Abstract
This review outlines the responsive polymer methods currently in use with their potential application to plant protection and puts forward plant-specific mechanisms as stimuli in newly devised methods for smart release of crop protection agents (CPAs). CPAs include chemicals (fungicides, insecticides, herbicides), biochemicals (antibiotics, RNA-based vaccines for plant viruses), semiochemicals (pheromones, repellents, allomones), microbial pesticides, growth regulators (insect and plant) or micronutrients, all with crop protection effects. This appraisal focuses on emerging uses of polymer nano-encapsulated CPAs. Firstly, the most interesting advances in controlled release methods are critically discussed with their advantages and drawbacks. Secondly, several plant-specific stimuli-based smart methods are anticipated for use alongside the polymer nano- or micro-capsules. These new CPA release methods are designed to (i) protect plants against infection produced by fungi or bacteria, and (ii) apply micro-nutrients when the plants need it the most. Thus, we foresee (i) the responsive release of nano- encapsulated bio-insecticides regulated by plant stress enzymes, and (ii) the delivery of micro-nutrients synchronized by the nature or intensity of plant root exudates. Such continued advances of nano-scale smart polymer-based CPAs for the protection of crops herald a “small revolution” for the benefit of sustainable agriculture.
1. Introduction
This review presents a critical analysis of the responsive polymer-based current methods for the release of crop protection agents (CPAs). CPAs include chemicals (fungicides, insecticides, herbicides), biochemical (antibiotics, RNA-based vaccines), semiochemicals (pheromones, repellents, allomones), microbial pesticides, growth regulators (insect or plant), micronutrients, all with beneficial effects on the protection of crops. Also, we recommend the most beneficial smart polymer-based methods for plants or crops and also offer newly envisioned “intelligent” polymer-based, nano-encapsulated CPAs, able to be self-activated by plant-specific stimuli.
Polymers and hydrogels have become established matrices for the responsive or controlled release of countermeasures, therapeutic agents, or active ingredients in general. Yet, their application in agriculture, for the delivery of CPAs, has been severely limited until quite recently. However, once micro- and nanotechnology were established and their benefits for agriculture became undeniable, then the polymer-based CPA responsive delivery at micro- or nano-scale began developing as enabling technologies, as was already the case in medical, veterinary or pharmaceutical practice.
The advantages of the responsive polymer and hydrogel based CPA include [1,2,37,41,42,43,44,45]:
- Stabilization against environmental degradation by light, air, humidity, or microorganisms;
- Decreased dosage, evaporation, leaching, leading to reduced environmental pollution and drift;
- Reduced irritation of the human mucous-membrane, and a lower phyto-toxicity;
- Increase in the number of target organisms, and ease of handling of harmful CPAs;
- Longer application intervals, actuated by CPA’s controlled release from responsive polymers;
- In addition, nano-encapsulation offers the in situ avenue of CPA release;
- Added benefits when employing specifically conductive polymers, as responsive materials.
While the interest in responsive polymers research and development generated a notable number of publications, it is apparent that the majority of literature to date accounts mostly for two of the stimuli: pH and temperature. Thus, our review does not cover these two classes of stimuli.
Existing CPA delivery methods of agricultural polymers occur typically through passive diffusion release, erosion of the capsule wall, or active diffusion via osmotic pressure. By contrast, the human and veterinary medicine has inspired the development of new and advanced stimuli responsive polymers, with positive impact for improving patient health and quality of life. One objective of this review is to stimulate interest in the transfer of the methods, configurations and designs of smart polymers—from medicine towards agricultural uses in plant protection. Based on emerging developments in Nano-Science and Plant Science, yet another goal herein is to put forward plant-specific mechanisms as new stimuli for the polymer-based, intelligent, self-regulated CPA release for future, sustainable crop protection.
Initially, we outline the main classes of responsive stimuli currently in use, the ultrasonic field, light, redox/thiol interactions, magnetic field, electric field, enzyme action antibody-antigen interactions. These stimuli are critically analyzed from their potential use as a matrix for the release of CPAs, with advantages and drawbacks. More importantly, this will enable us to put forward newly envisioned crop- and plant-specific strategies.
Next, the emerging advances in polymer-based nano-encapsulated CPAs are discussed, structured by their specific function and penetration mode to target, for the case of an in situ application, afforded by the minuscule dimension of such capsules.
Furthermore, the electroactive polymers (EAPs) are critically analyzed, from their perspective of being integrated within smart structures and also able to jointly exhibit both release/actuation and sensing functions simultaneously, as intelligent, self-regulated, integrated sensor-actuators. This specific approach of ours shows stimulating similarities with the hierarchical multi-functionalities evidenced by the natural living systems.
Finally, the critical screening of the above typical stimuli-responsive mechanisms, coupled with the practice of the emerging concepts from plant science and nanotechnology, has enabled the visionary design of methods based on plant-specific stimuli, applicable with nano-encapsulated, stimuli-responsive polymer-based CPA.
2. Classes of Stimuli for the Responsive Release Polymer and Hydrogels
We first outline the main categories of responsive stimuli currently in use that include the following stimuli: the ultrasonic field, light, redox/thiol interactions, magnetic field, electric field, enzyme action, and the antibody-antigen interactions. Next, these stimuli are critically analyzed from their potential use as a matrix for the release of CPAs, with their advantages and drawbacks. In addition, this will allow us to design newly envisioned plant-specific methods for CPAs release.
2.1. Ultrasound Responsive Polymers
This release mechanism is driven by cavitation: the ultrasound (ULS) energy generates both high and low pressure waves, resulting in an alternative growth and shrinkage of gas-filled microbubbles. These high-low pressure waves determine the periodical opening of the pores of the polymer, thus inducing delivery of the respective chemical agents.
The polymers employed for the ULS-responsive applications could be biodegradable or non‑biodegradable. The biodegradables include polyglycolides, polylactides, bis(p-carboxyphen-oxy) alkane anhydride with sebacic acid. The non-biodegradables include ethylene- vinyl acetate copolymers or the poly(lactide-co-glycolide) microspheres, PHEMA hydrogels, the PEO-b-PPO-b-PEO micelles and the poly(HEMA-co-DMAEMA) hydrogels [2,3,4,5,6,7,8]. An interesting report out-lines using perfluorcarbon gas-filled microbubbles, coated via the layer-by-layer (LbL) approach, with positively charged poly(allylamine hydrochloride) and negatively charged DNA.
We submit that the ULS method appears to be suitable for a targeted application of herbicides, using precision sprayers and, furthermore, controlled by sensors able to recognize the image of a crop plant. The precision sprayers would apply the herbicide that is previously incorporated in ULS responsive carriers. This spray will be smartly applied only to the weed, recognized by the sensors as not a crop plant. This will trigger an accelerated spray of herbicides through the weed leaf cuticles. Additional effects will result from the ULS cavitation within the water-containing leaf tissues, thus synergizing the herbicide effect on the weeds and also further reducing the application dose, for the sustainable benefit of the environment.
2.2. Light Responsive Polymers
The light responsive polymers are based, for the most part, on the photo-responsive polymer molecules which modify their release-related properties in response to a certain light wavelength, thereby inducing structural changes within the polymer. These polymers responsive to light include the well-known azobenzene groups incorporated in methylcellulose and methylcellulose-cyclodextrin inclusion complexes, as well as the spiropyran-containing photo-responsive polymers, such as PAA, PHPMA and PNIPAM. The photochromic derivatives of elastin-like polypeptides are also employed [9,10,11,12,13,14,15,16,17,18].
