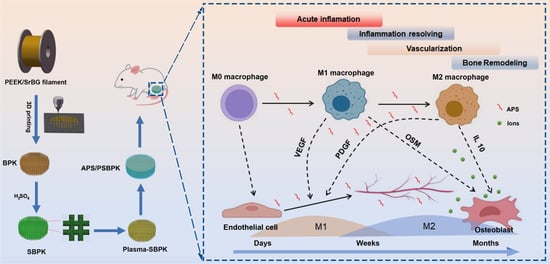
A Multifunctional PEEK Composite Scaffold with Immunomodulatory, Angiogenic, and Osteogenic Properties for Enhanced Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Composite Scaffolds
2.2. Characterization of Scaffolds
2.3. Cell Morphology and Proliferation
2.4. Alkaline Phosphatase (ALP) and Alizarin Red S Staining of rBMSCs
2.5. Flow Cytometry
2.6. In Vitro Angiogenic Activity
2.7. In Vivo Experiment
2.7.1. Subcutaneous Implantation Model
2.7.2. Rat Skull Defect Models
3. Results and Discussion
3.1. The Characterization of Scaffolds
3.2. In Vitro Mineralization and Ion/Drug Release
3.3. Effect of Scaffolds on Cell Proliferation and Osteogenic Differentiation of rBMSCs
3.4. Effect of the Scaffolds on Macrophage Polarization and Cytokine Secretion
3.5. Effects of the Scaffolds on the Angiogenic Activity of HUVECs
3.6. In Vivo Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Lu, Y.; Zhao, M.; Bosiakov, S.; Li, L. A Critical Review of Additive Manufacturing Techniques and Associated Biomaterials Used in Bone Tissue Engineering. Polymers 2022, 14, 2117. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, W.; Zhu, M.; Wu, C.; Zhu, Y. Bioceramic-Based Scaffolds with Antibacterial Function for Bone Tissue Engineering: A Review. Bioact. Mater. 2022, 18, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, J.; Wang, Y.; Wang, J.; Sun, Z.; Wei, F.; Wei, G.; Sun, Z. Recent Advances in the Design and Structural/Functional Regulations of Biomolecule-Reinforced Graphene Materials for Bone Tissue Engineering Applications. Small Sci. 2025, 5, 2400414. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, A.; Bai, J.; Liao, Q.; Wu, Y.; Zhang, W.; Guan, M.; Tong, L.; Geng, D.; Zhao, X.; et al. A Programmed Surface on Polyetheretherketone for Sequentially Dictating Osteoimmunomodulation and Bone Regeneration to Achieve Ameliorative Osseointegration under Osteoporotic Conditions. Bioact. Mater. 2022, 14, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, D.; Li, M.; Liu, X.; Zhang, Y.; Qian, S.; Peng, F. Osteogenesis, Angiogenesis and Immune Response of Mg-Al Layered Double Hydroxide Coating on Pure Mg. Bioact. Mater. 2021, 6, 91–105. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hu, Y.; Jing, Y.; Geng, Z.; Su, J. Bone Repair Materials: A Perspective from Immunomodulation. Adv. Funct. Mater. 2022, 32, 2208639. [Google Scholar] [CrossRef]
- Su, N.; Villicana, C.; Yang, F. Immunomodulatory Strategies for Bone Regeneration: A Review from the Perspective of Disease Types. Biomaterials 2022, 286, 121604. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal Stem Cell-Macrophage Crosstalk and Bone Healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving Concepts in Bone–Immune Interactions in Health and Disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Ma, H.; Han, H.; Zhao, X.; Ma, J.; Qu, X.; Lou, X.; Suonan, A.; Lei, B.; Zhang, Y. Engineering Multifunctional Polyether Ether Ketone Implant: Mechanics-Adaptability, Biomineralization, Immunoregulation, Anti-Infection, Osteointegration, and Osteogenesis. Adv. Healthc. Mater. 2023, 12, e2202799. [Google Scholar] [CrossRef]
