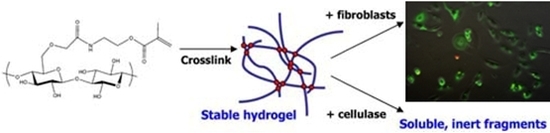
Synthesis and Characterization of Carboxymethylcellulose-Methacrylate Hydrogel Cell Scaffolds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impact of CMC-methacrylate molecular weight on gel synthesis

| CMC-methacrylate MW (kDa) | Total % copolymer a | % CMC methacrylate a | % PEG-DM a | Transparency b |
|---|---|---|---|---|
| 700 | 0.5 | 0.5 | 0 | +++ |
| 1.0 | 1.0 | 0 | +++ | |
| 90 | 12 | 5 | 7 | + |
| 8 | 4 | +/− | ||
| 20 | 8 | 12 | ++ | |
| 12 | 8 | + | ||
| 16 | 4 | +/− |
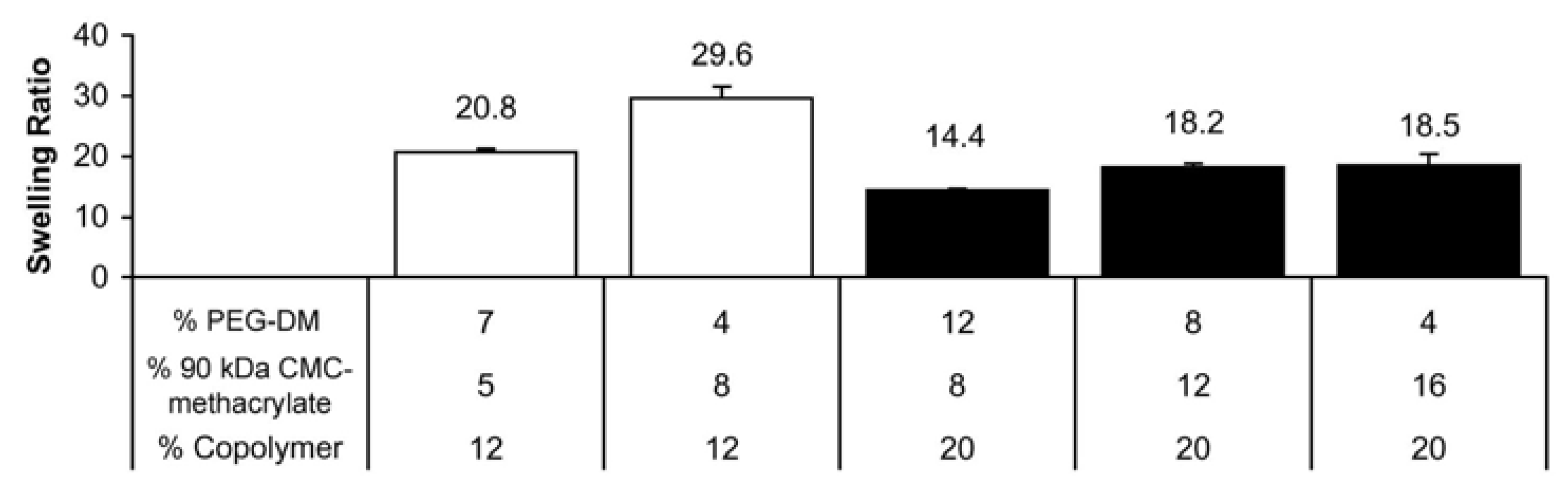
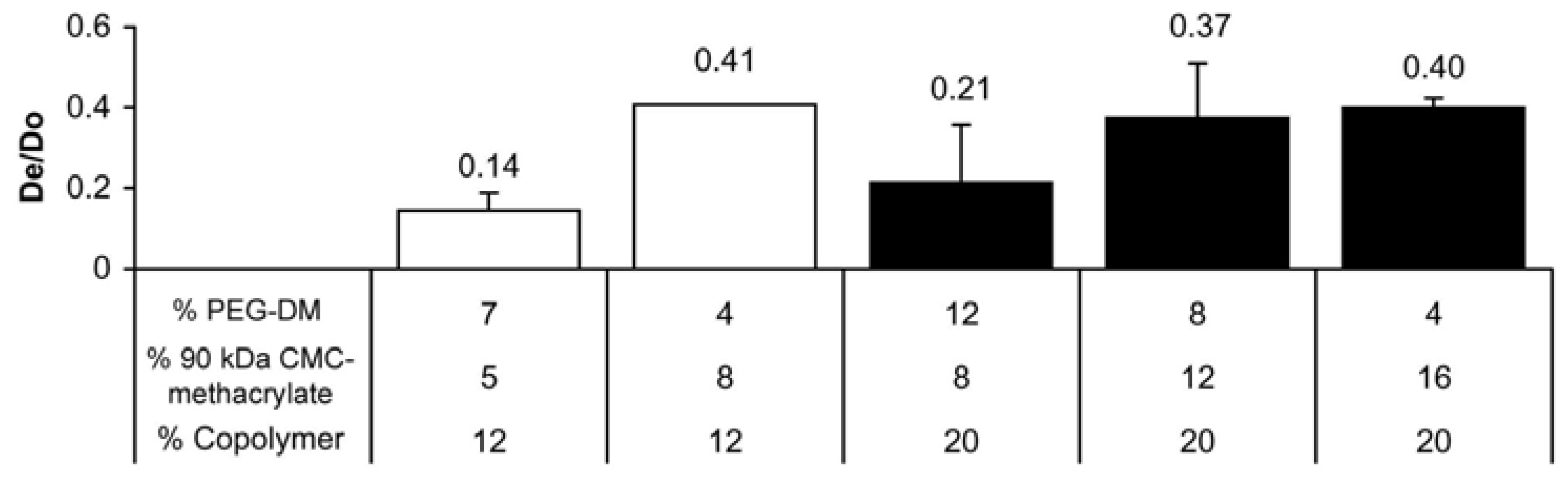
2.2. CMC-methacrylate:PEG-DM ratio determines hydrogel physical properties

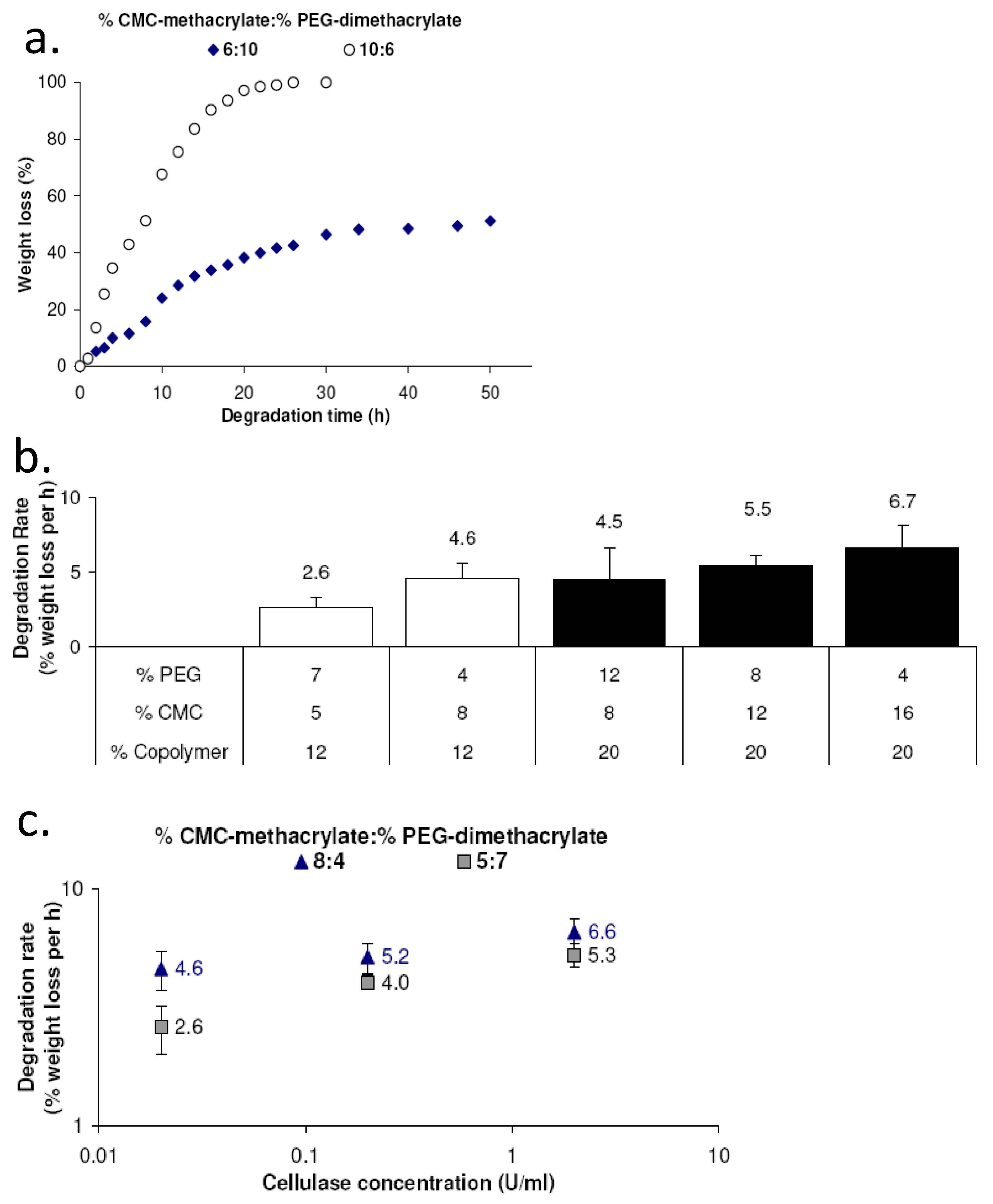
2.3. CMC-methacrylate content impacts enzymatic degradation of copolymer gels
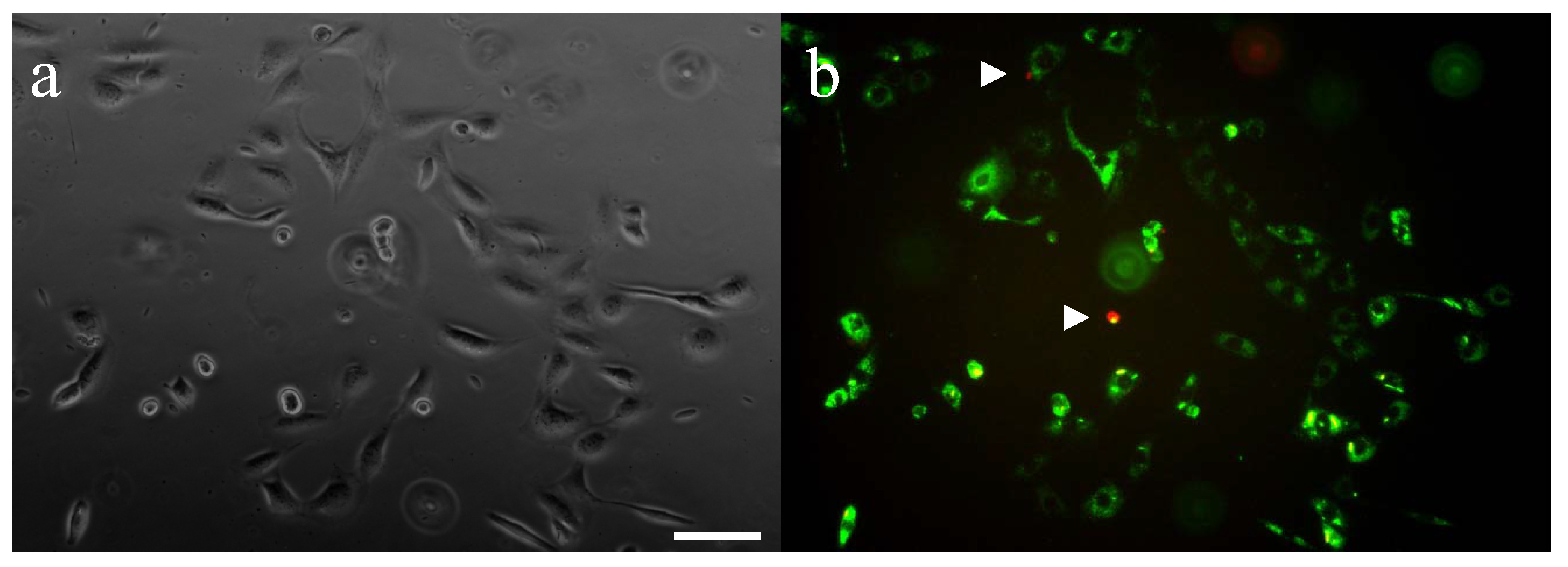
2.4. CMC-methacrylate/PEG-DM gels support cell adhesion and viability
3. Experimental Section
3.1. Materials
3.2. Synthesis of CMC-methacrylate
3.3. Synthesis of PEG-DM
3.4. Photocrosslinking of CMC hydrogels
3.5. Rheological characterization of hydrogel shear modulus
3.6. Swelling ratio
3.7. Release of BSA
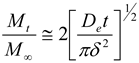
3.8. In vitro enzymatic degradation
3.9. Gel biocompatibility and support of mammalian cell adhesion
3.10. Statistical analysis
4. Conclusions
Acknowledgements
References
- Miyamoto, T.; Takahashi, S.; Ito, H.; Inagaki, H.; Noishiki, Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989, 23, 125–133. [Google Scholar] [PubMed]