Sunlight activated polymers could be, in principle, useful carriers for CPAs. Several photo-activable pre-polymers were described, more specifically a benzoin derivative, and methods of use with CPA microencapsulated within an initially nonporous polymer shell [19]. This could be used for the encapsulation of water-insoluble CPAs (i.e. thiadiazole fungicides, acetanilides, or chlorobutilate). These CPAs will be released by diffusion, through the shell pores that develop once the polymer is exposed to sunlight. Additionally, a dark colored film applied on each microcapsule should prevent the accidental activation of CPAs light responsive carriers. However, since the sunlight intensity is not easy to predict a priori, this concept seems to have an inherent drawback.
2.3. Redox/thiol Responsive Polymers
Due to the reversibility of their disulfide bonds, the polymers containing disulfide linkages can be considered both redox and thiol-responsive. As already known, disulfides can be converted to thiols by exposure to various reducing agents and/or undergo disulfide exchange in the presence of other thiols. There are several classes of polymers that are suitable to become redox and thiol-responsive. These include the shell crosslinked, disulfide based cystamine-containing triblock copolymer micelles, the polymer micelles with shells crosslinked via thiol-reducible disulfide bonds and also the block copolymers with hydrophilic PEG tethered to hydrophobic poly(propylene sulfide) via a disulfide bridge [2,20,21,22,23,24].
Redox/thiol responsive polymers could be a solution for the nano-encapsulation and targeted delivery of RNA based vaccines against plant viruses. These structures could deliver the siRNA when exposed to cysteine or glutathione, reductive amino-acid based molecules present at intracellular concentrations 50–1000 fold greater than those of the extracellular environment [26]. However, further studies are needed to develop a formulation adequate to deliver same, constant quantity of siRNA for different plants that are expected to have—individually—their own overall barrier to diffusion, integrating the flow resistances from their tissues and vessels.
2.4. Magnetic Field Responsive Polymers
This delivery method is the magnetic field. In one typical embodiment, magnetic micro- or nano-particles with adequate composition and size are embedded in a thermally responsive hydrogel. As soon as it is exposed to heat, gel conformation changes, pores open, and its “cargo” is released.
For example, the Fe3O4 magnetic nanoparticles were incorporated within PNIPAM-based microgels or PVA hydrogels. Another application reports on polymeric surfactants that stabilize magnetic nanoparticles, prepared by nitroxide-mediated radical polymerization. Also popular, is the synthesis of superparamagnetic iron oxide nanoparticles (SPION) of 10–20 nm diameter, carefully well dispersed in organic solvents. Interestingly, these SPIONs have been FDA approved as MRI contrast agents [25,26,27,28,29,30].
The magnetic field responsive polymers method appears interesting for plant protection; magnetic responsive carriers could be used for the nano or micro-encapsulation, allowing a very specific localization of the particles to release their load. A few works have been reported regarding the uptake, translocation and specific localization of magnetic nanoparticles (less than 50 nm) in pumpkin plants [31]. Magnetization signals of various strengths were observed from different portions (ranging from roots to leaves) of the treated plants, which clearly indicates the successful translocation of magnetic nanoparticles in the entire plant system irrespective of the area of application.
Magnetic field responsive polymers could be used as capping agents for porous carriers of various CPAs, so that their specific localization and uncapping process will be carried out using external magnets, hence releasing the carried chemicals at target site. Such methods are possible for specific treatments in fruit trees or high-input crops under green house conditions (since here it is easy to provide an external magnetic exposure that triggers the chemical release and the profitability could cover the cost of such a delivery system).
However, there are not enough data regarding the safety for crops using a combination of ferroloaded polymers and magnetic field. In the above cited papers, no toxicity on plant growth had been detected, thus suggesting the safe use of such magnetic responsive polymers delivery in plants. Furthermore, some of the treated plants were transplanted in pots and grown until maturity like normal plants. But there are others studies showing negative effects of combined nanoparticles and (electro)magnetic field [32]. Maize seeds were germinated in the presence of magnetic fluid followed by exposure to electromagnetic field (LM-EMF samples). For LM-EMF samples, a decrease in assimilatory pigments was observed with increased volume fraction of magnetic fluid solution. Exposure to electromagnetic field produced some local heating on the applied region due to the electromagnetic field energy absorbed by internalized magnetic nanoparticles in plant tissues that in turn affect the redox reactions involved in photosynthesis process. A pronounced increase in nucleic acid level was observed in LM-EMF samples due to the regeneration reactions of plant metabolism processes against local heating of vegetal tissues produced by the electromagnetic field energy.
Hence it is necessary to have an idea of suitable concentration ranges of Fe3O4 magnetic nanoparticles incorporated in magnetic field responsive polymers before designing the biotechnological tools for controlled release of CPAs on crop.
Also the effect of magnetic field responsive polymers entering the food chain must be considered and studied, especially in the reach area where people are overloaded with iron due to overuse of dietary supplement and/or iron fortified food.
2.5. Enzyme Responsive Polymers
It is well established that enzymes molecules exhibit selective, catalytic properties directed towards specific substrates. Thus, the enzyme-responsive hydrogels are typically made out of enzyme-sensitive substrates and another joint component able to direct or control specific interactions leading to macroscopic transitions. The enzyme catalytic action on the substrate leads to the swelling or collapse of the respective gels and/or the transformation of its surface properties [2,33,34]. This enzyme responsive class includes enzyme-degradable hydrogels: albumine and chitosan hydrogels N-acetyl–D-glucosamine, among others. For the proteases-responsive gels, a preformed network is typically exposed to a protease enzyme, and the hydrolysis of the protein or the peptide based cross-linkers in the network, subsequently leads to gel degradation followed by the release of its encapsulated contents.
The enzyme-base stimuli are definitely interesting because of their high selectivity and specificity. However, other characteristics are to be assessed as well: accuracy, response time, repeatability.
2.6. Antigen-antibody Responsive Polymers
A typical example of this class is a hydrogel swelling in the presence of a free antigen, rabbit IgG, because of competition between free and polymer-immobilized antigen. Upon removal of the free antigen, the hydrogel shrank thus exhibiting reversible behavior, correlated with the concentration of the free antigen concentration. The semi-interpenetrating polymer network consisted of polymer containing rabbit antibody (IgG) and goat anti-rabbit IgG as the antigen. Tanihara et al. demonstrated a responsive delivery system that detects Staphylococcus aureus through increased thrombin activity, releasing an appropriate amount of antibiotic. Also reported, antigen responsive hydrogels were based on polymerizable antibody Fab’ fragments. These polymerizable Fab fragments were co-polymerized with NIPAM and MBA. Yet another antigen-responsive hydrogel was membrane based, on a crosslinked dextran backbone grafted with both a fluorescein isothiocyanate (FITC) antigen and a sheep anti-FITC IgG antibody [34,35,36,37,38].
This molecular recognition approach involved on antigen-antibody responsive method seems attractive for targeted delivery of CPAs; however more specific tests developed for plants are needed before a verdict is made. Also the presence of an antigen or an antibody in close proximity, or within, any edible plant could raise some legitimate concerns related to potential new allergen developments.
2.7. Electric Field Responsive Polymers
Typically, electro-responsive polyelectrolyte hydrogels change conformation and deform under an electric field, due to anisotropic swelling and shrinking, as charged ions are directed towards the anode or cathode side of the gel. Various polymers are synthesized, using a combination of natural and synthetic components: semi interpenetrating polymer network (IPN) hydrogel of PHEMA and chitosan, purely synthetic IPN composed of poly(methyl methacrylate) (PMMA)/PVA, crosslinked strong acid hydrogels composed of sulfonated polystyrene or sulfonated poly(styrene-b-ethylene-co-butylene-b-styrene), and hydrogel based on PVA and poly(sodium maleate-cosodium acrylate) [38,39,40,41].
