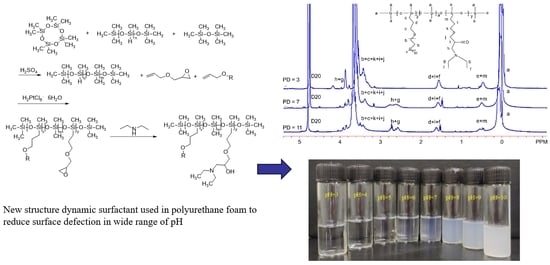
Polyether- and Tertiary Amine-Modified Silicone Surfactants: Synthesis and Surface Performance Across pH Ranges
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyether- and Tertiary Amine-Modified Silicone Surfactants
2.2.1. Synthesis of Low-Hydrogen Silicone Oil
2.2.2. Preparation of Polyether- and Epoxy-Modified Polysiloxane
2.2.3. Preparation of Polyether- and Tertiary Amine-Modified Silicone Surfactants
2.3. Structural Characterization
2.4. 1H NMR Spectroscopy at Different pH Values
2.5. Determination of Acid-Ionization Constant (pKa)
2.6. Static Surface Tension
2.7. Dynamic Surface Tension
2.8. Zeta Potential and Dynamic Light Scattering (DLS)
3. Results and Discussion
3.1. Acid-Ionization Constant (pKa) of PSiEO/(PO)-OH(CH3) Surfactants
3.2. 1H NMR Analysis at Different pH Values
3.3. Static Surface Tension of PSiEO/(PO)-OH(CH3) Surfactants
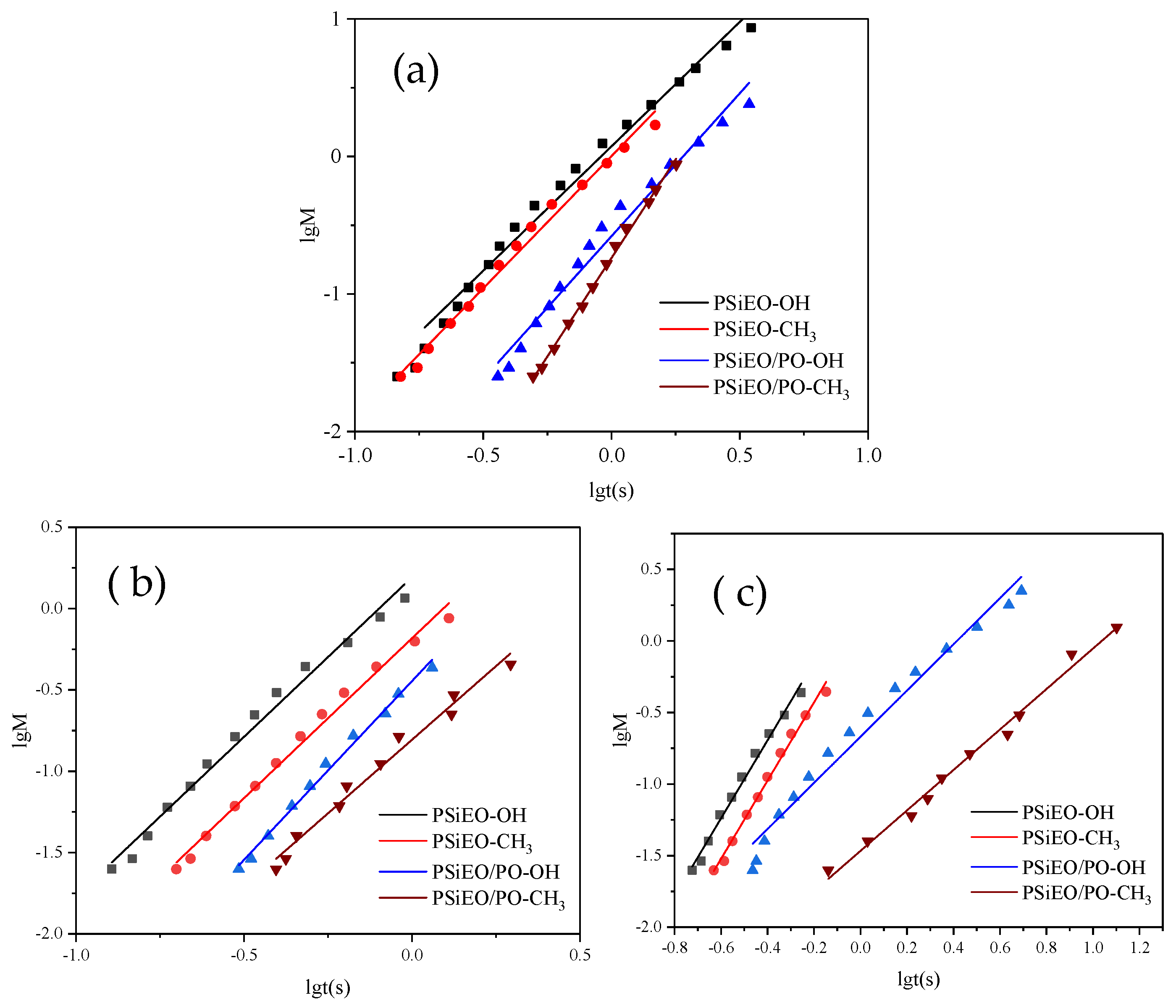
3.4. Dynamic Surface Tension of PSiEO/(PO)-OH(CH3) Surfactants
3.5. Thermodynamics of Micelles and Absorption of PSiEO/(PO)-OH(CH3) Surfactants
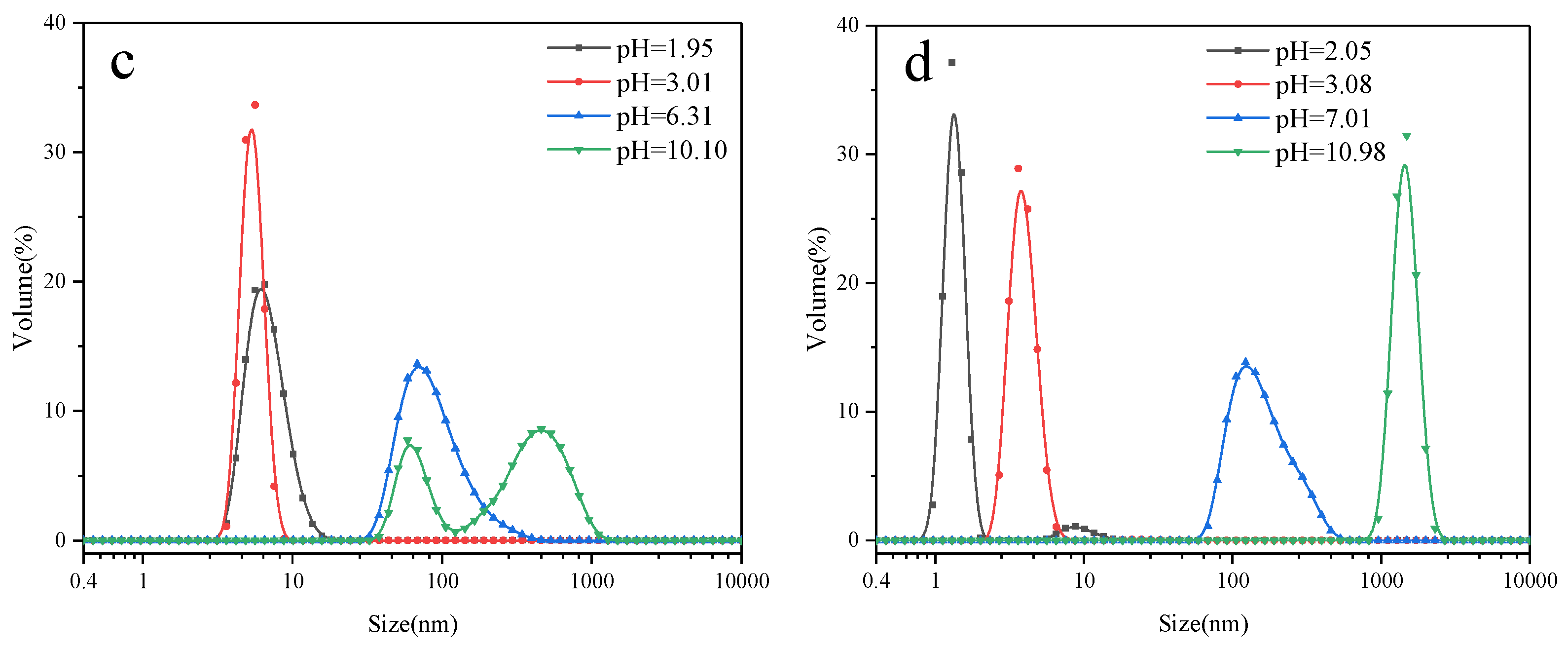
3.6. Aggregation Behavior of PSiEO/(PO)-OH(CH3) Surfactants
- At pH 3–5 (zeta potential > 30 mV): High electrostatic repulsion prevented aggregation (1–10 nm);
- At pH 6–8 (zeta potential 10–30 mV): Partial charge neutralization initiated micelle growth (10–100 nm);
- At pH > 9 (zeta potential < 10 mV): Electrostatic shielding allowed hydrophobic-driven aggregation (>100 nm).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Goddard, E.D.; Phillips, T.S.; Hannan, R.B. Water soluble polymer-surfactant interaction. Part I. J. Soc. Cosmet. Chem. 1975, 26, 461. [Google Scholar]
- Goddard, E.D.; Ananthapadmanabhan, K.P. (Eds.) Interactions of Surfactants with Polymers and Proteins; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Goddard, E.D.; Ananthapadmanabhan, K.P. Polymer-Surfactant Systems; Kwak, J.C.T., Ed.; Marcel Dekker: New York, NY, USA, 1998; pp. 21–64. [Google Scholar]
