Rational Designing of NiO Nanoparticles Anchored with PEG-WO3 for Enhanced Water Oxidation Performance
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
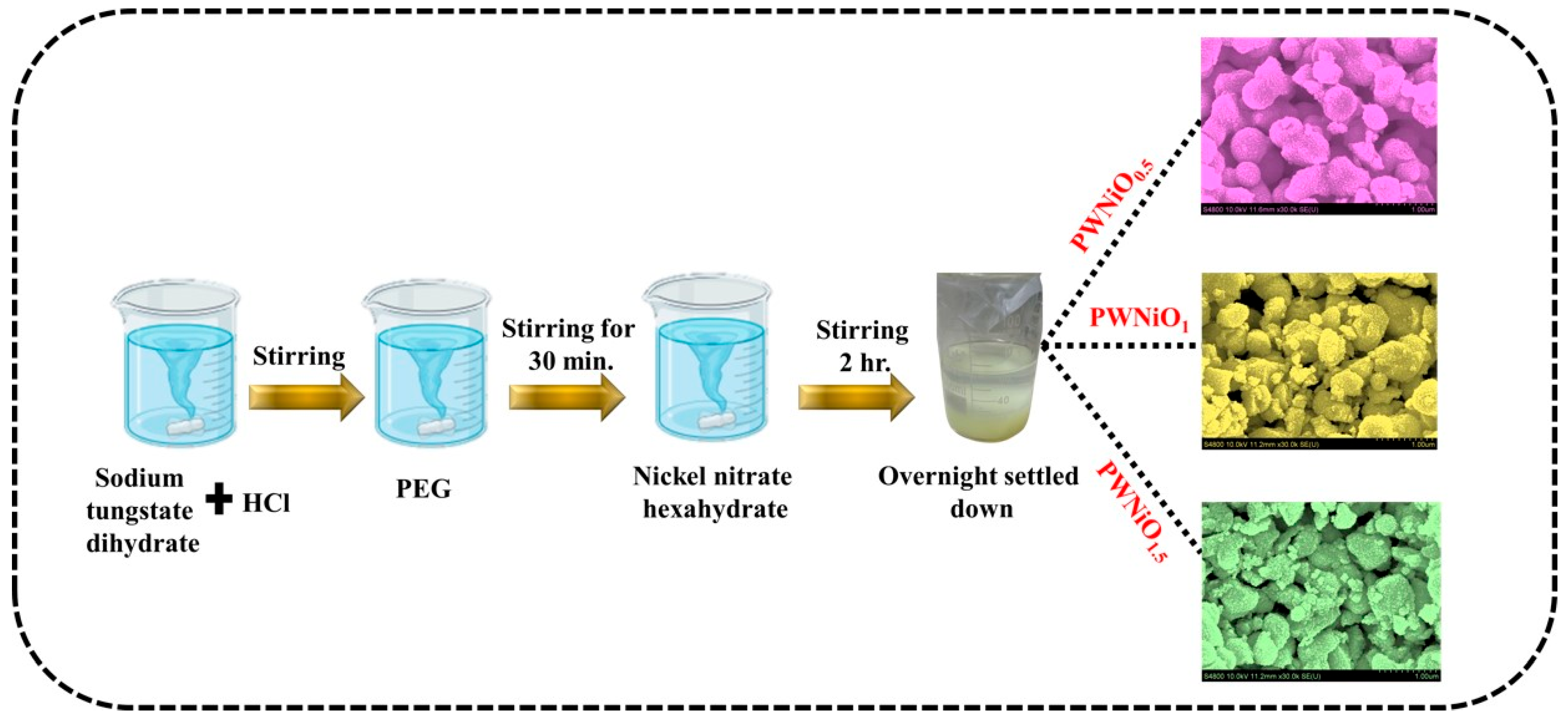
2.2. Preparation of PEG-WO3-NiO
2.3. Material Characterization
2.4. Electrochemical Analysis
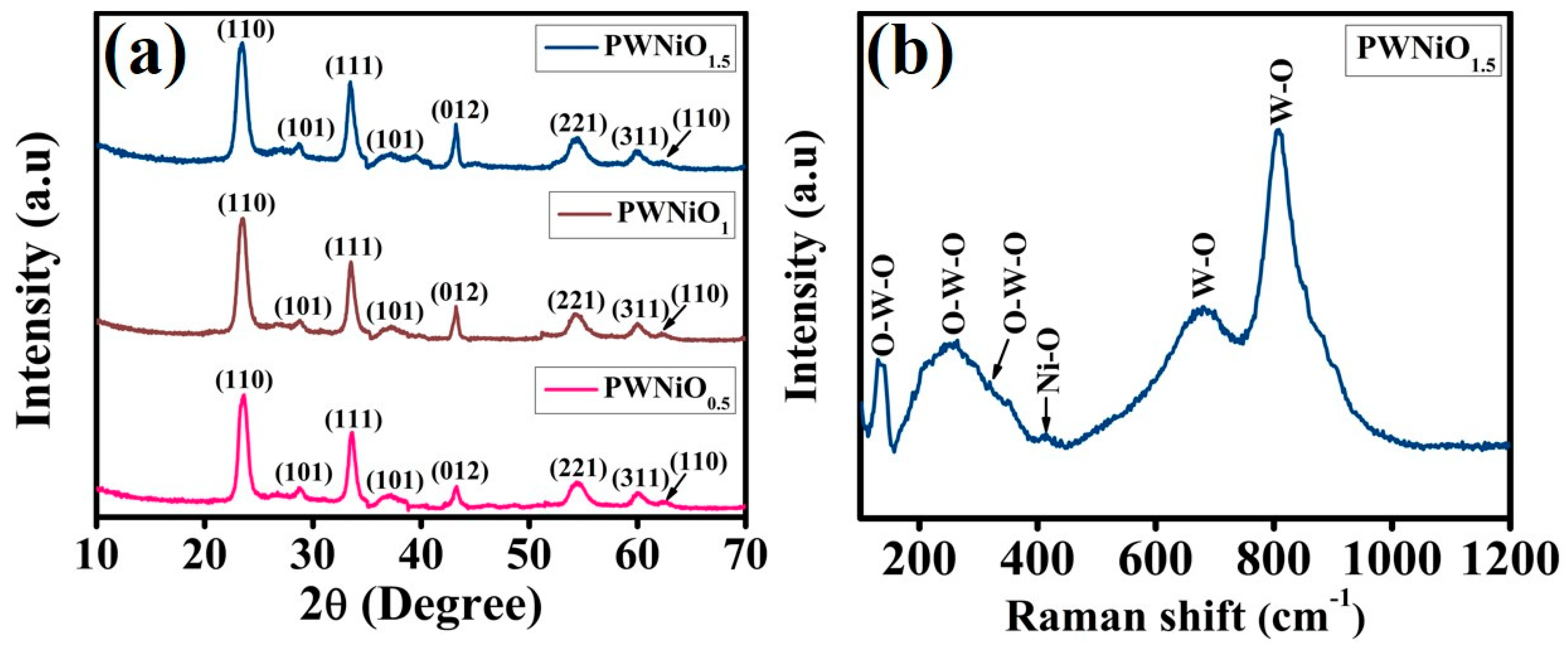
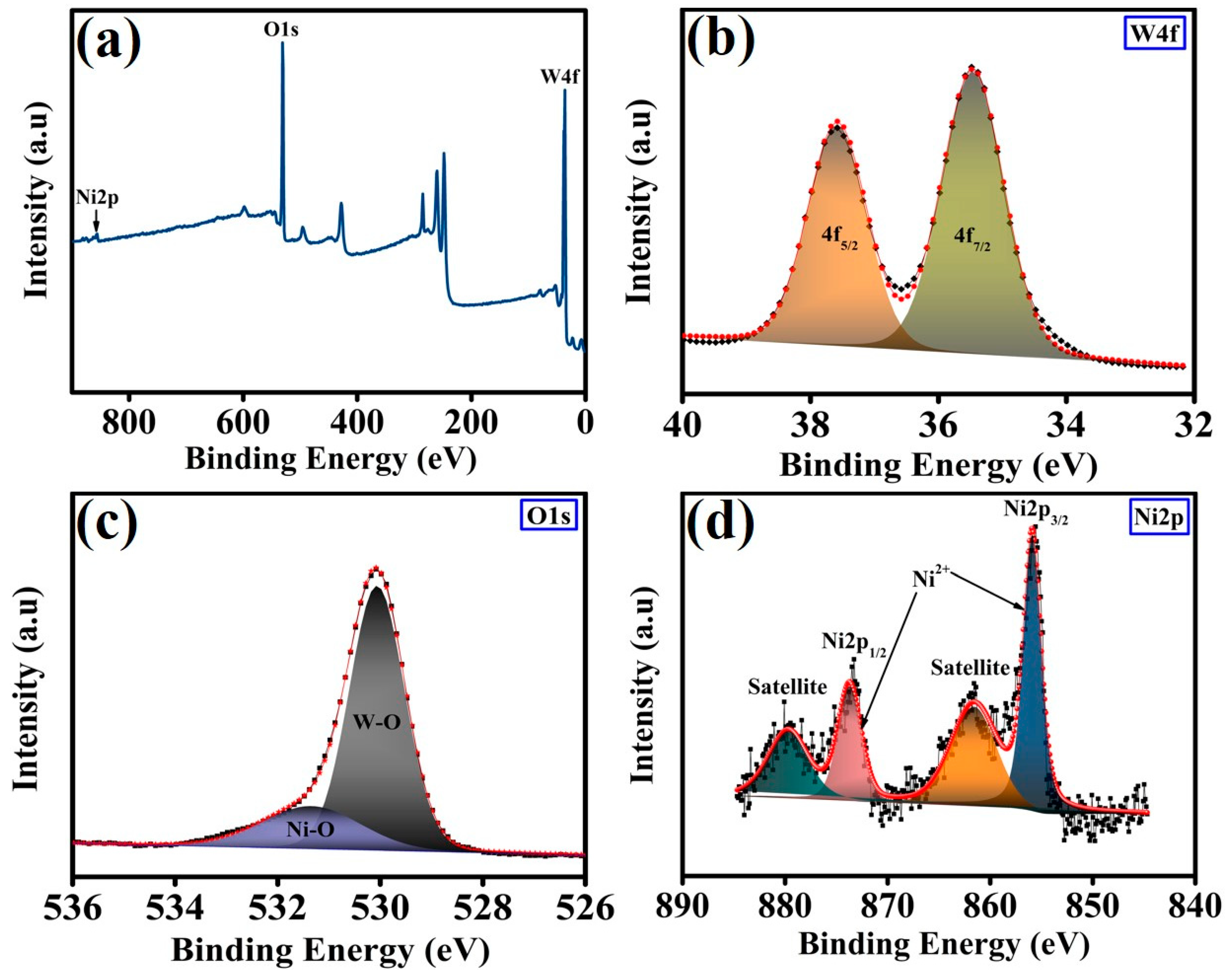
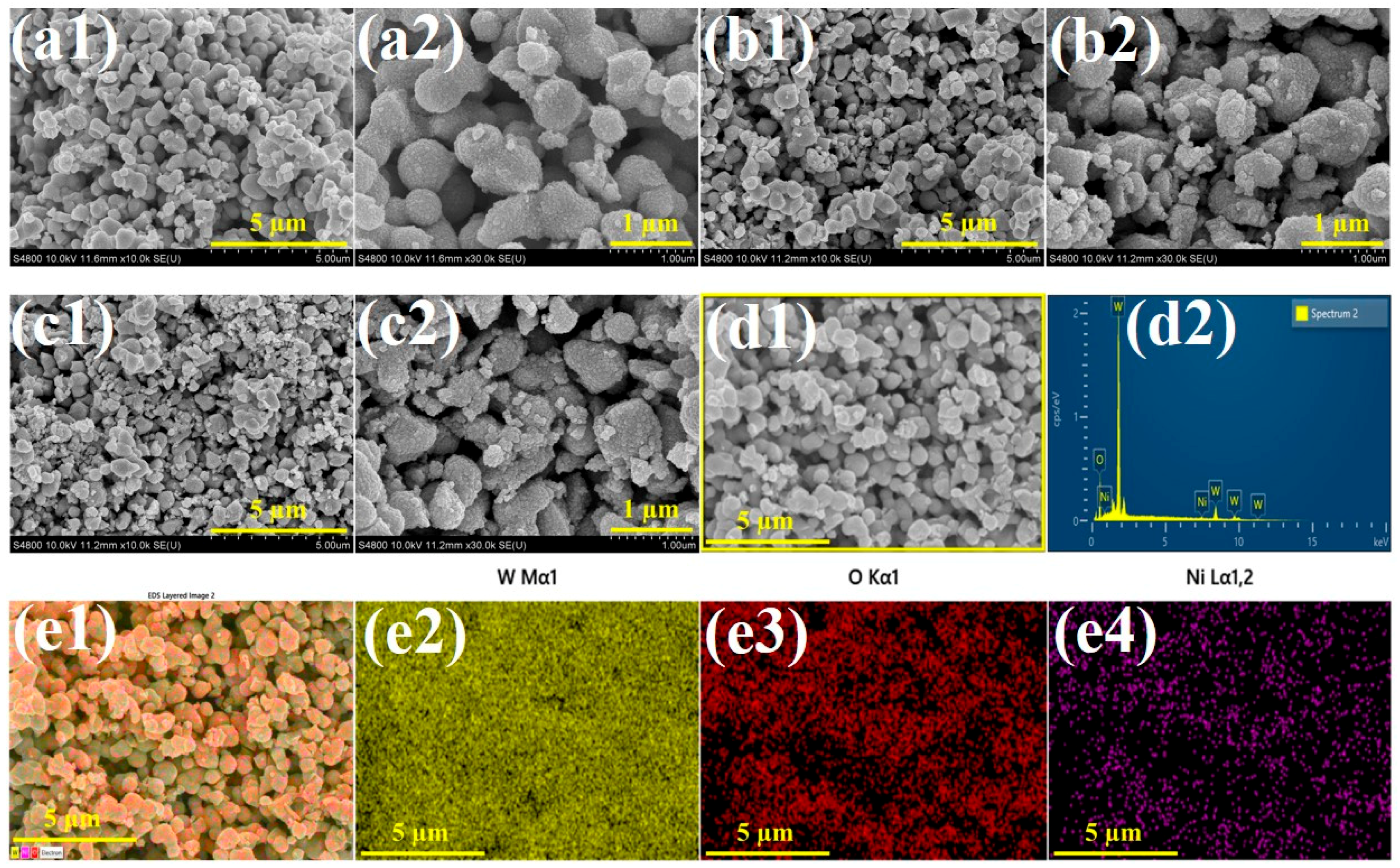
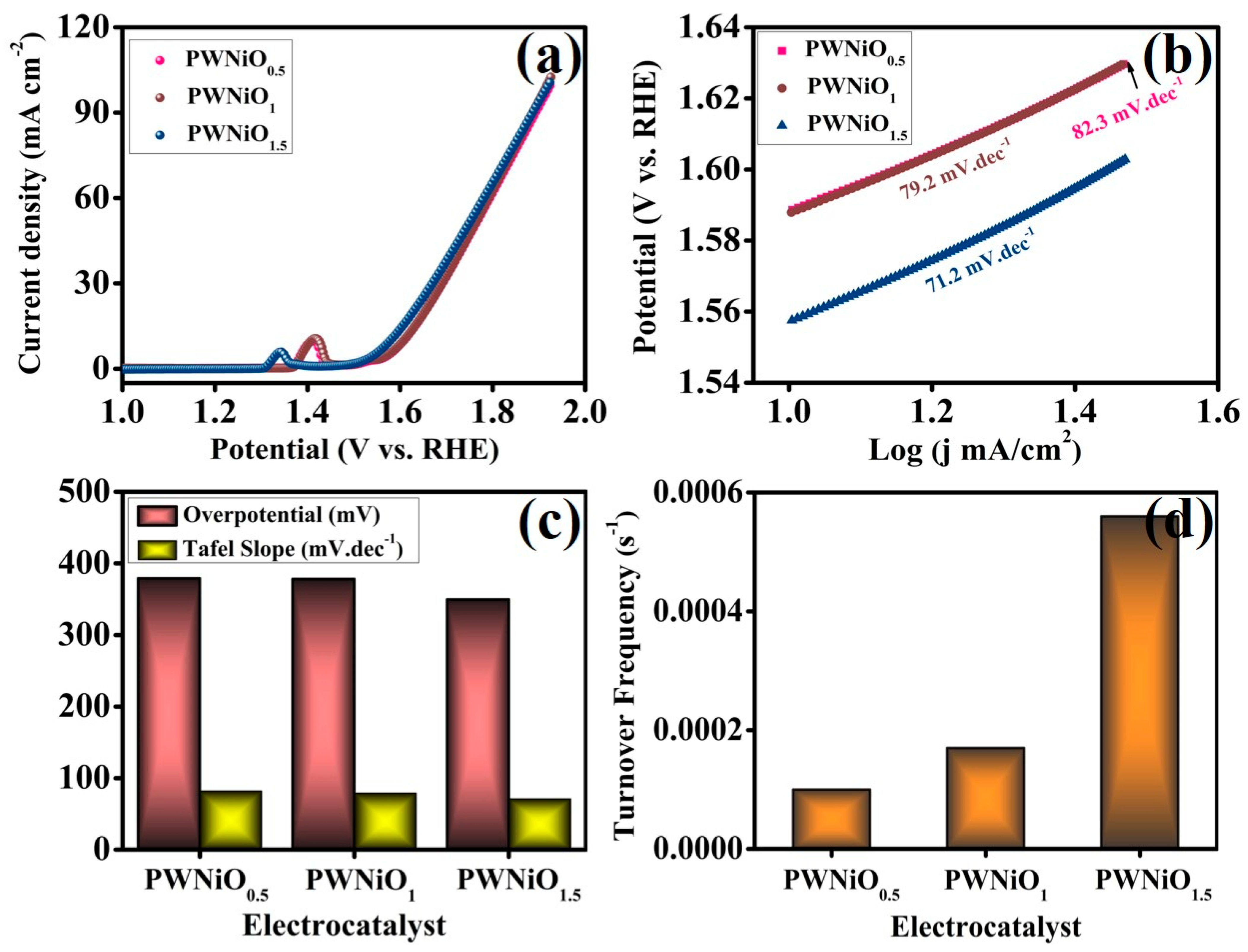
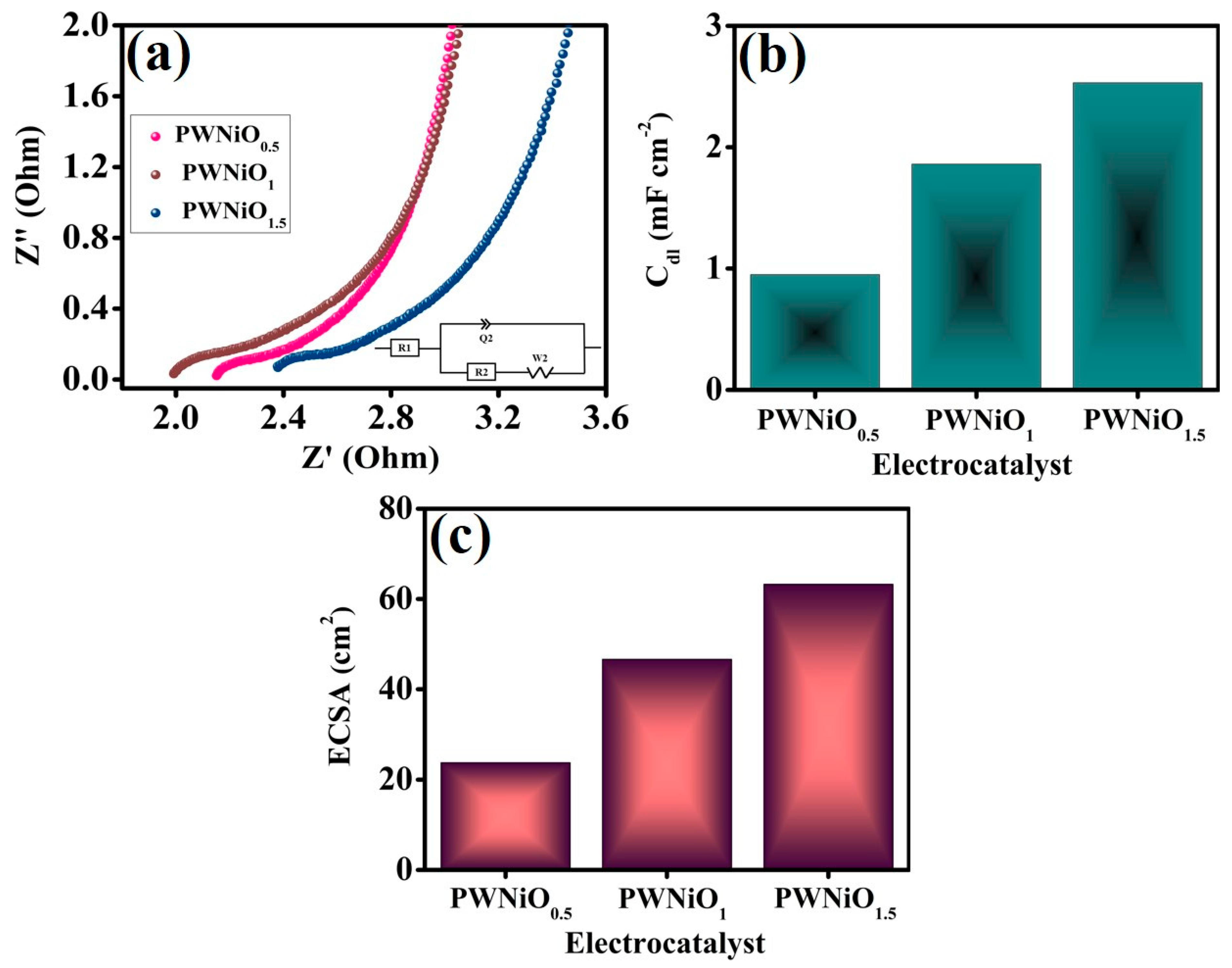
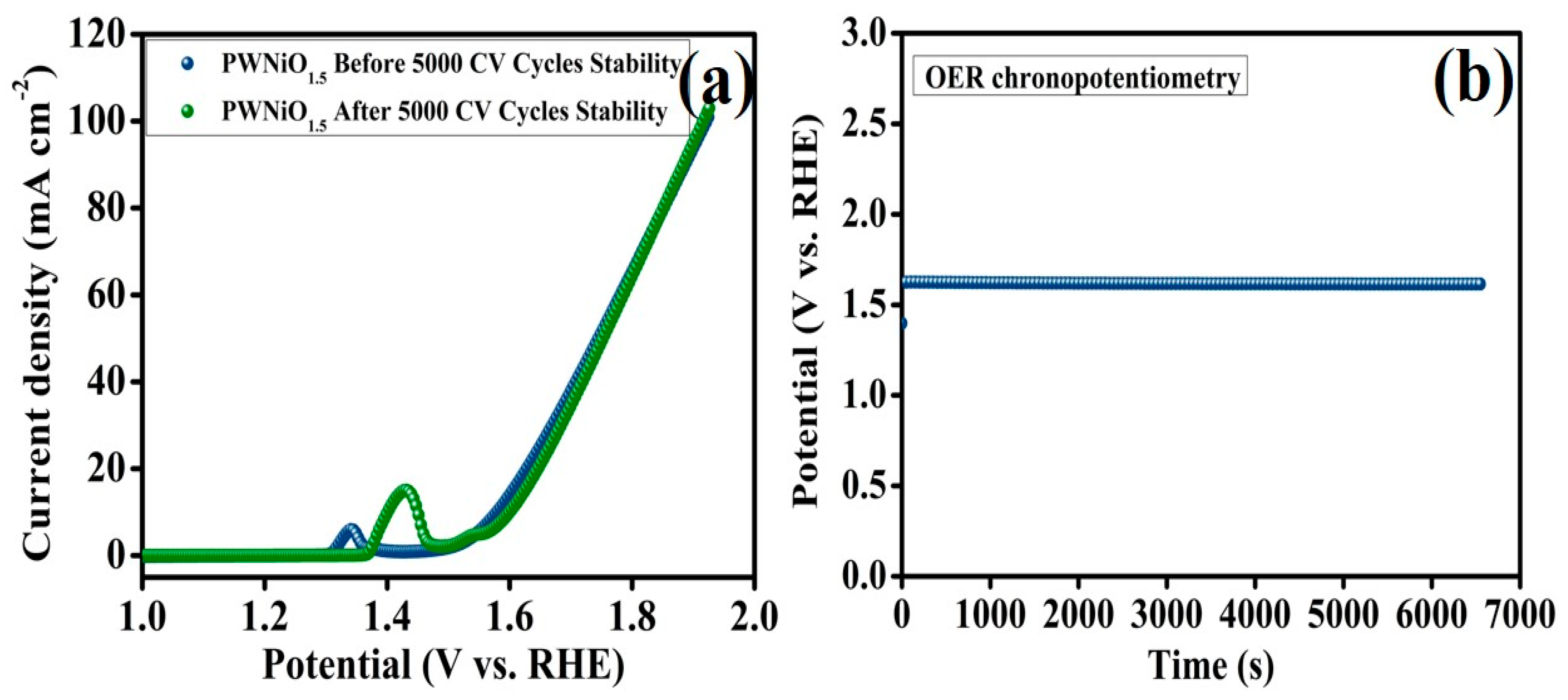
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arumugam, B.; Siddharthan, E.E.; Mannu, P.; Thapa, R.; Dong, C.-L.; Jeffery, A.A.; Kim, S.-C. Regulating the electronic structure of CoMoO4 via La doping for efficient and durable electrochemical water splitting reactions. J. Mater. Chem. A 2025, 13, 6749–6767. [Google Scholar] [CrossRef]
- Jang, J.-H.; Jeffery, A.A.; Min, J.; Jung, N.; Yoo, S.J. Emerging carbon shell-encapsulated metal nanocatalysts for fuel cells and water electrolysis. Nanoscale 2021, 13, 15116–15141. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O. The origin of ideas on a hydrogen economy and its solution to the decay of the environment. Int. J. Hydrogen Energy 2002, 27, 731–740. [Google Scholar] [CrossRef]
- Teli, A.M.; Mane, S.M.; Beknalkar, S.A.; Mishra, R.K.; Jeon, W.; Shin, J.C. Development of Electrochemical Water Splitting with Highly Active Nanostructured NiFe Layered Double Hydroxide Catalysts: A Comprehensive Review. Catalysts 2025, 15, 293. [Google Scholar] [CrossRef]
- Khalid, N.; Rehman, A.; Pervaiz, E. Electrochemical pathway to enhanced water splitting efficiency through cobalt Phosphide-MOFs hybrid. Int. J. Hydrogen Energy 2024, 73, 231–256. [Google Scholar] [CrossRef]