The synergistic integration of similar-size biomolecules and nano-materials resulted recently in novel crossbred bio-nano-materials, displaying an interesting set of electronic, photonic, catalytic and responsive functionalities. The advanced use of these conducting polymers in actuators and sensors has grown over the past decade, equally due to their compliance and compatibility with nano-fabrication. These soft, “synthetic metals” were proven to enhance sensitivity of nanobiosensors or to deliver actuation at true nanoscale [41,42,43,44,45].

Table 1.
Classes of stimuli-responsive polymers.
| Stimuli | Polymer | Example of Responsive-Polymer Releasing Mechanism | Reference |
|---|---|---|---|
| Ultrasound-responsive | Biodegradable polyglycolides, polylactides, and poly [bis(p-carboxyphenoxy) alkane anhydrides] with sebacic acid and non-biodegradable ethylene-vinyl acetate copolymers | The ultrasound energy generates high and low pressure waves resulting in an alternative growth and shrinkage of gas-filled microbubbles, phenomenon called
cavitation. (Reproduced with kind permission from Reference [8]). 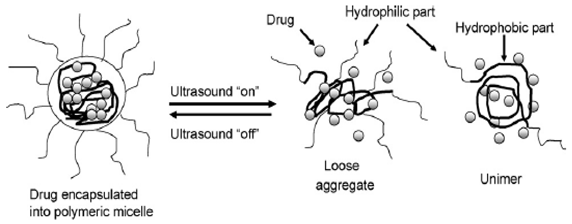
| [2,3,4,5,6,7,8] |
| Poly(lactide-co-glycolide) microspheres and poly(HEMA-co-DMAEMA) hydrogels | |||
| Ethylene-vinyl alcohol copolymer system | |||
| PHEMA hydrogels | |||
| PEO-b-PPO-b-PEO micelles | |||
| Perfluorcarbon gas-filled microbubbles, coated via the layer-by-layer (LbL) approach with positively charged poly(allylamine hydrochloride) and negatively charged DNA | |||
| Photo-responsive | Epoxy-based azobenzene-containing polymer colloids | The photo-responsive polymer molecules modify their property in response to a certain light wavelength inducing structural changes within the polymer (Reproduced with kind permission from Reference [18]). 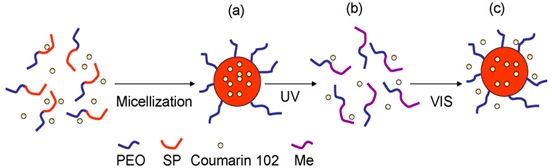
| [9,10,11,12,13,14,15,16,17,18] |
| Azobenzene groups incorporated in methylcellulose and methylcellulose-cyclodextrin inclusion complexes | |||
| Photochromicderivatives of elastin-like polypeptides [poly(VPGVG)] | |||
| Spiropyran-containing photo-responsive polymers, including, for example, PAA PHPMA, and PNIPAM | |||
| Redox/thiol- responsive | Polymer micelles with shells crosslinked via thiol-reducible disulfide bonds | Polymers containing disulfide linkages can be considered both redox and thiol-responsive because of the reversibility of their disulfide bonds. Disulfide can be converted to thiols by exposure to various reducing agents and/or undergo disulfide exchange in the presence of other thiols (Reproduced with kind permission from Reference [2]). 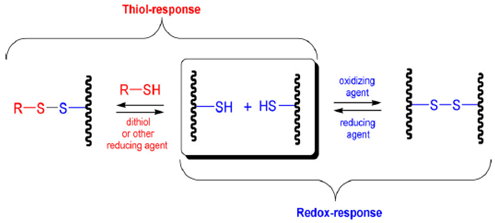
| [2,20,21,22,23,24] |
| Disulfide based cystamine-containing triblock copolymer micelles that were shell crosslinked | |||
| Block copolymers with hydrophilic PEG tethered to hydrophobic poly(propylene sulfide) via a disulfide bridge | |||
| Magnetic field-responsive | Incorporated magnetic nanoparticles (Fe3O4) within PNIPAM-based microgels or PVA hydrogels polymeric surfactants that stabilize magnetic nanoparticles prepared by nitroxide-mediated radical polymerization (NMP). | Magnetic nanoparticles with adequate composition and size are embedded in thermally responsive hydrogel. Exposed to heat, the gel changes conformation to open pores (Reproduced with kind permission from Reference [29]). 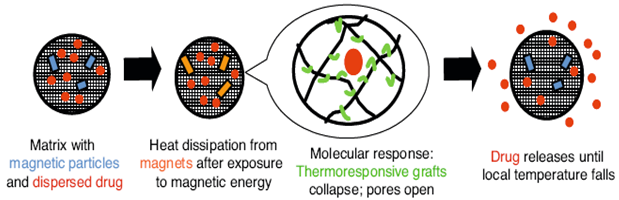
| [25,26,27,28,29,30] |
| The synthesis of superparamagnetic iron oxide nanoparticles (SPION) 10–20 nm diameter, which are well dispersed in organic solvents. SPIONs have been FDA approved as MRI contrast agents. | |||
| Enzyme-responsive | Enzyme-degradable hydrogels: albumine and chitosan hydrogels N-acetyl-D-glucosamine | Enzyme-responsive hydrogels are typically made out of enzyme-sensitive substrates and another component directing/controlling interactions leading to macroscopic transitions (Reproduced with kind permission from Reference [33]). 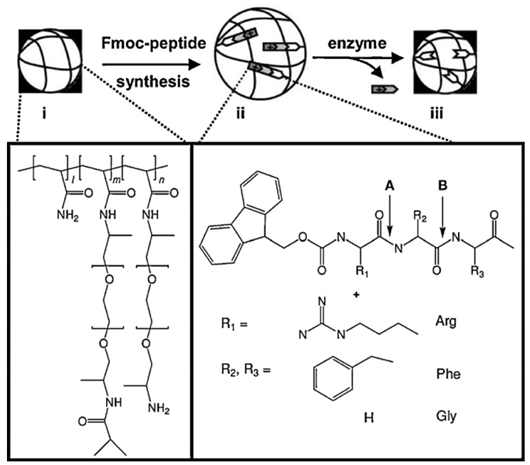
| [2,33,34] |
| Enzyme catalytic action on the substrate lead to gels swelling/collapse, or the transformation of surface properties | |||
| Proteases-responsive hydrogels: typically the preformed network is exposed to a protease enzyme, and hydrolysis of protein or peptide based cross-linkers in the network leads to gel degradation and subsequent release of encapsulated contents. | |||
| Antigen-antibody responsive | A semi-interpenetrating polymer network was prepared consisting of a polymer containing rabbit antibody (IgG) and goat anti-rabbit IgG as the antigen | This hydrogel swelled in the presence of the free antigen, rabbit IgG, because of competition between free and polymer-immobilized antigen. Upon removal of the free antigen, the hydrogel shrank, thus exhibiting reversible behavior that is dependent on free antigen concentration (Reproduced with kind permission from Reference [37]). 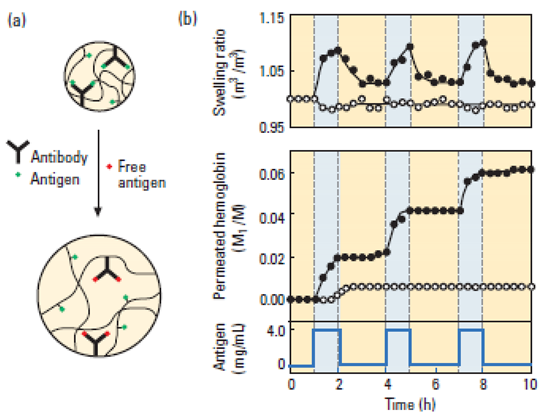
| [35,36,37,38] |
| Tanihara et al. also demonstrated a responsive delivery system that detects Staphylococcus aureus through increased thrombin activity and releases an appropriate amount of antibiotic | |||
| Antigen responsive hydrogels based on polymerizable antibody Fab’ fragments. The polymerizable Fab_ fragment was copolymerized with NIPAM and MBA | |||
| Antigen-responsive hydrogel membrane based on a crosslinked dextran backbonegrafted with both a fluorescein isothiocyanate (FITC) antigen and a sheep anti-FITC IgG antibody | |||