- Barbucci, R.; Leone, G.; Vecchiullo, A. Novel carboxymethylcellulose-based microporous hydrogels suitable for drug delivery. J. Biomater. Sci. Polym. Ed. 2004, 15, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Development of carboxymethyl cellulose acrylate for various biomedical applications. Biomed. Mater. 2006, 1, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Control. Release 2003, 86, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Ozkan, Y.; Savaser, A.; Baykara, T. In vitro release studies of chlorpheniramine maleate from gels prepared by different cellulose derivatives. Farmaco 2003, 58, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Yeo, Y.; Highley, C.B.; Bellas, E.; Benitez, C.A.; Kohane, D.S. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials 2007, 28, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Leach, R.E.; Burns, J.W.; Dawe, E.J.; SmithBarbour, M.D.; Diamond, M.P. Reduction of postsurgical adhesion formation in the rabbit uterine horn model with use of hyaluronate/carboxymethylcellulose gel. Fertil. Steril. 1998, 69, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Nho, Y.-C.; Lim, Y.-M.; Son, T.-I. Prevention of surgical adhesions with barriers of carboxymethylcellulose and poly(ethylene glycol) hydrogels synthesized by irradiation. J. Appl. Polym. Sci. 2005, 96, 1138–1145. [Google Scholar] [CrossRef]