- Kohli, N.; Ho, S.; Brown, S.J.; Sawadkar, P.; Sharma, V.; Snow, M.; García-Gareta, E. Bone Remodelling In Vitro: Where Are We Headed? A Review on the Current Understanding of Physiological Bone Remodelling and Inflammation and the Strategies for Testing Biomaterials In Vitro. Bone 2018, 110, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the Development of Advanced Bone Biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, J.; Wen, J.; Zhang, X.; Cao, L.; Zeng, D.; Liu, X.; Jiang, X. Novel Vascular Strategies on Polyetheretherketone Modification in Promoting Osseointegration in Ovariectomized Rats. Mater. Des. 2021, 202, 109526. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, Q.; Zhang, S.; Zhang, C.; Wu, Z. Enhancing Bone Regeneration with a Novel Bioactive Glass-Functionalized Polyetheretherketone Scaffold by Regulating the Immune Microenvironment. Smart Mater. Med. 2024, 5, 92–105. [Google Scholar] [CrossRef]
- He, M.; Huang, Y.; Xu, H.; Feng, G.; Liu, L.; Li, Y.; Sun, D.; Zhang, L. Modification of Polyetheretherketone Implants: From Enhancing Bone Integration to Enabling Multi-modal Therapeutics. Acta Biomater. 2023, 12, e2202799. [Google Scholar]
- Gao, A.; Liao, Q.; Xie, L.; Wang, G.; Zhang, W.; Wu, Y.; Li, P.; Guan, M.; Pan, H.; Tong, L.; et al. Tuning the Surface Immunomodulatory Functions of Polyetheretherketone for Enhanced Osseointegration. Biomaterials 2020, 230, 119642. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Wang, F.; Lyu, Z.; Hong, Q.; Nie, B.; Wei, J.; Wang, Y.; Zhang, J.; Yue, B. Dual-Functional Polyetheretherketone Surface Modification for Regulating Immunity and Bone Metabolism. Chem. Eng. J. 2021, 426, 130806. [Google Scholar] [CrossRef]
- Qi, D.; Wang, N.; Wang, S.; Liu, L.; Zhu, S.; She, P.; Yue, X. High-Strength Porous Polyetheretherketone/Hydroxyapatite Composite for the Treatment of Bone Defect. Compos. Commun. 2023, 38, 101473. [Google Scholar] [CrossRef]
- Yuan, X.; Ouyang, L.; Luo, Y.; Sun, Z.; Yang, C.; Wang, J.; Liu, X.; Zhang, X. Multifunctional Sulfonated Polyetheretherketone Coating with Beta-Defensin-14 for Yielding Durable and Broad-Spectrum Antibacterial Activity and Osseointegration. Acta Biomater. 2019, 86, 323–337. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Lui, S.C.; Chong, E.Y.W.; Wu, G.; Zhao, X.; Wang, C.; Pan, H.; Cheung, K.M.C.; Wu, S.; et al. Plasma Surface Functionalized Polyetheretherketone for Enhanced Osseointegration at Bone-Implant Interface. ACS Appl. Mater. Interfaces 2016, 8, 3901–3911. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Yu, Y.; Zhang, Z.; Wu, W.; Shao, Z.; Li, C.; Feng, J.; Xue, L.; Chen, F. Research and Application of Medical Polyetheretherketone as Bone Repair Material. Macromol. Biosci. 2023, 23, e2300032. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, D.; Zhang, Y.; Luo, Y.; Yang, L.; Xu, X.; Luo, F. Multifunctional Modifications of Polyetheretherketone Implants for Bone Repair: A Comprehensive Review. Biomater. Adv. 2023, 154, 213607. [Google Scholar] [CrossRef]
- Dejob, L.; Toury, B.; Tadier, S.; Grémillard, L.; Gaillard, C.; Salles, V. Electrospinning of In Situ Synthesized Silica-Based and Calcium Phosphate Bioceramics for Applications in Bone Tissue Engineering: A Review. Acta Biomater. 2021, 123, 123–153. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Roki, N.; Fenn, M.B. Bioactive Glasses: Importance of Structure and Properties in Bone Regeneration. J. Mol. Struct. 2014, 1073, 24–30. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Tsigkou, O.; Li, S.; Porter, A.E.; Jones, J.R. Human Mesenchymal Stem Cells Differentiate into an Osteogenic Lineage in Presence of Strontium Containing Bioactive Glass Nanoparticles. Acta Biomater. 2019, 90, 373–392. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, F.; Huang, D.; Fu, X.; Li, X.; Chen, X. Strontium-Substituted Submicrometer Bioactive Glasses Modulate Macrophage Responses for Improved Bone Regeneration. ACS Appl. Mater. Inter. 