- Xue, R.; Deng, R.; Li, Y.; Gao, M.; Wang, J.; Zhang, Q. Deep eutectic solvent-induced controllable synthesis of bifunctional Ni–Fe–P catalysts for electrochemical water splitting. Green Chem. Eng. 2025, 6, 93–101. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Li, R.; Fan, W.; Rao, P.; Luo, J.; Li, J.; Deng, P.; Wu, D.; Huang, W.; Jia, C.; Liu, Z.; et al. Multimetallic Single-Atom Catalysts for Bifunctional Oxygen Electrocatalysis. ACS Nano 2023, 17, 18128–18138. [Google Scholar] [CrossRef]
- Wondimu, T.H.; Bayeh, A.W.; Kabtamu, D.M.; Xu, Q.; Leung, P.; Shah, A.A. Recent progress on tungsten oxide-based materials for the hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2022, 47, 20378–20397. [Google Scholar] [CrossRef]
- Chae, M.J.; Jung, H.Y.; Suh, S.J. Cyclic Voltammetry-Assisted enhancement of water splitting activity in nanostructured WO3 catalysts through structural evolution and Pt substitution. Appl. Surf. Sci. 2024, 680, 161304. [Google Scholar] [CrossRef]
- Sharma, L.; Kumar, P.; Halder, A. Phase and Vacancy Modulation in Tungsten Oxide: Electrochemical Hydrogen Evolution. ChemElectroChem 2019, 6, 3420–3428. [Google Scholar] [CrossRef]
- Sekar, S.; Ahmed, A.T.A.; Pawar, S.M.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl. Surf. Sci. 2020, 508, 145127. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Liu, P.; Zhu, X.; Li, X.; Ali, R.N.; Xiang, B. Enhanced electrocatalytic activity of WO3@NPRGO composite in a hydrogen evolution reaction. Appl. Surf. Sci. 2018, 463, 275–282. [Google Scholar] [CrossRef]
- Yu, S.; Yu, X.; Yang, H.; Li, F.; Li, S.; Kang, Y.S.; Zheng, J.Y. Mechanism, modification and stability of tungsten oxide-based electrocatalysts for water splitting: A review. J. Energy Chem. 2024, 99, 23–49. [Google Scholar] [CrossRef]
