A Poly(Acrylamide-co-Acrylic Acid)-Encapsulated Nitrification Inhibitor with Good Soil-Loosening, Phosphorous-Solubilizing, and Nitrogen Fixation Abilities and High-Temperature Resistance
Abstract
1. Introduction
2. Experimental Sections
2.1. Fabrication Processes
2.2. Methods
3. Results and Discussion
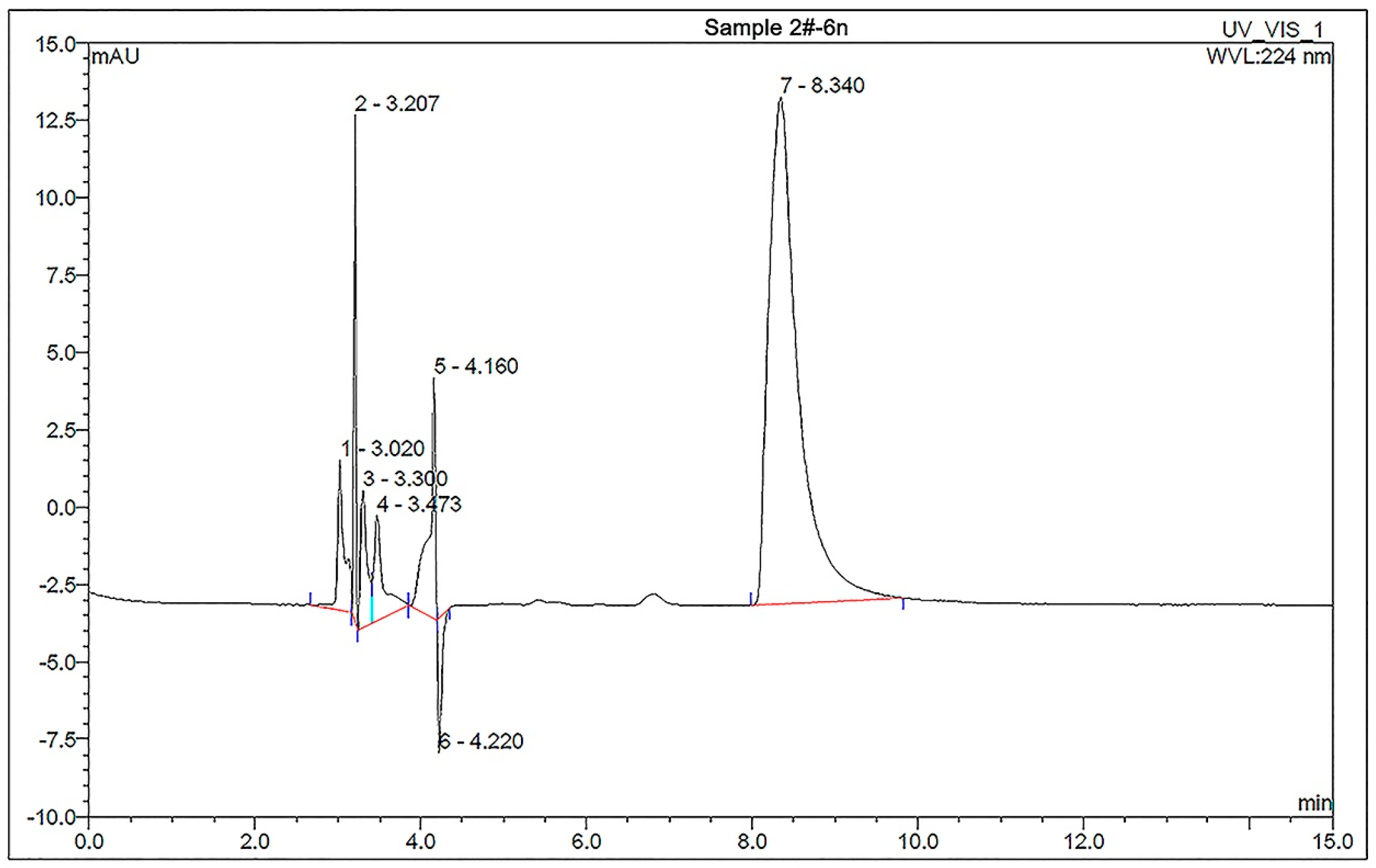
3.1. Content of DMPZ in P(AA-co-AM)-e-NI
3.2. Chemical Structures
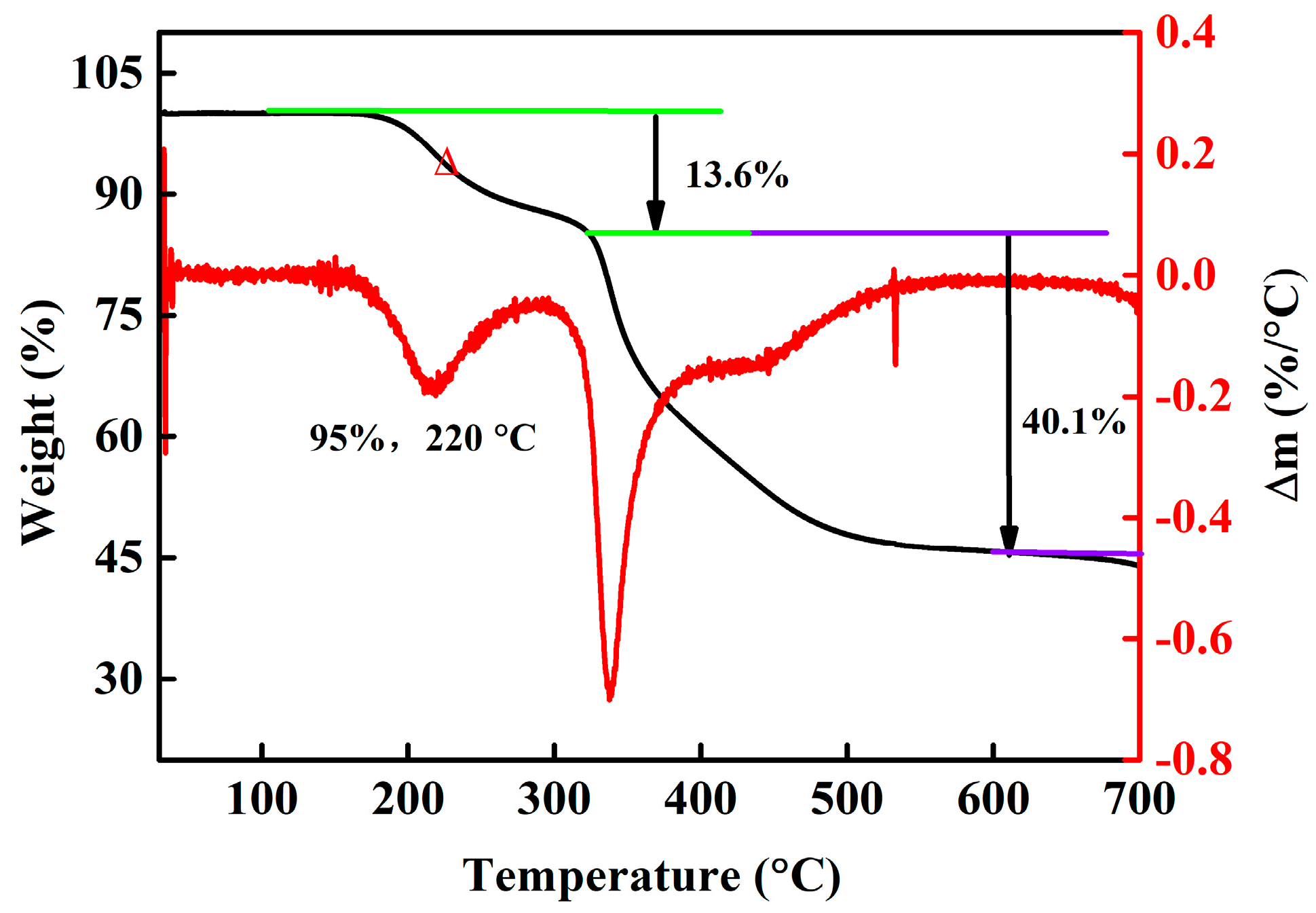
3.3. High-Temperature Resistance
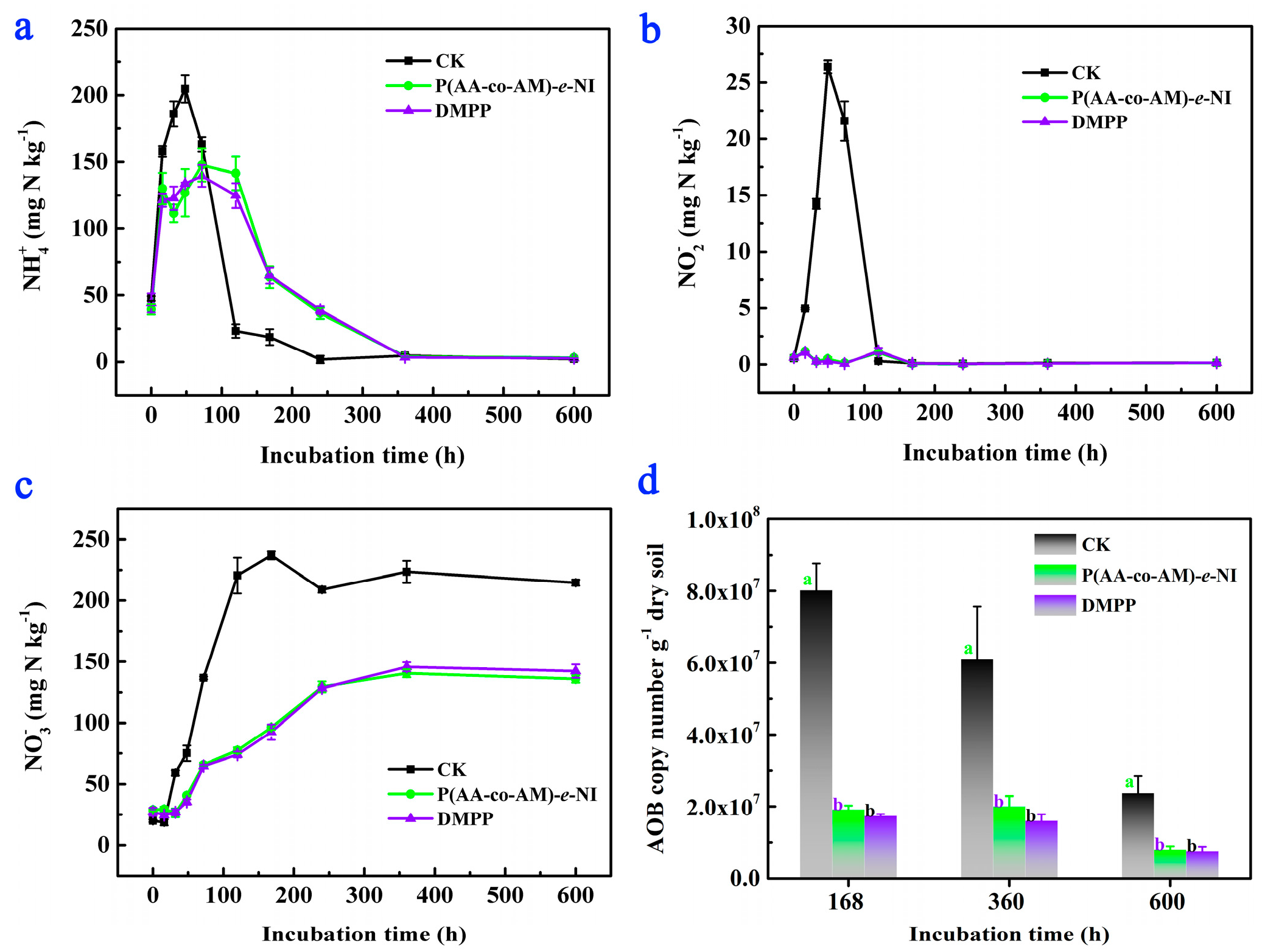
3.4. Nitrogen Fixation Ability
3.5. Phosphorous-Solubilizing Capacity
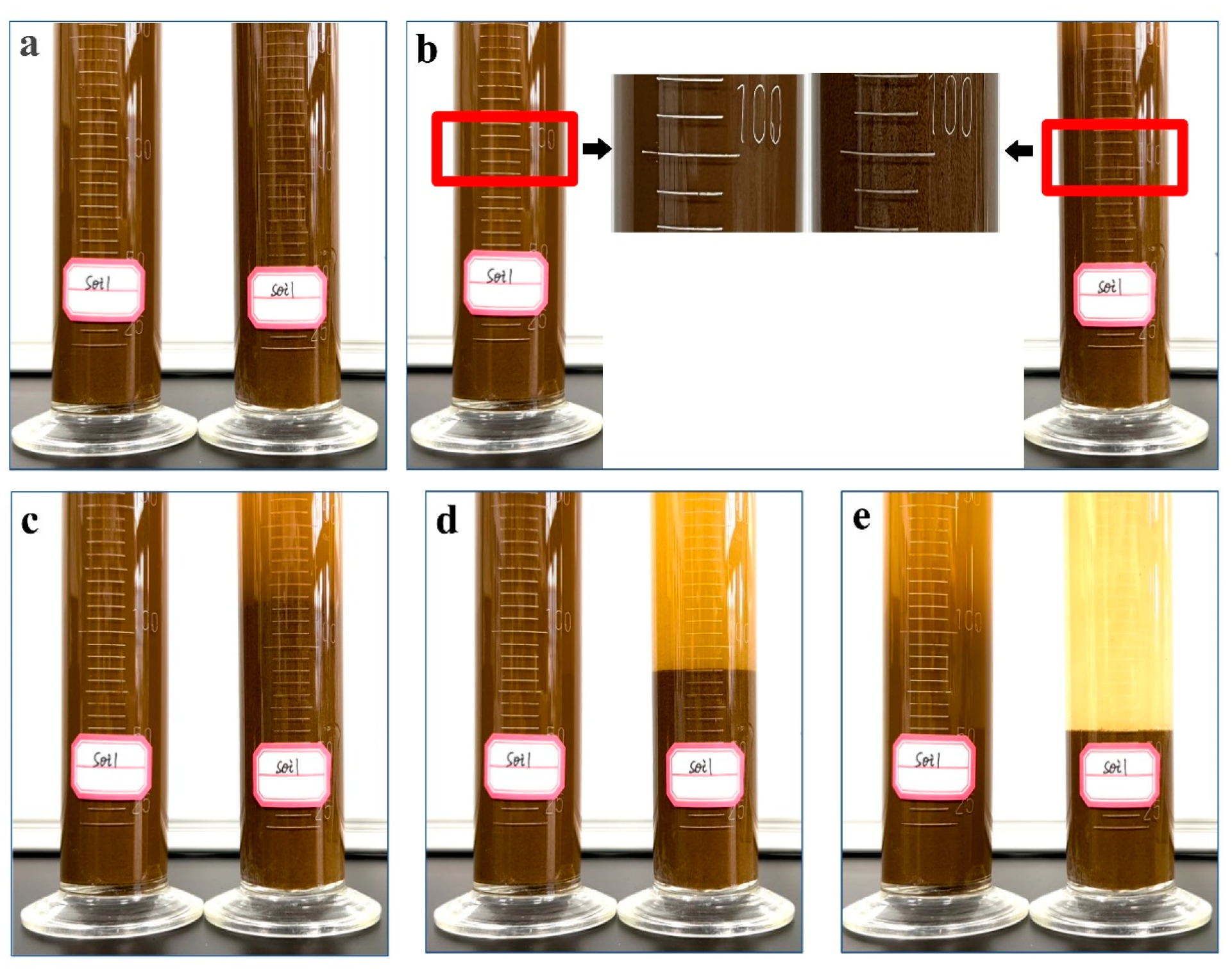
3.6. Soil-Loosening Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, Q.; Xu, J.; Liu, Y.; Wang, D.; Chen, S.; Qian, W.; He, M.; Chen, P.; Zhou, X.; Qi, Z. Nitrogen losses from soil as affected by water and fertilizer management under drip irrigation: Development, hotspots and future perspectives. Agric. Water. Manag. 2024, 296, 108791. [Google Scholar] [CrossRef]
- Ren, B.; Guo, Y.; Liu, P.; Zhao, B.; Zhang, J. Effects of urea-ammonium nitrate solution on yield, N2O emission, and nitrogen efficiency of summer maize under integration of water and fertilizer. Front. Plant Sci. 2021, 12, 700331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, R.; Karmakar, S.; Kumar, A.; Singh, A.K.; Kumar, A.; Singh, J. Impact of amide fertilizer on carbon sequestration under the agroforestry system in the eastern plateau region of India. Sustainability 2023, 15, 9775. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Z.; Bai, S.H.; Fan, H.; Zuo, J.; Zhang, L.; Hu, D.; Zhang, M. Non-targeted effects of nitrification inhibitors on soil free-living nitrogen fixation modified with weed management. Sci. Total Environ. 2024, 912, 169005. [Google Scholar] [CrossRef]
- Deng, M.; Zhao, X.; Senbati, Y.; Song, K.; He, X. Nitrogen removal by heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas sp. DM02: Removal performance, mechanism and immobilized application for real aquaculture wastewater treatment. Bioresour. Technol. 2021, 322, 124555. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Z.; Bai, S.H.; Zhou, M.; Tang, W.; Ma, B.; Zhang, M. Asymmetries among soil fungicide residues, nitrous oxide emissions and microbiomes regulated by nitrification inhibitor at different moistures. J. Hazard. Mater. 2024, 470, 134301. [Google Scholar] [CrossRef]
- Bundy, L.G.; Bremner, J.M. Effects of nitrification inhibitors on transformations of urea nitrogen in soils. Soil Biol. Biochem. 1974, 6, 369–376. [Google Scholar] [CrossRef]
- Ren, B.; Huang, Z.; Liu, P.; Zhao, B.; Zhang, J. Urea ammonium nitrate solution combined with urease and nitrification inhibitors jointly mitigate NH3 and N2O emissions and improves nitrogen efficiency of summer maize under fertigation. Field Crop. Res. 2023, 296, 108909. [Google Scholar] [CrossRef]