2015, 8, 30747–30758. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, H.; Li, J.; Yang, Z.; Zhuan, X.; Li, X.; Chen, Y.; Huo, S.; Zhou, X. Zinc-doped Bioactive Glass-functionalized Polyetheretherketone to Enhance the Biological Response in Bone Regeneration. J. Biomed. Mater. Res. A 2024, 112, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Huo, S.; Lu, L.; Zhou, C.; Li, Z. Magnesium Bioactive Glass Hybrid Ffunctionalized Polyetheretherketone with Immunomodulatory Function to Guide Cell Fate and Bone Regeneration. Colloids Surf. B Biointerfaces 2023, 230, 113523. [Google Scholar] [CrossRef]
- Jia, N.; Qiao, H.; Zhu, W.; Zhu, M.; Meng, Q.; Lu, Q.; Zu, Y. Antioxidant, Immunomodulatory, Oxidative Stress Inhibitory and Iron Supplementation Effect of Astragalus Membranaceus Polysaccharide-Iron (III) Complex on Iron-Deficiency Anemia Mouse Model. Int. J. Biol. Macromol. 2019, 132, 213–221. [Google Scholar] [CrossRef]
- Yang, J.; Qin, L.; Huang, J.; Li, Y.; Xu, S.; Wang, H.; Zhu, S.; Wang, J.; Zhu, B.; Li, F.; et al. Astragalus Polysaccharide Attenuates LPS-Related Inflammatory Osteolysis by Suppressing Osteoclastogenesis by Reducing the MAPK Signaling Pathway. J. Cell. Mol. Med. 2021, 25, 6800–6814. [Google Scholar] [CrossRef]
- Lee, J.; Lin, Y.; Kuo, T.; Lee, A.K.; Chen, C.; Shie, M. Synergistic Effects of Astragalus on 3D-Printed Calcium Silicate/Poly-ε-Caprolactone Scaffolds to Regulate Inflammation/Osteogenesis for Bone Regeneration. Mater. Adv. 2024, 5, 8927–8936. [Google Scholar] [CrossRef]
- Du, Y.; Wan, H.; Huang, P.; Yang, J.; He, Y. A Critical Review of Astragalus Polysaccharides: From Therapeutic Mechanisms to Pharmaceutics. Biomed. Pharmacother. 2022, 147, 112654. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Guo, D.; Sun, X.; Wang, J.; Ouyang, J.M.; Gui, B. Repair Effects of Astragalus Polysaccharides with Different Molecular Weights on Oxidatively Damaged HK-2 Cells. Sci. Rep. 2019, 9, 9871. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, L.; He, X.; Wei, Y.; Wang, M.; Ma, B.; Hu, D.; Shi, Z. Promotion of Angiogenesis in Vitro by Astragalus Polysaccharide via Activation of TLR4 Signaling Pathway. J. Food Biochem. 2022, 46, e14329. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, G.; Zhang, S.; Chen, B.; Wu, Z.; Zhang, C. A Bioactive Poly(Ether-Ether-Ketone) Nanocomposite Scaffold Regulates Osteoblast/Osteoclast Activity for the Regeneration of Osteoporotic Bone. J. Mater. Chem. B 2022, 10, 8719–8732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hoke, E.; Eisenberg, R.; Wang, D. Transition Metal Sulfide Hydrogen Evolution Catalysts for Hydrobromic Acid Electrolysis. Langmuir 2013, 29, 480–492. [Google Scholar]
- Kaur, A.; Chahal, P.; Hogan, L. Selective Fabrication of SiC/Si Diodes by Excimer Laser under Ambient Conditions. IEEE Electron. Device Lett. 2015, 37, 142–145. [Google Scholar] [CrossRef]
- Peighambardoust, N.; Aydemir, U. Electrophoretic Deposition and Characterization of Self-Doped SrTiO Thin Films. Turk. J. Chem. 2021, 45, 323–332. [Google Scholar] [CrossRef]
- Cizaire, L.; Martin, J.M.; Le Mogne, T.; Gresser, E. Chemical Analysis of Overbased Calcium Sulfonate Detergents by Coupling XPS, ToF-SIMS, XANES, and EFTEM. Colloids Surf. A Physicochem. Eng. Asp. 2004, 238, 151–158. [Google Scholar] [CrossRef]