| Electro-responsive Electro-responsive | Combination of natural and synthetic components: semi interpenetrating polymer network (IPN) hydrogel of PHEMA and chitosan, purely synthetic IPN composed of poly(methyl methacrylate) (PMMA)/PVA, crosslinked strong acid hydrogels composed of sulfonated polystyrene or sulfonated poly(styrene-b-ethylene-co-butylene-b-styrene), hydrogel based on PVA and poly(sodium maleate-cosodium acrylate) Neutral polymers: lightly crosslinked poly(dimethyl siloxane) gel containing electrosensitive colloidal TiO2 particles Electro-responsive polymers can transform electric energy into mechanic energy, with promising applications in artificial muscle actuation, sensing, energy transduction and controlled CPA delivery. | Typically, the electro-responsive polyelectrolyte hydrogels deform under an electric field due to anisotropic swelling and deswelling as charged ions are directed towards the anode or cathode side of the gel. An artificial muscle-based micropump is seen in figure below (Reproduced with kind permission from Reference [40]). 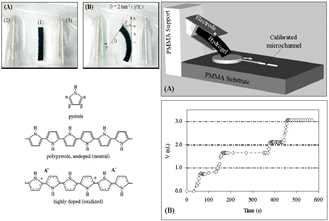
| [38,39,40,41] |
2.8. Suitability and Applicability of Each Stimulus for CPA Release: Advantages and Drawbacks
The categories of responsive polymers discussed above, from Table 1, need to be further critically examined for the potential to be applied to the protection of crops and plants. Indeed, agriculture essentially involves large areas of cultivated land that are being open to the elements: atmospheric pressure, ions concentration in the air, and all the other environmental changes including heat-cold cycles, daylight-nightlight cycles, complex rain-snow-dry cycles, or a superposition of these, etc.
From this perspective, one would need to take a step back and examine all the stimuli for the responsive polymers, first, in controlled “greenhouse” conditions for an unbiased preliminary assessment based on objective parameters, including: response time, reproducibility, accuracy, specificity, possible interferences, and also make a scientific and financial risk-benefits analysis.
Since the validation and use of these stimuli seem to become complicated and appear to raise some issues, one could not help but ask the question: is there another way? What if we consider the plant-specific mechanism as stimuli?
2.9. The Plant-specific Mechanisms, Engineered as Newly Tailored Stimuli for Responsive Polymers
In the case of the CPA release for crop and plant protection and for the current application format, we expect that the most capable stimuli are the ones highly specific, coupled with a strong potential to deliver in complex environments. These stimuli include: the enzyme, antigen-antibody, magnetic field, and electric field. However, few of the above stimuli, if any, in their current designed format, are applicable to the crop or plants per se.
By contrast, the critical analysis of the current typical stimuli-responsive mechanisms, coupled with practicing emerging concepts from plant science and nanotechnology, has enabled us the vision to design plant-specific methods applicable by using nano-encapsulated, stimuli-responsive polymer-based CPA. These novel applications will be based on some emerging advances discussed in Section 3 and then our specific ideas will be detailed in Section 4.
3. Emerging Advances in Stimuli Responsive Polymers for Micro- and Nanocapsule-based CPAs
3.1. Microncapsulation Methods
Encapsulation methods are broadly categorized as either physical (extrusion, fluidized bed, pan coating, atomization, spinning disk, spray drying, spray chilling/congealing) or chemical (solvent loss, phase separation, coacervation, polymerization, precipitation, liposomes, sol-gel and nano-encapsulation).
Encapsulation techniques include: micelles, polymersomes, phase inversion/precipitation, solvent evaporation, poly-electrolyte complexes, layer-by-layer deposition, controlled precipitation, surfactant-free particle formation, templating, or molecular encapsulation. Next, we will focus on the nanocapsules for in situ application of CPAs.
3.2. Nanocapsules for in situ Application of CPAs
Nanoencapsulation is an area of especially intense R&D in medicine, including for small molecule targeted delivery, extending the circulatory half-life, or for triggered release of proteins and DNA.
The correlation between the capsule size and the fabrication process is illustrated in Figure 1. Typical particle sizes range from 10–100 nm, also conferring the advantage of a high surface area per volume ratio. Additionally, nanocapsules can be synthesized with chemically functionalized, hydrophobic or hydrophilic, surface areas, and are packed with especially low payloads.
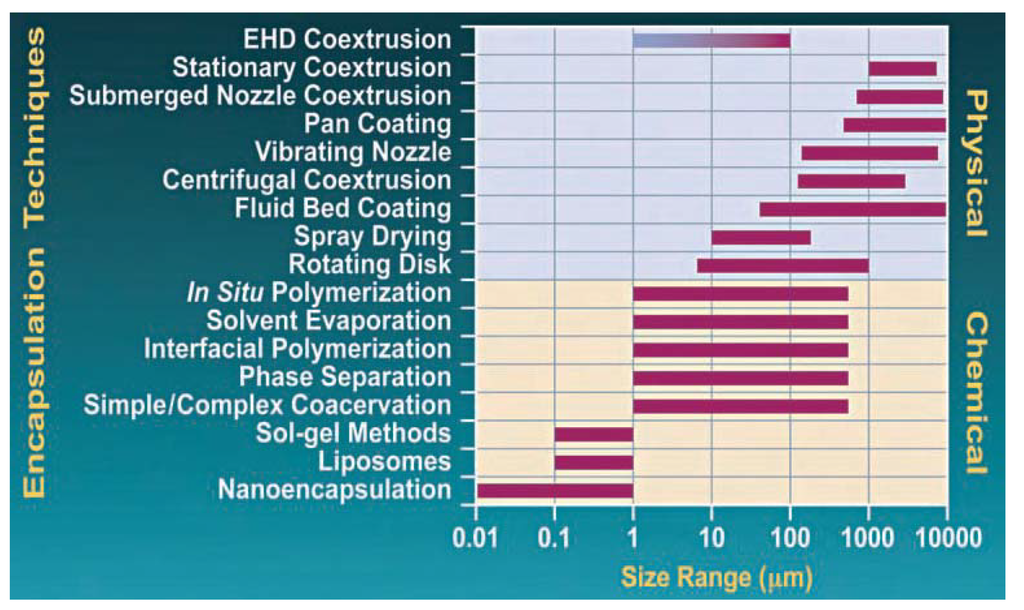
Figure 1.
The correlation between the microcapsule size and the fabrication process. Recommended guidelines reproduced with kind permission of the Southwest Research Institute [46].
Although not used for polymer-based CPAs at the present time, the nanoencapsulation has, in our opinion, a great future for crop and plant protection. Interestingly, the nanocapsules can be applied to plants in-situ, being shown to infiltrate the plant tissues and could move through the plant vessels, just as the nutrients-filled water does. Furthermore, the nanocapsules are able to diffuse in the soil, with the water, and consequently be absorbed and engulfed by the plant root hairs.