- Liu, L.S.; Berg, R.A. Adhesion barriers of carboxymethylcellulose and polyethylene oxide composite gels. J. Biomed. Mater. Res. 2002, 63, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.C.; Shear, D.A.; Hoffman, S.W.; Stein, D.G.; LaPlaca, M.C. Biocompatibility of methylcellulose-based constructs designed for intracerebral gelation following experimental traumatic brain injury. Biomaterials 2001, 22, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Krochta, J.M.; Hsieh, Y.L.; Kurth, M.J. Mechanism and characteristics of protein release from lactitol-based cross-linked hydrogel. J. Agric. Food Chem. 2000, 48, 5658–5665. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Powell, C.; Ahmed, S.M.; Alsberg, E. Biodegradable, Photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng. A 2010, in press. [Google Scholar]
- Leach, J.B.; Bivens, K.A.; Patrick, C.W., Jr.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.B.; Schmidt, C.E. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials 2005, 26, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1975. [Google Scholar]
- Narasimhan, B.; Mallapragada, S.K.; Peppas, N.A. Release kinetics, data interpretation. In Encyclopedia of Controlled Drug Delivery; Mathiowitz, E., Ed.; Wiley Interscience: Hoboken, NJ, USA, 1999; pp. 921–935. [Google Scholar]
- Siddiqui, K.S.; Azhar, M.J.; Rashid, M.H.; Rajoka, M.I. Stability and identification of active-site residues of carboxymethylcellulases from Aspergillus niger and Cellulomonas biazotea. Folia Microbiol. 1997, 42, 312–318. [Google Scholar] [CrossRef]