- Liu, F.; Feng, Z.; Zhang, X.; Cui, L.; Liu, J. One-step achievement of Fe-doped and interfacial Ru nanoclusters co-engineered Ni(OH)2 electrocatalyst on Ni foam for promoted oxygen evolution reaction. J. Colloid Interface Sci. 2023, 638, 498–505. [Google Scholar] [CrossRef]
- Li, Y.; Bao, X.; Chen, D.; Wang, Z.; Dewangan, N.; Li, M.; Xu, Z.; Wang, J.; Kawi, S.; Zhong, Q. A Minireview on Nickel-Based Heterogeneous Electrocatalysts for Water Splitting. ChemCatChem 2019, 11, 5913–5928. [Google Scholar] [CrossRef]
- Kitiphatpiboon, N.; Chen, M.; Li, X.; Liu, C.; Li, S.; Wang, J.; Peng, S.; Abudula, A.; Guan, G. Heterointerface engineering of Ni3S2@NiCo-LDH core-shell structure for efficient oxygen evolution reaction under intermittent conditions. Electrochim. Acta 2022, 435, 141438. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, L.; Li, L.; Yang, W.; Zou, H.; Chen, S. Effect of PEG on Performance of NiMnO Catalyst for Hydrogen Evolution Reaction. Front. Chem. 2020, 8, 281. [Google Scholar] [CrossRef]
- Babar, P.; Lokhande, A.; Gang, M.; Pawar, B.; Pawar, S.; Kim, J.H. Thermally oxidized porous NiO as an efficient oxygen evolution reaction (OER) electrocatalyst for electrochemical water splitting application. J. Ind. Eng. Chem. 2018, 60, 493–497. [Google Scholar] [CrossRef]
- Chen, Y.; Rui, K.; Zhu, J.; Dou, S.X.; Sun, W. Recent Progress on Nickel-Based Oxide/(Oxy)Hydroxide Electrocatalysts for the Oxygen Evolution Reaction. Chem. A Eur. J. 2019, 25, 703–713. [Google Scholar] [CrossRef]