- Wang, X.; Bai, J.; Xie, T.; Wang, W.; Zhang, G.; Yin, S.; Wang, D. Effects of biological nitrification inhibitors on nitrogen use efficiency and greenhouse gas emissions in agricultural soils: A review. Ecotoxicol. Environ. Safe 2021, 220, 112338. [Google Scholar] [CrossRef]
- Peixoto, L.; Petersen, S.O. Efficacy of three nitrification inhibitors to reduce nitrous oxide emissions from pig slurry and mineral fertilizers applied to spring barley and winter wheat in Denmark. Geoderma Reg. 2023, 32, e00597. [Google Scholar] [CrossRef]
- Rose, T.J.; Kearney, L.J.; Zeng, Y.; Van Zwieten, L.; Rose, M.T. DMPP-urea restricts nitrification in the first month without improving agronomic N use efficiency. Nutr. Cycl. Agroecosys. 2023, 126, 115–125. [Google Scholar] [CrossRef]
- Yu, Q.; Ma, J.; Zou, P.; Lin, H.; Sun, W.; Yin, J.; Fu, J. Effects of combined application of organic and inorganic fertilizers plus nitrification inhibitor DMPP on nitrogen runoff loss in vegetable soils. Environ. Sci. Pollut. Res. 2015, 22, 472–481. [Google Scholar] [CrossRef]
- Tufail, M.A.; Naeem, A.; Arif, M.S.; Farooq, T.H.; Shahzad, S.M.; Dar, A.A.; Albasher, G.; Shakoor, A. Unraveling the efficacy of nitrification inhibitors (DCD and DMPP) in reducing nitrogen gases emissions across agroecosystems: A three-decade global data synthesis (1993–2021). Fuel 2022, 324, 124725. [Google Scholar] [CrossRef]