This nano-enabled in situ application has the strong potential to remove some of the complexity owed to the external environmental changes (hot-cold, daylight-nightlight, rain-snow-dry cycles) as discussed in Section 2.8 above. To facilitate a smooth transition to Section 3.3 “Intelligent responsive polymers with integrated sensing-release dual function”, and to Section 4 “Envisioned applications”, the specific domain of nano-capsules for plant protection should first be clarified.
These nanocapsules should incorporate CPAs as nutrition and growth promoters, low-temperature-shield fluids, herbicides (against harmful weeds, fungi or bacteria) or insecticides (insect growth regulators, pheromones, insect sexual repellents). These multi-functional pills become preventive counter-measures against a variety of possible plant diseases, once present in the plant in situ, as it also becomes apparent from the data shown in Table 2.

Table 2.
Agricultural uses of the nano- and micro-capsule-based controlled release polymers [41,47,48]
| No | Specific Area of Crop Protection | Entrapped CPA | Polymer Based CPA Action Mode and Functionality | Polymer Shape | Penetration Mode to Target | Stimulus or Stimuli to Activate Control Release |
|---|---|---|---|---|---|---|
| 1 | Parasitic weed control | Systemic herbicides |
|
| Penetrate cuticle and tissue |
|
| 2 | Perennial weed control | Magnets |
| Nanocapsule |
| Magnetic field |
| 3 |
|
| Triggers plant defense mechanisms | Nanocapsule (i.e., viral capsid) | Penetrate through plant cuticle, stomata and tissue | Enzymes |
| 4 | Pesticides | Pesticide, once present in crop plant, will protect it against potential attack | Nanocapsule | Penetration
|
| |
| 5 | Pest control | Insecticide |
|
| Pest ingest product |
|
| 6 |
|
| Product is ingested or respired by insect |
| ||
| 7 | Insect growth regulators |
|
| Product ingested by insect |
| |
| 8 | Pest control |
|
|
|
| Temperature interval when insects are active |
| 9 | Bio-pesticides | Microcapsule |
| |||
| 10 | Nutrition | Fertilizer | The active compound offers plant the oligo, micro or macro nutrients the plant needs |
| Penetrate through stomata, cuticle and tissue, or root hairs |
|
| 11 | Growth | Growth hormone |
| Nanocapsule | Penetrate through stomata, cuticle and tissue |
|
| 12 |
| Inhibitor i.e. abscisic acid (ABA) | Induces dormancy in plants | Penetrate through cuticle and tissue | Low temperature |
3.3. Intelligent Responsive Polymers with Integrated Sensing-release Dual Function
The advanced use of conducting (also called electroactive) polymers in biological sensors and actuators has grown over the past decade, also due to their compliance and compatibility with nanofabrication. Electroactive polymers (EAPs) such as polypyrrole, polyaniline, polyethylene- dioxythiophene (PEDOT), exhibit a high conductivity, mediate rapid electron transfer and can be synthesized under mild conditions through simple deposition onto conductive surfaces from monomer solutions with precise electrochemical control [43].
Furthermore, the synergistic integration of similar-size biomolecules and nanomaterials resulted recently in novel cross-bred bionanomaterials, displaying an unusual set of electronic, photonic, catalytic and recognitive properties and functionalities. More specifically, the nano-EAPs integrated with other (non)polymeric nanoentities, and receptors, enzymes, antibodies, whole cells, and nucleic acids in their matrix, lead to uniquely advanced smart or so-called intelligent materials, due to their ability to self-regulate in response to a given agent or challenge [38].
These EAP-based intelligent materials were proven to enhance biosensing or to deliver actuation, both at nanoscale. More specifically, we have reported for the first time the actuation of individual polypyrrole nanowires of 50 nm diameter, when triggered electrochemically in solution by volume change, as the result of its oxidation [42,44]. Moreover, very recently, a nano-PEDOT-based interface has evidenced a 50 times increase in sensitivity of electrochemical detection events, as a result of the catalytic synergy of the nano-PEDOT with a metallo-porphyrin [43].
In fact, these data, together with other ones emerging, seemingly show that same EAP-based smart polymer will be able to jointly exhibit both functions simultaneously and reliably, as a truly intelligent self-regulated sensor-actuator. This is intrinsically similar to multi-level hierarchical functions found in natural living systems, that bio-inspired engineers are now trying to mimic on a finer level, with newly-found enthusiasm [42,49].
4. Envisioned Applications
The specific methods outlined below are meant to protect plants against infection produced by fungi or bacteria, and to apply micro-nutrients when the plant needs it the most. As such, we foresee and actually explain and illustrate below, the responsive release of nano-encapsulated bio-insecticides regulated by plant stress enzymes, or the delivery of micro-nutrients synchronized by the nature or intensity of plant root exudates.
4.1. Nanocapsules Enter Plants through Stomata Orifices and Prevent Infection
This method includes the following steps: the CPAs are nanoencapsulated and the resulted polymer nanocapsules are sprayed onto the leaf tissue. These nanocapsules enter the plant through the stomata orifices. The nanocapsule’s chemical bonds of the polymer wall can be weakened or broken by a critical amount of stress enzymes present. Plant cell stress enzymes are activated by mechanical, thermal, chemical or biological stress. This stress sensitizes the plant during an attack and infection from fungi and bacteria. As illustrated in Figure 2, these polymer based CPA nanocapsules sprays are able to prevent this infection: in this case, the plant cell stress enzymes are the stimuli triggering the CPA release.
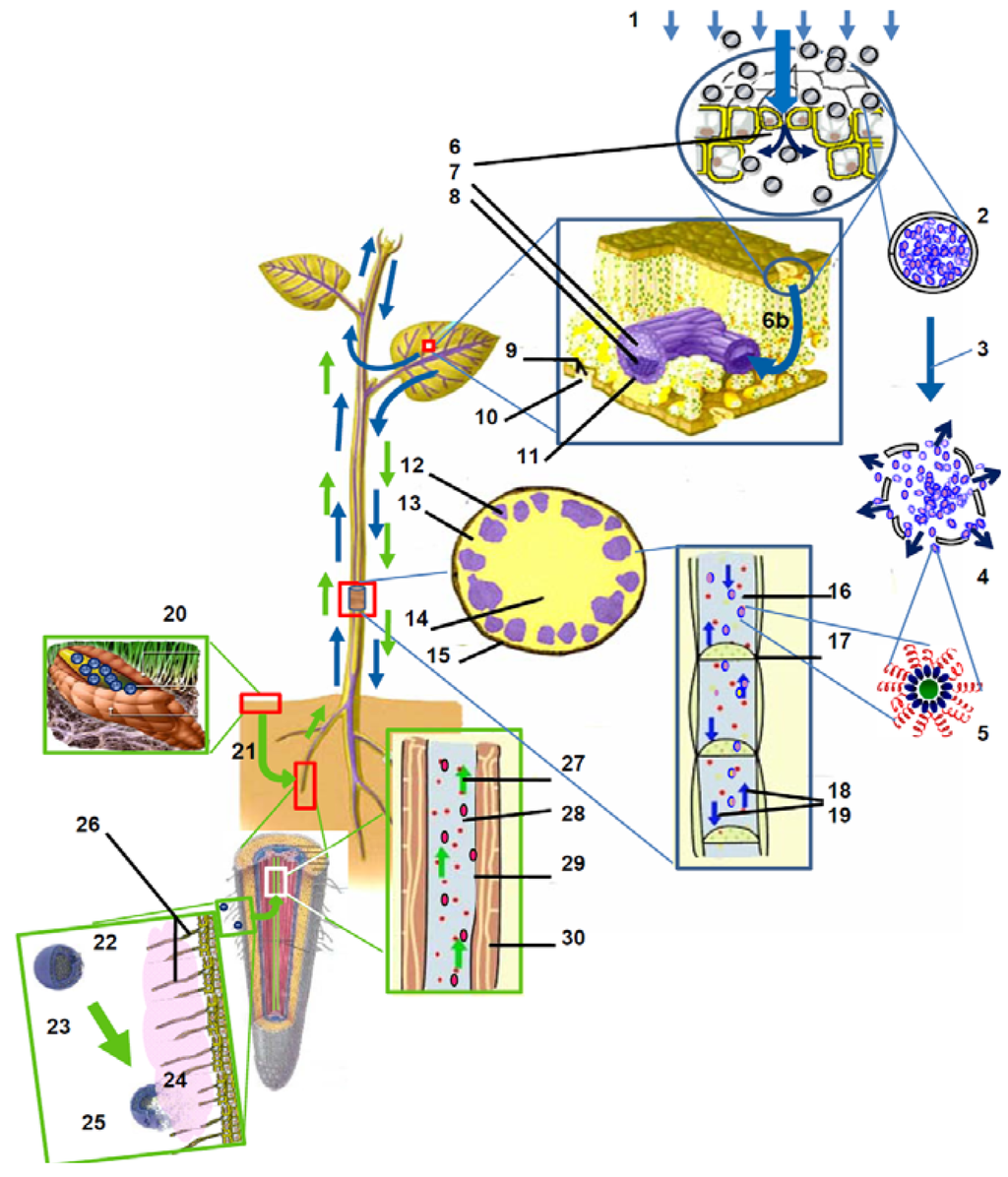
Figure 2.
