Mechanical, Thermal, and Flammability Properties of Eco-Friendly Nanocomposites from Recycled PET/PA-11 Blends Reinforced with Graphene Nanoplatelets
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Extrusion Processing
2.3. Injection Moulding and Formulation of GNP Nanocomposites
2.4. Characterization
3. Results and Discussion
3.1. FTIR Analysis of GNP-Filled RPET/PA-11 Nanocomposites
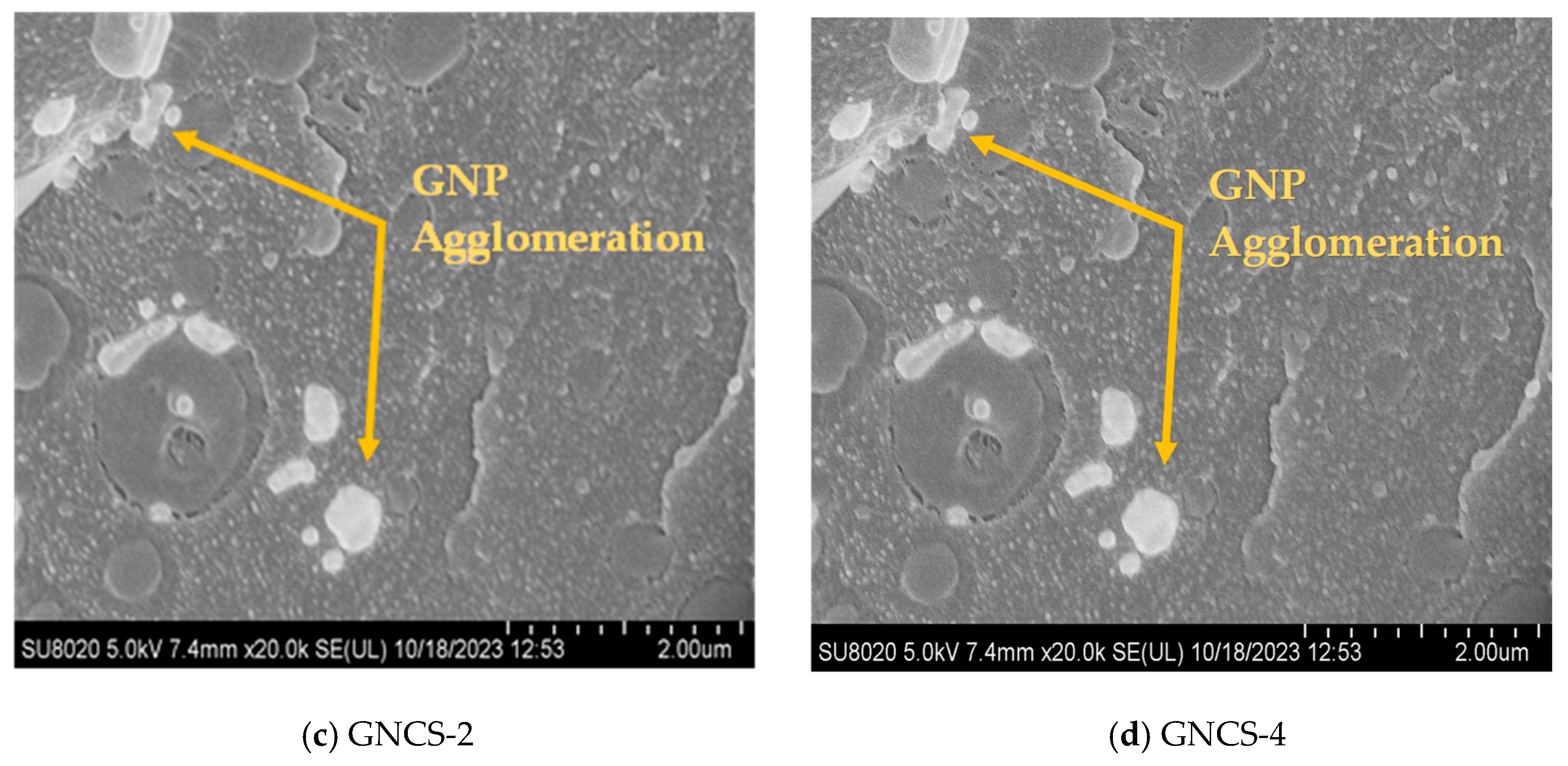
3.2. Morphology of GNP Nanocomposites
3.3. Mechanical Properties of GNP Nanocomposites
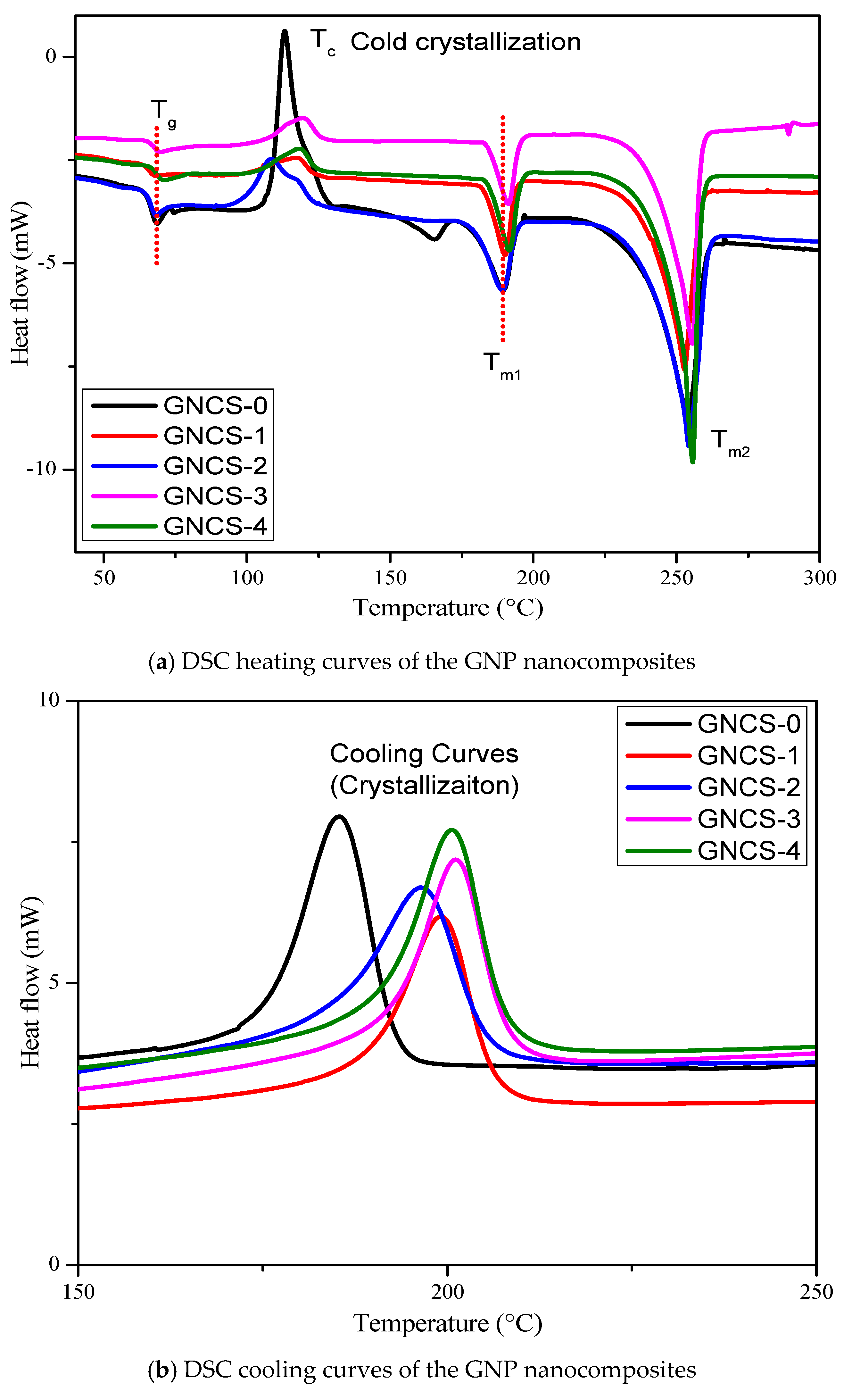
3.4. Differential Scanning Calorimetry (DSC)
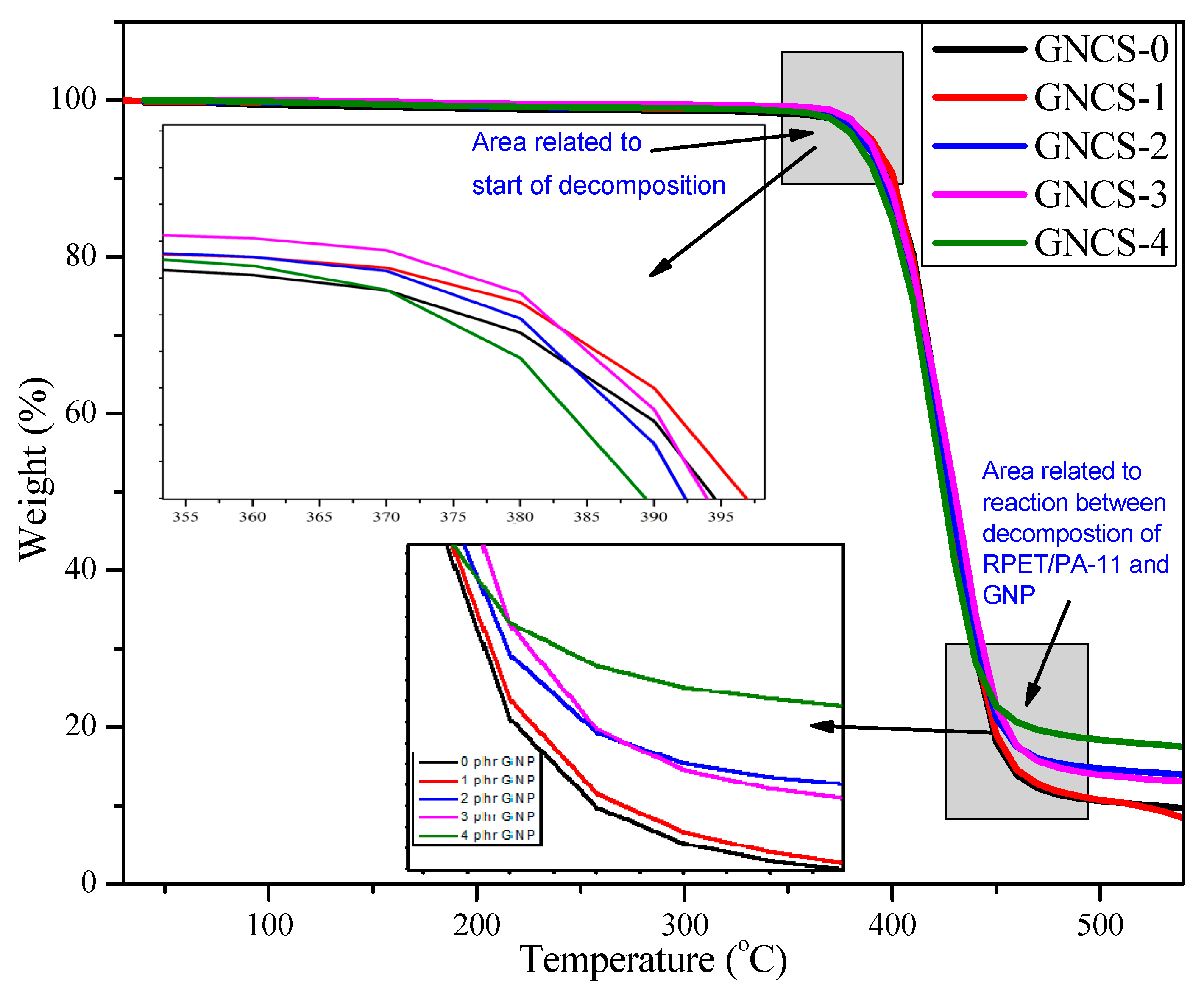
3.5. Thermal Gravimetric Analysis (TGA)