- Casali, L.; Broll, V.; Ciurli, S.; Emmerling, F.; Braga, D.; Grepioni, F. Facilitating nitrification inhibition through green, mechanochemical synthesis of a novel nitrapyrin complex. Cryst. Growth Des. 2021, 21, 5792–5799. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Xu, F. Synthesis of a novel polymer nitrification inhibitor with acrylic acid and 3,4-Dimethylpyrazole. J. Agric. Food Chem. 2021, 69, 3307–3311. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Y.; Huang, W.; Xu, F. A novel high temperature resistant and multifunctional nitrification inhibitor: Synthesis, characterization, and application. J. Agric. Food Chem. 2022, 70, 13832–13838. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, H.; Liu, S.; Li, J.; Kong, F. Synthesizing a water-soluble polymeric nitrification inhibitor with novel soil-loosening ability. Polymers 2023, 16, 107. [Google Scholar] [CrossRef]
- Kebede, B.; Tsunekawa, A.; Haregeweyn, N.; Tsubo, M.; Mulualem, T.; Mamedov, A.I.; Meshesha, D.T.; Adgo, E.; Fenta, A.A.; Ebabu, K.; et al. Effect of polyacrylamide integrated with other soil amendments on runoff and soil loss: Case study from northwest Ethiopia. Int. Soil Water Conservat. 2022, 10, 487–496. [Google Scholar] [CrossRef]