- Manjunath, V.; Bimli, S.; Biswas, R.; Didwal, P.N.; Haldar, K.K.; Mahajan, M.; Deshpande, N.G.; Bhobe, P.A.; Devan, R.S. Experimental investigations on morphology controlled bifunctional NiO nano-electrocatalysts for oxygen and hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 39018–39029. [Google Scholar] [CrossRef]
- Mondal, A.; Paul, A.; Srivastava, D.N.; Panda, A.B. NiO hollow microspheres as efficient bifunctional electrocatalysts for Overall Water-Splitting. Int. J. Hydrogen Energy 2018, 43, 21665–21674. [Google Scholar] [CrossRef]
- Shaghaghi, Z.; Akbari, S. Hydrogen and oxygen production on Ag2O/NiO hybrid nanostructures via electrochemical water splitting. Int. J. Hydrogen Energy 2023, 51, 936–949. [Google Scholar] [CrossRef]
- Pan, U.N.; Singh, T.I.; Paudel, D.R.; Gudal, C.C.; Kim, N.H.; Lee, J.H. Covalent doping of Ni and P on 1T-enriched MoS2 bifunctional 2D-nanostructures with active basal planes and expanded interlayers boosts electrocatalytic water splitting. J. Mater. Chem. A 2020, 8, 19654–19664. [Google Scholar] [CrossRef]
- Guo, J.; Wei, Z.; Wang, K.; Zhang, H. Synergistic coupling of CoFe-layered double hydroxide nanosheet arrays with reduced graphene oxide modified Ni foam for highly efficient oxygen evolution reaction and hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 27529–27542. [Google Scholar] [CrossRef]
- Wang, P.; Yu, Y.; Yan, Y.; Qin, B.; Ye, Z.; Zhong, W.; Cai, W.; Zheng, X. N plasma assisted Fe doped NiCo nanosheet arrays for alkaline electrocatalytic oxygen evolution. J. Alloys Compd. 2023, 941, 168954. [Google Scholar] [CrossRef]
- Tang, C.; Zhong, L.; Xiong, R.; Xiao, Y.; Cheng, B.; Lei, S. Regulable in-situ autoredox for anchoring synergistic Ni/NiO nanoparticles on reduced graphene oxide with boosted alkaline electrocatalytic oxygen evolution. J. Colloid Interface Sci. 2023, 648, 181–192. [Google Scholar] [CrossRef]