Illustration of two revolutionary avenues for the responsive polymer based delivery of the crop protection agents (CPAs) at nano- and microscale: (i) responsive release of bioinsecticides from polymer nanocapsules (PNC) regulated by plant stress enzymes, and (ii) smart delivery of nutrients from polymer microcapsules (PMC), self-adjusted by the intensity of plant root exudates *.
4.2. Microelements Enter Plant through Root-hairs and Deliver Nutrients
The step-by-step method includes the following: the microelements, such as Ca, Mg, Fe, S, Zn are encapsulated into microspheres and these polymer microcapsules are, with time, incorporated and dissolved into the soil. As shown in Figure 2, once close to the root, the chemical bonds of the microcapsule’s wall polymer are broken down by the organic acids or phenolic substances from the root exudates. These root exudates are typically released to enhance plant feeding during the .plant growth process and represent the stimuli activating CPA release.
5. Challenges for the Responsive Polymer Nanocapsules Application in Crop Protection
There are two apparent challenges that limit the extent of the large scale in use of nano-encapsulation: toxicity and cost. The potential toxicity of polymer nano-encapsulated CPAs for crops is a crucial topic to be addressed before any larger-scale cultivation.
5.1. Toxicity of Nanocapsules
First off, there is little knowledge on the nanoparticles toxicity in plants, since most, if not all, studies were being focused on humans or animals: we found less than 2–3 studies. For example, it seems that ultrahigh concentrations (2,000 mg/L) of nanoparticles can affect root growth of some plants. Also, a study of interactions between plant cells and magnetic nanoparticles seemed to indicate a possible interference with the photosynthetic system or a likely growth of chromosomal aberrations [47].
However, not all nanocapsules have the same toxicity potential: those made from lipids, chitin, other natural organic compounds, are expected to be less damaging than the ones metal-made. Moreover, cyto-toxicity does not necessarily imply phyto-toxicity [47], since it is possible that low concentrations of certain nanoparticles could affect plant cells only locally, with the whole plant left undamaged.
Additional questions include: Do the nanocapsules accumulate in fruits, leaves, etc.? Are they destroyed or excreted after some time? Are they incorporated into the food chain? What happens to the animals and humans, should they typically eat such plants?
5.2. Cost of Nanocapsules Production
Another important limitation for nanocarriers at this moment in time is the production scale and cost. Nowadays, the production costs for large enough quantities of polymer nano-encapsulated CPA is still prohibitive. As expected, the nanoencapsulation and liposome batch processes are currently amongst the most complex, and thus expensive as confirmed by the Figure 3 data [46].
The biggest players who can afford to invest in such nanoscale research will be rewarded by being the first to get their products to market. Additionally, increasing the manufacture yield of polymer nanocapsules shall in time bring the cost down.
Meanwhile, it is likely that the two first such applications will be directed towards higher value plants or trees, including the (i) high-value individual fruit trees and (ii) greenhouse-based intensive agriculture of high-input crops.
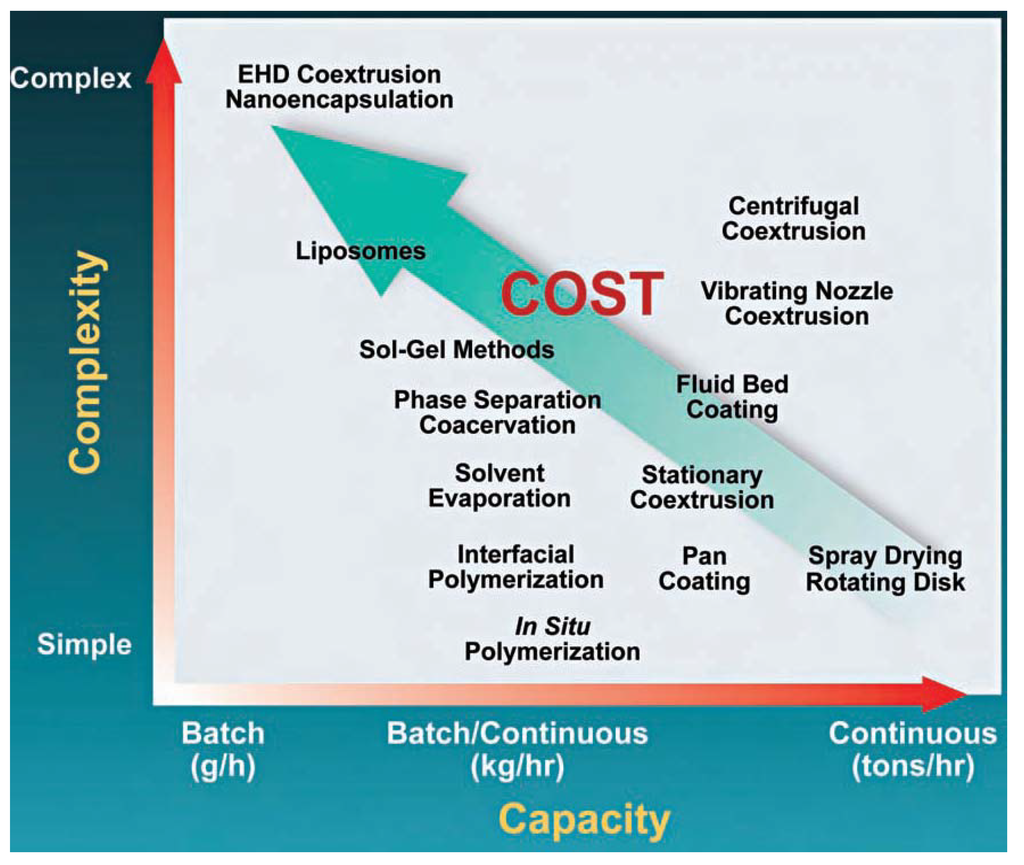
Figure 3.
Encapsulation processes offer different levels of complexity, capacity. The operating cost rent of different fabrication methods is shown in coordinates of capacity (in grams to tons per hour) and complexity (from simple to complex). Reproduced with kind permission of the Southwest Research Institute from [46].