- Ng, C.W.W.; So, P.S.; Lau, S.Y.; Zhou, C.; Coo, J.L.; Ni, J.J. Influence of biopolymer on gas permeability in compacted clay at different densities and water contents. Eng. Geol. 2020, 272, 105631. [Google Scholar] [CrossRef]
- Abulaiti, A.; She, D.; Liu, Z.; Sun, X.; Wang, H. Application of biochar and polyacrylamide to revitalize coastal saline soil quality to improve rice growth. Environ. Sci. Pollut. R 2023, 30, 18731–18747. [Google Scholar] [CrossRef]
- Pan, Y.; She, D.; Shi, Z.; Chen, X.; Xia, Y. Do biochar and polyacrylamide have synergistic effect on net denitrification and ammonia volatilization in saline soils? Environ. Sci. Pollut. Res. 2021, 28, 59974–59987. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xu, P.; Zhang, P.; Wang, S. Numerical investigation of the anti-infiltration and anti-erosion performance of composite layers mixed with polyacrylamide and basalt fibre for the protection of silt subgrade slopes. Front. Earth Sci. 2022, 10, 815602. [Google Scholar] [CrossRef]
- Huang, J.; Kogbara, R.B.; Hariharan, N.; Masad, E.A.; Little, D.N. A state-of-the-art review of polymers used in soil stabilization. Constr. Build. Mater. 2021, 305, 124685. [Google Scholar] [CrossRef]
- Hobza, P.; Havlas, Z. Blue-shifting hydrogen bonds. Chem. Rev. 2000, 100, 42534–43264. [Google Scholar] [CrossRef] [PubMed]