- Dickinson, E.; Rodríguez Patino, J.M. Food Emulsions and Foams: Interfaces, Interactions and Stability; Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Wüstneck, R.; Krägel. Characterisation of gelatine-surfactant interactions and its relevance to liquid film coating. J. Stud. Interface Sci. 1998, 7, 433. [Google Scholar]
- Cyboran-Mikołajczyk, S.; Bonarska-Kujawa, D.; Kleszczyńska, H.; Łuczyński. Effects of Interaction of Gemini Ester Quat Surfactants with Biological Membranes. J. Tenside Surfactants Deterg. 2016, 53, 20–28. [Google Scholar] [CrossRef]
- Robb, I.D. Anionic Surfactants: Physical Chemistry of Surfactant Action; Lucassen-Reynders, E.H., Ed.; Marcel Dekker: New York, NY, USA, 1981; pp. 109–142. [Google Scholar]
- Goddard, E.D. Polymer/Surfactant Interaction: Interfacial Aspects. J. Colloid Interface Sci. 2002, 256, 228. [Google Scholar] [CrossRef]
- Miller, R.; Fainerman, V.B.; Makievski, A.V.; Krägel, J.; Grigoriev, D.O.; Kazakov, V.N.; Sinyachenko, O.V. Dynamics of protein and mixed protein/surfactant adsorption layers at the water/fluid interface. Adv. Colloid Interface Sci. 2000, 86, 39. [Google Scholar] [CrossRef] [PubMed]
- Yao, E.D.; Liang, T.B.; Li, Y.; Sun, J.X.; Zhou, F.J. Development of a New Multifunctional Cationic Surfactant System with Corrosion Inhibiting Ability. Geofluids 2017, 2849356. [Google Scholar] [CrossRef]
- Liao, S.; Jia, S.C.; Yue, Y.H.; Zeng, H.; Lin, J.J.; Liu, P. Advancements in pH-Responsive nanoparticles for osteoarthritis treatment: Opportunities and challenges. Front. Bioeng. Biotechnol. 2024, 12, 1426794. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Eastoe, J.; Dalton, J.S. Dynamic surface tension and adsorption mechanisms of surfactants at the air–water interface. Adv. Colloid Interface Sci. 2000, 85, 103. [Google Scholar] [CrossRef]
- Moosavi, S.S.; Zolghadr, A.R. Structural Transitions of Anionic, Cationic, and Nonionic Surfactant Solutions Confined between Amorphous SiO2 Slabs: Molecular Dynamics Simulations. Ind. Eng. Chem. Res. 2022, 61, 50. [Google Scholar] [CrossRef]
- Taylor, D.J.F.; Thomas, R.K.; Penfold, J. Polymer/surfactant interactions at the air/water interface. J. Adv. Colloid Interface Sci. 2007, 132, 69. [Google Scholar] [CrossRef]