- Hern, D.L.; Hubbell, J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J. Biomed. Mater. Res. 1998, 39, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.B.; Bivens, K.A.; Collins, C.N.; Schmidt, C.E. Development of photocrosslinkable hyaluronic acid-polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J. Biomed. Mater. Res. A 2004, 70, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Carrino, D.; Gershman, H. Division of BALB/c mouse 3T3 and simian virus 40-transformed 3T3 cells in cellular aggregates. Proc. Natl. Acad. Sci. USA 1977, 74, 3874–3878. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reeves, R.; Ribeiro, A.; Lombardo, L.; Boyer, R.; Leach, J.B. Synthesis and Characterization of Carboxymethylcellulose-Methacrylate Hydrogel Cell Scaffolds. Polymers 2010, 2, 252-264. https://doi.org/10.3390/polym2030252
Reeves R, Ribeiro A, Lombardo L, Boyer R, Leach JB. Synthesis and Characterization of Carboxymethylcellulose-Methacrylate Hydrogel Cell Scaffolds. Polymers. 2010; 2(3):252-264. https://doi.org/10.3390/polym2030252
Chicago/Turabian StyleReeves, Robert, Andreia Ribeiro, Leonard Lombardo, Richard Boyer, and Jennie B. Leach. 2010. "Synthesis and Characterization of Carboxymethylcellulose-Methacrylate Hydrogel Cell Scaffolds" Polymers 2, no. 3: 252-264. https://doi.org/10.3390/polym2030252
APA StyleReeves, R., Ribeiro, A., Lombardo, L., Boyer, R., & Leach, J. B. (2010). Synthesis and Characterization of Carboxymethylcellulose-Methacrylate Hydrogel Cell Scaffolds. Polymers, 2(3), 252-264. https://doi.org/10.3390/polym2030252