- Pullanchery, S.; Kulik, S.; Rehl, B.; Hassanali, A.; Roke, S. Charge transfer across C–H…O hydrogen bonds stabilizes oil droplets in water. Science 2021, 374, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Qin, S.; Song, Y.; Liu, Z.; Yang, P.; Li, S.; Liu, C.; Shen, C. Zwitterion functionalized graphene oxide/polyacrylamide/polyacrylic acid hydrogels with photothermal conversion and antibacterial properties for highly efficient uranium extraction from seawater. Adv. Funct. Mater. 2023, 33, 2301773. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Fawell, P.D.; van Bronswijk, W. An in situ FTIR-ATR study of polyacrylate adsorbed onto hematite at high pH and high ionic strength. Langmuir 2004, 20, 4093–4100. [Google Scholar] [CrossRef]
- Sundaraganesan, N.; Kavitha, E.; Sebastian, S.; Cornard, J.P.; Martel, M. Experimental FTIR, FT-IR (gas phase), FT-Raman and NMR spectra, hyperpolarizability studies and DFT calculations of 3,5-dimethylpyrazole. Spectrochim. Acta Part A 2009, 74, 788–797. [Google Scholar] [CrossRef]

| Dosage (mL) | 0.1 | 0.5 | 1.0 | 2.0 | 3.0 | 5.0 | |
| Solubilizing Ability | |||||||
| Degree | turbidity | turbidity | clarification | clarification | clarification | clarification | |
| Time (s) | ∞ | ∞ | 10 | 7 | 11 | 15 | |
| Sample | Density (g/cm3) | Compactness (N/cm2) | Porosity (%) |
|---|---|---|---|
| Without P(AA-co-AM)-e-NI | 1.41 ± 0.06 a | 419 ± 9 a | 46.8% |