- Mortensen, K. Phase behaviour of poly (ethylene oxide)-poly (propyleneoxide)-poly (ethylene oxide) triblock-copolymer dissolved in water. Europhys. Lett. 1992, 19, 599. [Google Scholar] [CrossRef]
- Szymańska, M.; Hoppe, J.; Dutkiewicz, M.; Sobolewski, P.; Palacz, M.; Janus, E.; Zielińska, B.; Drozd, R. Silicone polyether surfactant enhances bacterial cellulose synthesis and water holding capacity. Int. J. Biol. Macromol. 2022, 208, 642. [Google Scholar] [CrossRef]
- Dworakowska, S.; Bogdał, D.; Zaccheria, F.; Ravasio, N. The role of catalysis in the synthesis of polyurethane foams based on renewable raw materials. Catal. Today 2014, 223, 148. [Google Scholar] [CrossRef]
- Wu, H.R.; Chen, X.; Tan, R.; Luo, Y.L.; Hu, Y.; Li, Y.C.; Hou, J.R.; Kang, W.L. Controllable regulation of emulsion stability by a pH-responsive zwitterionic/anionic surfactant system. Feul 2022, 312, 122921. [Google Scholar] [CrossRef]
- Pukale, D.D.; Bansode, A.S.; Jadhav, N.L.; Pinjari, D.V.; Kulkarni, R.R. Review on Silicone Surfactants: Silicone-based Gemini Surfactants, Physicochemical Properties and Applications. Tenside Surfactants Deterg. 2019, 56, 268. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, F.; Luan, Y.; Cao, W.; Ji, X.; Zhao, L.; Zhang, L.; Li, Z. Formation of drug/surfactant catanionic vesicles and their application in sustained drug release. Int. J. Pharm. 2012, 436, 806. [Google Scholar] [CrossRef] [PubMed]
- Šegota, S.; Težak, D. Spontaneous formation of vesicles. Adv. Colloid Interface Sci. 2006, 121, 51. [Google Scholar] [CrossRef] [PubMed]
- Zana, R. Alkanediyl-α, ω-bis (dimethylalkylammonium bromide) surfactants. 10. Behavior in aqueous solution at concentrations below the critical micellization concentration: An electrical conductivity study. J. Colloid Interface Sci. 2002, 246, 182. [Google Scholar] [CrossRef]
- Chung, D.-W.; Lim, J.C. Study on the effect of structure of polydimethylsiloxane grafted with polyethyleneoxide on surface activities. Colloids Surf. A 2009, 336, 35. [Google Scholar] [CrossRef]