- Bhosale, M.; Thangarasu, S.; Magdum, S.S.; Jeong, C.; Oh, T.-H. Enhancing the electrocatalytic performance of vanadium oxide by interface interaction with rGO and NiO nanostructures for electrochemical water oxidation. Int. J. Hydrogen Energy 2023, 54, 1449–1460. [Google Scholar] [CrossRef]
- Mohan, L.; Avani, A.; Kathirvel, P.; Marnadu, R.; Packiaraj, R.; Joshua, J.R.; Nallamuthu, N.; Shkir, M.; Saravanakumar, S. Investigation on structural, morphological and electrochemical properties of Mn doped WO3 nanoparticles synthesized by co-precipitation method for supercapacitor applications. J. Alloys Compd. 2021, 882, 160670. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Chavan, G.T.; Teli, A.M.; Dalavi, D.S.; Jeon, C.-W. Improved electrochromic performance of potentiostatically electrodeposited nanogranular WO3 thin films. J. Alloys Compd. 2023, 945, 169363. [Google Scholar] [CrossRef]
- Balčiūnaitė, A.; Upadhyay, K.K.; Radinović, K.; Santos, D.M.F.; Montemor, M.F.; Šljukić, B. Steps towards highly-efficient water splitting and oxygen reduction using nanostructured β-Ni(OH)2. RSC Adv. 2022, 12, 10020–10028. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, X.; Gao, Y.; Liu, Z. Co/Cu-modified NiO film grown on nickel foam as a highly active and stable electrocatalyst for overall water splitting. Dalton Trans. 2020, 49, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, W.; Liu, J.; Lou, G.; Zhang, C.; Liu, Y.; Li, J. Controlling crystallographic orientation in h-WO3 films to maximize photoelectrochemical water splitting efficiency. J. Mater. Chem. A 2025, 13, 7284–7294. [Google Scholar] [CrossRef]