| With P(AA-co-AM)-e-NI | 1.28 ± 0.05 a | 375 ± 8 a | 51.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Fu, Y.; Wang, T.; Liu, M.; Mao, J.; Xu, F. A Poly(Acrylamide-co-Acrylic Acid)-Encapsulated Nitrification Inhibitor with Good Soil-Loosening, Phosphorous-Solubilizing, and Nitrogen Fixation Abilities and High-Temperature Resistance. Polymers 2025, 17, 1280. https://doi.org/10.3390/polym17091280
Gao H, Fu Y, Wang T, Liu M, Mao J, Xu F. A Poly(Acrylamide-co-Acrylic Acid)-Encapsulated Nitrification Inhibitor with Good Soil-Loosening, Phosphorous-Solubilizing, and Nitrogen Fixation Abilities and High-Temperature Resistance. Polymers. 2025; 17(9):1280. https://doi.org/10.3390/polym17091280
Chicago/Turabian StyleGao, Hui, Yuli Fu, Tianyu Wang, Meijia Liu, Jianzhen Mao, and Feng Xu. 2025. "A Poly(Acrylamide-co-Acrylic Acid)-Encapsulated Nitrification Inhibitor with Good Soil-Loosening, Phosphorous-Solubilizing, and Nitrogen Fixation Abilities and High-Temperature Resistance" Polymers 17, no. 9: 1280. https://doi.org/10.3390/polym17091280
APA StyleGao, H., Fu, Y., Wang, T., Liu, M., Mao, J., & Xu, F. (2025). A Poly(Acrylamide-co-Acrylic Acid)-Encapsulated Nitrification Inhibitor with Good Soil-Loosening, Phosphorous-Solubilizing, and Nitrogen Fixation Abilities and High-Temperature Resistance. Polymers, 17(9), 1280. https://doi.org/10.3390/polym17091280