- Venzmer, J. Superspreading-20 years of physicochemical research. Curr. Opin. Colloid Interface Sci. 2011, 16, 335. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, F.; Jia, Q.; Wang, Q. Norm descriptors for predicting the hydrophile-lipophile balance (HLB) and critical micelle concentration (CMC) of anionic surfactants. Colloids Surf. A 2019, 583, 123967. [Google Scholar] [CrossRef]
- Zana, R.; Xia, J. (Eds.) Gemini Surfactants: Synthesis, Interfacial and Solution Phase Behavior & Applications; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Rosen, M.J.; Zhu, Z.H.; Gu, B.; Murphy, D.S. Relationship of structure to properties of surfactants. 14. Some N-Alkyl-2-pyrrolidones at Various Interfaces. Langmuir 1988, 4, 1273. [Google Scholar] [CrossRef]
- Cai, B.; Li, X.; Yang, Y.; Dong, J. Surface properties of Gemini surfactants with pyrrolidinium head groups. J. Colloid Interface Sci. 2012, 370, 111. [Google Scholar] [CrossRef]
- Castro, M.J.L.; Kovensky, J.; Cirelli, A.F. New family of nonionic Gemini surfactants. Determination and analysis of interfacial properties. Langmuir 2002, 18, 2477. [Google Scholar] [CrossRef]
- Klasner, S.A.; Metto, E.C.; Roman, G.T.; Culbertson, C.T. Synthesis and Characterization of a Poly (Dimethylsiloxane)- Poly (Ethylene Oxide) Block Copolymer for Fabrication of Amphiphilic Surfaces on Micorfluidic Devices. Langmuir 2009, 25, 10390. [Google Scholar] [CrossRef] [PubMed]
- Myers, D. Surfactant Science and Technology, 2nd ed.; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Chernyshev, V.S.; Chuprov-Netochin, R.N.; Tsydenzhapova, E.; Van Devener, B.; Leonov, S.; Gorin, D.; Skliar, M. Measurements of extracellular vesicle concentration by a dynamic surface tension probe. Biochem. Biophys. Res. Commun. 2022, 609, 189. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Hung, S.-H.; Tseng, W.-C.; Tsay, R.-Y.; Noskov, B.; Lin, S.-Y. The effect of buffer concentration on the dynamic/equilibrium surface tension of bovine serum albumin solution. J. Mol. Liq. 2023, 381, 121837. [Google Scholar] [CrossRef]