- Chen, L.; Yao, Z.G.; Zhang, S.; Tang, K.; Yang, Q.; Wang, Y.; Li, B.; Nie, Y.; Tian, X.; Sun, L. Biomaterial-Induced Macrophage Polarization for Bone Regeneration. Chin. Chem. Lett. 2023, 34, 107925. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Zhou, Y. Strontium Functionalized in Biomaterials for Bone Tissue Engineering: A Prominent Role in Osteoimmunomodulation. Front. Bioeng. Biotechnol. 2022, 10, 928799. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Li, C.; Wang, Z.; Xu, Y.; Sun, Y.; Zhang, W.; Liu, H.; Wang, J. Advanced Applications of Strontium-Containing Biomaterials in Bone Tissue Engineering. Mater. Today Bio 2023, 20, 100636. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, F.; Yin, D.; Fang, Z.; Huang, L. Astragalus Polysaccharide-Loaded Fibrous Mats Promote the Restoration of Microcirculation in/Around Skin Wounds to Accelerate Wound Healing in a Diabetic Rat Model. Colloids Surf. B Biointerfaces 2015, 136, 111–118. [Google Scholar] [CrossRef]
- Liu, J.; Duan, S.; Liu, L.; Zhang, G.; Peng, X. Reprogrammed Epigenetic Landscape-Prophesied Functions of Bioactive Polysaccharides in Alleviating Diseases: A Pilot Study of DNA Methylome Remodeling in Astragalus Polysaccharide (APS)-Improved Osteoporosis in a Rat Model. J. Agric. Food Chem. 2020, 68, 15449–15459. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Zhou, Z.; Hou, M.; Hong, L.; Li, H.; Qian, Z.; Gao, X.; Liu, M. Research Progress of Vascularization Strategies of Tissue-Engineered Bone. Front. Bioeng. Biotech. 2024, 11, 2296–4185. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Han, Q.; Sun, B.; Liu, Y.; Zhang, A.; Fan, D.; Xia, P.; Wang, J. Recent Advancement in Vascularized Tissue-Engineered Bone Based on Materials Design and Modification. Mater. Today Bio 2023, 23, 100858. [Google Scholar] [CrossRef]
- Shi, G.; Li, Y.; Luo, Y.; Jin, J.; Sun, Y.; Zheng, L.; Lai, Y.; Li, L.; Fu, G.; Qin, L.; et al. Bioactive PLGA/Tricalcium Phosphate Scaffolds Incorporating Phytomolecule Icaritin Developed for Calvarial Defect Repair in Rat Model. J. Orthop. Translat. 2020, 24, 112–120. [Google Scholar] [CrossRef]
- Khallaf, R.; Emam, A.; Mostafa, A.; Nassif, M.; Hussein, T. Strength and Bioactivity of PEEK Composites Containing Multiwalled Carbon Nanotubes and Bioactive Glass. J. Mech. Behav. Biomed. Mater. 2023, 144, 105964. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Yang, H.; Yang, Q.; Zhang, C.; Liu, J.; Wu, Z.; Wang, L.; Zhang, W.; Wang, B.; Liu, W. A Multifunctional PEEK Composite Scaffold with Immunomodulatory, Angiogenic, and Osteogenic Properties for Enhanced Bone Regeneration. Polymers 2025, 17, 1206. https://doi.org/10.3390/polym17091206
Zhao M, Yang H, Yang Q, Zhang C, Liu J, Wu Z, Wang L, Zhang W, Wang B, Liu W. A Multifunctional PEEK Composite Scaffold with Immunomodulatory, Angiogenic, and Osteogenic Properties for Enhanced Bone Regeneration. Polymers. 2025; 17(9):1206. https://doi.org/10.3390/polym17091206
Chicago/Turabian StyleZhao, Mengen, Han Yang, Qianwen Yang, Chao Zhang, Jie Liu, Zhaoying Wu, Lijun Wang, Wei Zhang, Bing Wang, and Wenliang Liu. 2025. "A Multifunctional PEEK Composite Scaffold with Immunomodulatory, Angiogenic, and Osteogenic Properties for Enhanced Bone Regeneration" Polymers 17, no. 9: 1206. https://doi.org/10.3390/polym17091206
APA StyleZhao, M., Yang, H., Yang, Q., Zhang, C., Liu, J., Wu, Z., Wang, L., Zhang, W., Wang, B., & Liu, W. (2025). A Multifunctional PEEK Composite Scaffold with Immunomodulatory, Angiogenic, and Osteogenic Properties for Enhanced Bone Regeneration. Polymers, 17(9), 1206. https://doi.org/10.3390/polym17091206