- Baby, N.; Murugan, N.; Thangarasu, S.; Kim, Y.A.; Oh, T.-H. Synergistic insights into the electrocatalytic mechanisms of ZIF-derived Co3S4 on 1T-WS2/WO3 for electrochemical water splitting. Int. J. Hydrogen Energy 2024, 94, 1005–1017. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Teli, A.M.; Beknalkar, S.A.; Jeon, C.-W. Exploring electrochromic performance via layered deposition of tungsten oxide on niobium oxide composite electrode. J. Power Sources 2024, 613, 234930. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Jiao, Y.; Wang, Z.; Lu, Z.; Vasileff, A.; Qiao, S. NiO as a Bifunctional Promoter for RuO2 toward Superior Overall Water Splitting. Small 2018, 14, e1704073. [Google Scholar] [CrossRef]
- Hu, Q.; Li, W.; Abouelamaiem, D.I.; Xu, C.; Jiang, H.; Han, W.; He, G. Hollow Cu-doped NiO microspheres as anode materials with enhanced lithium storage performance. RSC Adv. 2019, 9, 20963–20967. [Google Scholar] [CrossRef]
- Yu, M.; Budiyanto, E.; Tüysüz, H. Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar] [CrossRef]
- Zhai, Y.; Ren, X.; Sun, Y.; Li, D.; Wang, B.; Liu, S. Synergistic effect of multiple vacancies to induce lattice oxygen redox in NiFe-layered double hydroxide OER catalysts. Appl. Catal. B Environ. 2022, 323, 122091. [Google Scholar] [CrossRef]
- Roy, S.S.; Karmakar, A.; Madhu, R.; Nagappan, S.; Dhandapani, H.N.; Kundu, S. Three-Dimensional Sm-Doped NiCu-LDH on Ni Foam as a Highly Robust Bifunctional Electrocatalyst for Total Water Splitting. ACS Appl. Energy Mater. 2023, 6, 8818–8829. [Google Scholar] [CrossRef]
- Pitchai, C.; Chen, C.-M. Electrochemically enhanced oxygen evolution and urea oxidation reactions with advanced high-entropy LDH nanoneedles. Sustain. Energy Fuels 2025, 9, 1829–1838. [Google Scholar] [CrossRef]