6. Conclusions
The development of nanocapsules for controlled release and systemic application of herbicides has a strong potential to greatly increase their sustainable use for plant protection, such as against parasitic plants for example, by allowing the use of herbicides with different modes of action, i.e. the contact herbicides. While accepting the cost as a current issue for nanoencapsulation, it is expected, as with most technologies, this cost will decrease with time as knowledge progresses and new fabrication methods—on a larger-scale and thus less expensive—become available.
The potential of polymer nanocapsules for use as smart vectors for self-regulated release is impressive. Moreover, their prospects for genetic and molecular work is unprecedented: they could deliver nucleic acids in a controlled way, and for activating or silencing specific genes, thus opening new avenues for the development of resistant plants “vaccinated” preventatively against a wide area of phyto-pathogens.
As we have ascertained herein perhaps for the first time, the continued advances of nano-scale intelligent polymer-based self-regulated CPA release, coupled with recent progress in plant sciences, are heralding the propagation of a “small revolution” in sustainable agriculture. However true, one has to make sure that the nano-toxicological risks are (i) first known and then (ii) reduced to below a critical level (iii) yet to be defined.
Last but not least, the authors submit that the intelligent, EAP polymer-based nanocapsulated CPAs activated by plant-specific stimuli arguably represent the new bright future of protecting economically important plants, trees and crops, once the Plant experts synergize effectively with the Nanotechnology specialists.
Glossary of Terms
| Allomone: | a pheromone that induces a behavioral or physiological change in a member of another species that is of benefit to the producer. |
| Bio-insecticide: | the natural substance made and used for eliminating plant-eating insects. |
| Cuticle: | the layer of cutin (waxy waterproof substance, consisting of derivatives of fatty acids) covering the epidermis of the aerial parts of plants. |
| Elicitor: | a substance that induces the defense mechanisms in higher plants. |
| Epidermis: | the outer protective layer of plant cells. |
| Parasitic plant: | a plant that derives some or all of its sustenance from another plant. |
| Perennial plant: | a plant that lives for more than two years. |
| Phloem: | a plant tissue that conducts organic nutrients substances to all parts of the plant. |
| Phytopathogen: | an organism that causes a disease in a plant. |
| RNA-based vaccines for plant viruses: | Double-stranded RNA (dsRNA) is a RNA with two complementary strands, which is converted to small interfering (siRNA), involved in the RNA interference (RNAi) pathway, including plant viruses RNA silencing. |
| Root exudate: | any substance released through the pores of a diseased or injured plant root. |
| Root hair: | one of the hair-like outgrowths of the root epidermis with a function of absorption. |
| Semiochemical: | any chemical substance or mixture that carries a message. |
| Stoma: | one of the pores on a leaf or stem of a plant allowing fluids access in and out. |
Abbreviations
| AAm | acrylamide; |
| DMAEMA | N,N-dimethylaminoethyl methacrylate; |
| FITC | fluo-rescein isothiocyanate; |
| HEMA | 2-hydroxyethyl methacrylate; |
| MAA | methyl acrylic acid; |
| MBA | N,N′-methylene bis acrylamide; |
| MMA | methyl methacrylate; |
| NIPAAm | N-isopro-pyl acrylamide; |
| NMP | N-methyl-2-pyrrolidone; |
| NSA | N-succinimidyl acrylate; |
| N-tBAAm | N-tert-butylacrylamide; |
| PAA | poly- (acrylic acid); |
| PCIPAAm | poly (2-carboxyisopro-pylacrylamide); |
| PDEAAm | poly(N,N-diethylacrylamide); |
| PDMAAm | poly(N,N'-dime-thylacrylamide); |
| PEO | poly(ethylene oxide); |
| PHEMA | poly(hydroxyethyl methacrylate); |
| PHPMA | poly(2-hydroxypropyl methacrylate); |
| PMAA | polymethacrylic acid; |
| PNVP | poly-N-vinylpyrrolidone; |
| PPO | poly(propylene oxide); |
| PVA | poly (vinyl alcohol); |
| Poly(I-HMPMAAm) | poly(N–(I)-hydroxymethylpropylmethacrylamide). |
References
- Rudzinski, W.E.; Dave, A.M.; Vaishnav, U.H.; Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, T.M. Hydrogels as controlled release devices in agriculture. Des. Monom. Polym. 2002, 5, 39–65. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. J. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Lavon, I.; Kost, J. Mass transport enhancement by ultrasound in non-degradable polymeric controlled release systems. J. Control. Release 1998, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Kost, J.; D’Emanuele, A.; Langer, R. Experimental approach to elucidate the mechanism of ultrasound-enhanced polymer erosion and release of incorporated substances. Macromolecules 1992, 25, 123–128. [Google Scholar] [CrossRef]
- Norris, P.; Noble, M.; Francolini, I.; Vinogradov, A.M.; Stewart, P.S.; Ratner, B.D.; Costerton, J.W.; Stoodley, P. Ultrasonically controlled release of ciprofloxacin from self-assembled coatings on poly(2-hydroxyethyl methacrylate) hydrogels for Pseudomonas aeruginosa biofilm prevention. Antimicrob. Agent. Chemother. 2005, 49, 4272–4279. [Google Scholar] [CrossRef]
- Rapoport, N.Y.; Herron, J.N.; Pitt, W.G.; Pitina, L. Micellar delivery of doxorubicin and its paramagnetic analog, ruboxyl, to hl-60 cells: effect of micelle structure and ultrasound on the intracellular drug uptake. J. Control. Release 1999, 58, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.L.; Roeder, B.L.; Carmen, J.C.; Roloff, F.; Pitt, W.G. Ultrasonically activated chemotherapeutic drug delivery in a rat model. Cancer Res. 2002, 62, 7280–7283. [Google Scholar] [PubMed]
- Rapoport, N.Y.; Christensen, D.A.; Fain, H.D.; Barrows, L.; Gao, Z. Ultrasound-triggered drug targeting of tumors in vitro and in vivo. Ultrasonics 2004, 42, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Sabey, C.J.; Fernando, G.F. Photoresponsive polymers: an investigation of their photoinduced temperature changes during photoviscosity measurements. Polymer 2007, 48, 255–263. [Google Scholar] [CrossRef]
- Nagasaki, T. Photoresponsive polymeric materials for drug delivery systems: double targeting with photoresponsive polymers. Drug Deliv. Syst. 2008, 23, 637–643. [Google Scholar] [CrossRef]
- Leclerc, E.; Furukawa, K.S.; Miyata, F.; Sakai, Y.; Ushida, T.; Fujii, T. Fabrication of microstructures in photosensitive biodegradable polymers for tissue engineering applications. Biomaterials 2004, 25, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, Y.; Tong, X.; Wang, X. Photoinduced deformation of amphiphilic azo polymer colloidal spheres. J. Am. Chem. Soc. 2005, 127, 2402–2403. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Kawabata, Y. Changes in the sol-gel transformation behavior of azobenzene moiety-containing methyl cellulose irradiated with UV light. Macromol. Rapid Commun. 1995, 16, 875–880. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, P.J.; Zhao, X.Y.; Li, L.; Tam, K.C.; Gan, L.H. Preparation, characterization and novel photoregulated rheological properties of azobenzene functionalized cellulose derivatives and their [alpha]-cd complexes. Polymer 2004, 45, 6219–6225. [Google Scholar] [CrossRef]