- Le, T.T.-Y.; Tsay, R.-Y.; Lin, S.-Y. A study on the dynamic surface tension of surfactant solutions at dilute concentrations. J. Mol. Liq. 2021, 324, 115112. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 2nd ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Paula, S.; Sues, W.; Tuchtenhagen, J.; Blume, A. Thermodynamics of Micelle Formation as a Function of Temperature: A High Sensitivity Titration Calorimetry Study. J. Phys. Chem. 1995, 99, 11742. [Google Scholar] [CrossRef]
- Harwell, J.H.; Roberts, B.L.; Scamehorn, J.F. Thermodynamics of adsorption of surfactant mixtures on minerals. Colloids Surf. 1988, 32, 1. [Google Scholar] [CrossRef]
- Liu, G.; Gu, D.; Liu, H.; Ding, W.; Luan, H.; Lou, Y. Thermodynamic properties of micellization of Sulfobetaine-type Zwitterionic Gemini Surfactants in aqueous solutions—A free energy perturbation study. J. Colloid Interface Sci. 2012, 375, 148. [Google Scholar] [CrossRef] [PubMed]






| Surfactant | pKa |
|---|---|
| PSiEO-OH | 4.40/8.55 |
| PSiEO-CH3 | 4.19/8.60 |
| PSiEO/PO-CH3 | 4.35/8.07 |
| PSiEO/PO-OH | 4.30/8.19 |
| Surfactant | pH | CMC [g L−1] | γCMC [mN m−1] | pC20 | Γmax [μmoL m−2] | Amin [nm2] |
|---|---|---|---|---|---|---|
| PSiEO-OH | 3.0 | 0.48 | 29.83 | 1.28 | 1.71 | 0.97 |
| 7.0 | 0.38 | 28.22 | 1.47 | 2.00 | 0.83 | |
| 11.0 | 0.33 | 25.56 | 1.66 | 2.21 | 0.75 | |
| PSiEO-CH3 | 3.0 | 0.60 | 29.20 | 1.36 | 1.34 | 1.23 |
| 7.0 | 0.33 | 27.44 | 1.60 | 1.66 | 1.00 | |
| 11.0 | 0.24 | 24.50 | 1.74 | 2.09 | 0.79 | |
| PSiEO/PO-OH | 3.0 | 0.37 | 27.80 | 1.63 | 1.87 | 0.89 |
| 7.0 | 0.14 | 25.26 | 1.81 | 2.47 | 0.67 | |
| 11.0 | 0.11 | 24.39 | 1.85 | 2.69 | 0.62 | |
| PSiEO/PO-CH3 | 3.0 | 0.42 | 28.06 | 1.52 | 1.64 | 1.01 |
| 7.0 | 0.13 | 27.57 | 1.65 | 2.70 | 0.62 | |
| 11.0 | 0.12 | 22.95 | 1.80 | 3.41 | 0.49 |
| pH | Surfactant | γm [mN m−1] | n | t* [s] | R1/2 [mN m−1 s−1] |
|---|---|---|---|---|---|
| 3.0 | PSiEO-OH | 35.1 | 1.81 | 0.72 | 25.6 |
| PSiEO-CH3 | 33.8 | 1.92 | 1.00 | 19.1 | |
| PSiEO/PO-OH | 31.6 | 2.08 | 1.56 | 12.9 | |
| PSiEO/PO-CH3 | 32.7 | 2.87 | 1.81 | 10.9 | |
| 7.0 | PSiEO-OH | 31.4 | 1.96 | 0.80 | 25.4 |