- Hanan, A.; Shu, D.; Aftab, U.; Cao, D.; Laghari, A.J.; Solangi, M.Y.; Abro, M.I.; Nafady, A.; Vigolo, B.; Tahira, A.; et al. Co2FeO4@rGO composite: Towards trifunctional water splitting in alkaline media. Int. J. Hydrogen Energy 2022, 47, 33919–33937. [Google Scholar] [CrossRef]
- Ma, L.; Sui, S.; Zhai, Y. Preparation and characterization of Ir/TiC catalyst for oxygen evolution. J. Power Sources 2008, 177, 470–477. [Google Scholar] [CrossRef]
- Jo, S.G.; Kim, C.-S.; Kim, S.J.; Lee, J.W. Phase-Controlled NiO Nanoparticles on Reduced Graphene Oxide as Electrocatalysts for Overall Water Splitting. Nanomaterials 2021, 11, 3379. [Google Scholar] [CrossRef]
- Wang, C.P.; Kong, L.J.; Sun, H.; Zhong, M.; Cui, H.J.; Zhang, Y.H.; Wang, D.H.; Zhu, J.; Bu, X.H. Carbon Layer Coated Ni3S2/MoS2 Nanohybrids as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. ChemElectroChem 2019, 6, 5603–5609. [Google Scholar] [CrossRef]
- Tian, L.; Liu, H.; Zhang, B.; Liu, Y.; Lv, S.; Pang, L.; Li, J. Ni and CeO2 Nanoparticles Anchored on Cicada-Wing-like Nitrogen-Doped Porous Carbon as Bifunctional Catalysts for Water Splitting. ACS Appl. Nano Mater. 2021, 5, 1252–1262. [Google Scholar] [CrossRef]
- Liang, D.; Zhang, H.; Ma, X.; Liu, S.; Mao, J.; Fang, H.; Yu, J.; Guo, Z.; Huang, T. MOFs-derived core-shell Co3Fe7@Fe2N nanopaticles supported on rGO as high-performance bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Mater. Today Energy 2020, 17, 100433. [Google Scholar] [CrossRef]
- Upadhyay, S.; Mir, R.A.; Pandey, O.P. Ni(OH)2 nanosheets and highly stable Ni(OH)2/GO nanocomposite for its improved OER performance. Int. J. Hydrogen Energy 2023, 48, 36687–36693. [Google Scholar] [CrossRef]