- Strzegowski, L.A.; Martinez, M.B.; Gowda, D.C.; Urry, D.W.; Tirrell, D.A. Photo modulation of the inverse temperature transition of a modified elastin poly(pentapeptide). J. Am. Chem. Soc. 1994, 116, 813–814. [Google Scholar] [CrossRef]
- Ivanov, A.E.; Eremeev, N.L.; Wahlund, P.O.; Galaev, I.Y.; Mattiasson, B. Photosensitive copolymer of n-isopropylacrylamide and methacryloyl derivative of spirobenzopyran. Polymer 2002, 43, 3819–3823. [Google Scholar] [CrossRef]
- Lee, H.-I.; Wu, Y.; Oh, J.K.; Mueller, I.; Sherwood, G.; Peteanu, L. Light-induced reversible formation of polymeric micelles. Angew. Chem. Int. Ed. 2007, 46, 2453–2457. [Google Scholar] [CrossRef]
- Stowell, M.H.B. Photo-Responsive Microencapsulation materials, compositions and methods of use thereof. US Patent 20090202652, 13 August 2009. [Google Scholar]
- Koo, A.N.; Lee, H.J.; Kim, S.E.; Chang, J.H.; Park, C.; Kim, C.; Park, J.H.; Lee, S.C. Disulfide-cross-linked peg-poly(amino acid)s copolymer micelles for glutathione-mediated intracellular drug delivery. Chem. Commun. 2008, 48, 6570–6572. [Google Scholar] [CrossRef]
- Li, Y.; Lokitz, B.S.; Armes, S.P. McCormick, C.L. Synthesis of reversible shell cross-linked micelles for controlled release of bioactive agents. Macromolecules 2006, 39, 2726–2728. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Quinn, J.F.; Caruso, F. Disulfide cross-linked polymer capsules: en route to biodeconstructible systems. Biomacromolecules 2006, 7, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Napoli, A.; Valentini, M.; Tirelli, N.; Müller, M.; Hubbell, J.A. Oxidation-responsive polymeric vesicles. Nat. Mater. 2004, 3, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Napoli, A.; Tirelli, N.; Kilcher, G.; Hubbell, A. New synthetic methodologies for amphiphilic multiblock copolymers of ethylene glycol and propylene sulfide. Macromolecules 2001, 34, 8913–8917. [Google Scholar] [CrossRef]
- Zrinyi, M. Intelligent polymer gels controlled by magnetic fields. Colloid Polym. Sci. 2000, 278, 98–103. [Google Scholar] [CrossRef]
- Matsumoto, S; Christie, R.J.; Nishiyama, N.; Miyata, K.; Ishii, A. Environment-responsive block co- polymer micelles with a disulfide cross-linked core for enhanced siRNA delivery. Biomacromolecules 2009, 10, 119–127. [Google Scholar]
- Sewell, M.K.; Fugit, K.D.; Ankareddi, I.; Zhang, C.; Hampel, M.L.; Kim, D.-H.; Brazel, C.S. Magnetothermally-triggered drug delivery using hydrogels with imbedded cobalt ferrite, iron platinum or manganese ferrite nanoparticles. PMSE Preprints 2008, 98, 694–695. [Google Scholar]
- Zhang, J.; Xu, S.; Kumacheva, E. Polymer microgels: reactors for semiconductor, metal, and magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 7908–7914. [Google Scholar] [CrossRef] [PubMed]
- Brugger, B.; Richtering, W. Magnetic, thermosensitive microgels as stimuli-responsive emulsifiers allowing for remote control of separability and stability of oil in water-emulsions. Adv. Mater. 2007, 19, 2973–2978. [Google Scholar] [CrossRef]
- Brazel, C.S. Magnetothermally-responsive nanomaterials: combining magnetic nanostructures thermally-sensitive polymers for triggered drug release. Pharma. Res. 2008, 26, 644–656. [Google Scholar] [CrossRef]
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, translocation and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008, 10, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Racuciu, M.; Miclaus, S.; Creanga, D. The response of plant tissues to magnetic fluid and electromagnetic exposure. Rom. J. Biophys. 2009, 19, 73–82. [Google Scholar]
- Shalaby, W.S.; Park, K. Biochemical and mechanical characterization of enzyme-digestible hydrogels. Pharm. Res. 1993, 7, 816–823. [Google Scholar] [CrossRef]
- Thornton, P.D.; McConnel, G.; Ulijin, R.V. Enzyme-responsive polymer hydrogel beads. Chem. Commun. 2005, 5913–5915. [Google Scholar]
- Miyata, T.; Asami, N.; Uragami, T. Preparation of an antigen-sensitive hydrogel using antigen-antibody bindings. Macromolecules 1999, 32, 2082–2084. [Google Scholar] [CrossRef]
- Miyata, T.; Asami, N.; Uragami, T. A reversibly antigen-responsive hydrogel. Nature 1999, 399, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bowyer, A.; Eisenthal, R.; Hubble, J. A smart membrane based on an antigen-responsive hydrogel. Biotechnol. Bioeng. 2007, 97, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.K.; Moschou, E.A.; Peteu, S.F.; Bachas, L.G.; Madou, M.J.; Eisenhardt, P.E.; Daunert, S. Responsive drug delivery systems: new challenges for analytical chemists. Anal. Chem 2003, 75, A207–A213. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, S.; Wu, R.; Wang, J.; Wei, J. Preparation and characteristic of electric stimuli responsive hydrogel composed of polyvinyl alcohol/poly (sodium maleate-co-sodium acrylate). J. Appl. Polym. Sci. 2008, 107, 391–395. [Google Scholar] [CrossRef]
- Homma, M.; Seida, Y.; Nakano, Y. Design and optimization of high performance electrically-driven polymer hydrogel systems. J. Appl. Polym. Sci. 2000, 75, 111–118. [Google Scholar] [CrossRef]
- Peteu, S.F. Responsive materials configured for micro- and nano-actuation. J. Int. Mater. Syst. Struct. 2007, 18, 147–152. [Google Scholar] [CrossRef]
- Peteu, S.F. Micro- to nano-biosensors and actuators integrated for responsive delivery of countermeasures. IEEE CAS. Proc. 2010. in print. [Google Scholar]
- Peteu, S.F.; Gebremichael, E.; Peiris, P.; Bayachou, M. Nanostructured poly(ethylenedioxy-thiophene)-metalloporphyrin catalytic platform for the detection of peroxynitrite. Biosens. Bioelectron. 2010, 25, 1914–1921. [Google Scholar]
- Lee, A.S.; Peteu, S.F.; Ly, J.V.; Requicha, A.A.G.; Thompson, M.E.; Zhou, C. Actuation of polypyrrole nanowires. Nanotechnology (IOP) 2008, 19, 254–261. [Google Scholar]
- Daunert, S.; Peteu, S.F.; Bachas, L.G.; Madou, M.J.; Moschou, E.A. Artificial muscle blends electro-actuated near neutral pH, actuating devices and methods using the same. U.S. Patent 7,482,381, 27 January 2009. [Google Scholar]
- Microencapsulation and Controlled Release Section. In Micro & Nano Encapsulation; The South-west Research Institute (SwRI): San Antonio, TX, USA, 2008.
- Perez-de-Luque, A.; Rubiales, D. Nanotechnology for parasitic plant control. Pest Manag. Sci. 2009, 65, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, M.; Yanase, M.; Suzuki, M.; Honda, H.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T. Intracellular hyperthermia for cancer using magnetite cationic liposomes. J. Magn. Magn. Mater. 1999, 194, 176–184. [Google Scholar] [CrossRef]
- Peteu, S.F. Cnidaria nematocysts soft microvalves. In Proceedings of World Congress of Biomimetics and Artificial Muscles, Albuquerque, NM, USA, 9–11 December 2002.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).