| PSiEO-CH3 | 29.2 | 1.97 | 1.24 | 17.3 | |
| PSiEO/PO-OH | 27.1 | 2.20 | 1.90 | 11.8 | |
| PSiEO/PO-CH3 | 20.7 | 1.80 | 2.82 | 9.1 | |
| 11.0 | PSiEO-OH | 31.7 | 2.73 | 0.91 | 22.1 |
| PSiEO-CH3 | 28.7 | 2.73 | 1.39 | 15.6 | |
| PSiEO/PO-OH | 28.2 | 1.61 | 2.60 | 8.4 | |
| PSiEO/PO-CH3 | 20.2 | 1.41 | 10.90 | 2.4 |
| Surfactant | pH | [kJ mol−1] | [kJ mol−1] | [J mol−1 K−1] | [kJ mol−1] | [kJ mol−1] | [J mol−1 K−1] |
|---|---|---|---|---|---|---|---|
| PSiEO-OH | 3.0 | −23.61 | 13.72 | 104.36 | −21.15 | 9.96 | 125.20 |
| 7.0 | −26.42 | 7.90 | 106.83 | −24.23 | 7.62 | 115.11 | |
| 11.0 | −28.68 | 4.46 | 108.18 | −26.58 | 5.67 | 111.15 | |
| PSiEO-CH3 | 3.0 | −27.27 | 11.51 | 107.21 | −24.10 | 7.87 | 130.06 |
| 7.0 | −28.26 | 6.95 | 108.80 | −25.58 | 6.86 | 118.10 | |
| 11.0 | −30.63 | 3.56 | 111.32 | −28.37 | 4.82 | 114.65 | |
| PSiEO/PO-OH | 3.0 | −26.66 | 9.87 | 109.70 | −24.30 | 8.41 | 122.55 |
| 7.0 | −29.59 | 6.48 | 113.96 | −27.70 | 6.27 | 120.99 | |
| 11.0 | −33.08 | 3.49 | 117.31 | −31.30 | 3.67 | 122.67 | |
| PSiEO/PO-CH3 | 3.0 | −27.65 | 8.37 | 112.76 | −24.98 | 8.64 | 120.83 |
| 7.0 | −30.55 | 5.95 | 115.07 | −28.89 | 5.42 | 122.41 | |
| 11.0 | −34.53 | 3.20 | 119.62 | −33.09 | 2.58 | 126.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Yao, C. Polyether- and Tertiary Amine-Modified Silicone Surfactants: Synthesis and Surface Performance Across pH Ranges. Polymers 2025, 17, 1204. https://doi.org/10.3390/polym17091204
Guo Y, Yao C. Polyether- and Tertiary Amine-Modified Silicone Surfactants: Synthesis and Surface Performance Across pH Ranges. Polymers. 2025; 17(9):1204. https://doi.org/10.3390/polym17091204
Chicago/Turabian StyleGuo, Yi, and Cheng Yao. 2025. "Polyether- and Tertiary Amine-Modified Silicone Surfactants: Synthesis and Surface Performance Across pH Ranges" Polymers 17, no. 9: 1204. https://doi.org/10.3390/polym17091204
APA StyleGuo, Y., & Yao, C. (2025). Polyether- and Tertiary Amine-Modified Silicone Surfactants: Synthesis and Surface Performance Across pH Ranges. Polymers, 17(9), 1204. https://doi.org/10.3390/polym17091204