwald, H.; Sukhorukov, G.B. The role of metal nanoparticles in remote release of encapsulated materials. Nano Lett. 2005, 5, 1371–1377. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Karageorgiev, P.; Bedard, M.F.; Sukhorukov, G.B.; Mohwald, H. Reversibly permeable nanomembranes of polymeric microcapsules. J. Am. Chem. Soc. 2008, 130, 11572–11573. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Schmidt, S.; Fernandes, P.; Larionova, N.I.; Sukhorukov, G.B.; Duschl, C.; Möhwald, H.; von Klitzing, R. One-step formulation of protein microparticles with tailored properties: Hard templating at soft conditions. Adv. Funct. Mater. 2012, 22, 1914–1922. [Google Scholar] [CrossRef]
- Schmidt, S.; Behra, M.; Uhlig, K.; Madaboosi, N.; Hartmann, L.; Duschl, C.; Volodkin, D. Mesoporous protein particles through colloidal CaCO3 templates. Adv. Funct. Mater. 2013, 23, 116–123. [Google Scholar]
- Schmidt, S.; Volodkin, D. Microparticulate biomolecules by mild CaCO3 templating. J. Mater. Chem. B 2013, 1, 1210–1218. [Google Scholar] [CrossRef]
- Schmidt, S.; Uhlig, K.; Duschl, C.; Volodkin, D. Stability and cell uptake of calcium carbonate templated insulin microparticles. Acta Biomater. 2014, 10, 1423–1430. [Google Scholar]
- Volodkin, D. CaCO3 templated micro-beads and -capsules for bioapplications. Adv. Colloid Interface Sci. 2014, 207, 306–324. [Google Scholar]
- Volodkin, D.V.; von Klitzing, R.; Möhwald, H. Pure protein microspheres by calcium carbonate templating. Angew. Chem. Int. Ed. 2010, 49, 9258–9261. [Google Scholar] [CrossRef]
- Behra, M.; Azzouz, N.; Schmidt, S.; Volodkin, D.V.; Mosca, S.; Chanana, M.; Seeberger, P.H.; Hartmann, L. Magnetic porous sugar-functionalized PEG microgels for efficient isolation and removal of bacteria from solution. Biomacromolecules 2013, 14, 1927–1935. [Google Scholar] [CrossRef]
- Behra, M.; Schmidt, S.; Hartmann, J.; Volodkin, D.V.; Hartmann, L. Synthesis of porous PEG microgels using CaCO3 microspheres as hard templates. Macromol. Rapid Commun. 2012, 33, 1049–1054. [Google Scholar] [CrossRef]
- Sergeeva, A.; Gorin, D.; Volodkin, D. Polyelectrolyte microcapsule arrays: Preparation and biomedical applications. BioNanoScience 2013, 4, 1–14. [Google Scholar] [CrossRef]
- Volodkin, D. Colloids of pure proteins by hard templating. Colloid Polym. Sci. 2014, in press. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Volodkin, D.; Von Klitzing, R.; Moehwald, H. Polyelectrolyte Multilayers: Towards Single Cell Studies. Polymers 2014, 6, 1502-1527. https://doi.org/10.3390/polym6051502
Volodkin D, Von Klitzing R, Moehwald H. Polyelectrolyte Multilayers: Towards Single Cell Studies. Polymers. 2014; 6(5):1502-1527. https://doi.org/10.3390/polym6051502
Chicago/Turabian StyleVolodkin, Dmitry, Regine Von Klitzing, and Helmuth Moehwald. 2014. "Polyelectrolyte Multilayers: Towards Single Cell Studies" Polymers 6, no. 5: 1502-1527. https://doi.org/10.3390/polym6051502
APA StyleVolodkin, D., Von Klitzing, R., & Moehwald, H. (2014). Polyelectrolyte Multilayers: Towards Single Cell Studies. Polymers, 6(5), 1502-1527. https://doi.org/10.3390/polym6051502