- Sprengel, S.; Amiri, M.; Bezaatpour, A.; Nouhi, S.; Baues, S.; Wittstock, G.; Wark, M. One-Pot Synthesis of Ni-MOF/Co-MOF Hybrid as Electrocatalyst for Oxygen Evolution Reaction. J. Electrochem. Soc. 2022, 169, 124504. [Google Scholar] [CrossRef]
- Sreekanth, T.; Dillip, G.; Nagajyothi, P.; Yoo, K.; Kim, J. Integration of Marigold 3D flower-like Ni-MOF self-assembled on MWCNTs via microwave irradiation for high-performance electrocatalytic alcohol oxidation and oxygen evolution reactions. Appl. Catal. B Environ. 2021, 285, 119793. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Ji, W.-B.; Hu, B.-C.; Liang, H.-W.; Xu, X.-X.; Yu, Z.-L.; Li, B.-Y.; Yu, S.-H. Partially oxidized Ni nanoparticles supported on Ni-N co-doped carbon nanofibers as bifunctional electrocatalysts for overall water splitting. Nano Energy 2018, 51, 286–293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, M.; Morankar, P.J.; Amate, R.U.; Jeon, C.-W. Rational Designing of NiO Nanoparticles Anchored with PEG-WO3 for Enhanced Water Oxidation Performance. Polymers 2025, 17, 1281. https://doi.org/10.3390/polym17091281
Bhosale M, Morankar PJ, Amate RU, Jeon C-W. Rational Designing of NiO Nanoparticles Anchored with PEG-WO3 for Enhanced Water Oxidation Performance. Polymers. 2025; 17(9):1281. https://doi.org/10.3390/polym17091281
Chicago/Turabian StyleBhosale, Mrunal, Pritam J. Morankar, Rutuja U. Amate, and Chan-Wook Jeon. 2025. "Rational Designing of NiO Nanoparticles Anchored with PEG-WO3 for Enhanced Water Oxidation Performance" Polymers 17, no. 9: 1281. https://doi.org/10.3390/polym17091281
APA StyleBhosale, M., Morankar, P. J., Amate, R. U., & Jeon, C.-W. (2025). Rational Designing of NiO Nanoparticles Anchored with PEG-WO3 for Enhanced Water Oxidation Performance. Polymers, 17(9), 1281. https://doi.org/10.3390/polym17091281