3.6. Limiting Oxygen Index (LOI) of the HNT/GNP Hybrid Nanocomposites
3.7. UL-94 Vertical Burning Test of the GNP Nanocomposites
4. Conclusions
- The incorporation of 1 phr GNPs (GNCS-1) resulted in a well-balanced combination of tensile strength, flexural strength, and impact resistance. Higher GNP loadings (≥3 phr) led to increased stiffness but also promoted agglomeration, negatively affecting tensile and impact properties. Young’s modulus increased from 785.75 ± 171.5 MPa (GNCS-0) to 1210.6 ± 183.3 MPa (GNCS-2), while the flexural modulus improved from 3090.4 ± 273.4 MPa (GNCS-0) to 4170 ± 217.79 MPa (GNCS-4).
- The DSC analysis revealed that GNPs acted as a nucleating agent, influencing the cold crystallization behaviour of the blend. The crystallization temperature varied non-linearly with the GNP content, indicating complex filler–polymer interactions. The crystallinity (Xc) increased from 4.19% (GNCS-0) to 25.73% (GNCS-1) and 31.28% (GNCS-2), confirming the nucleating effect of GNPs. The TGA results showed a moderate improvement in thermal stability, with Tonset increasing from 386 °C (GNCS-0) to 398 °C (GNCS-1) and Tmax from 410 °C to 430 °C.
- The LOI results confirmed the flame-retardant role of GNPs, with values increasing from 19 (GNCS-0) to 23 (GNCS-1) and 24 (GNCS-2). However, at higher GNP loadings (≥3 phr), agglomeration limited further improvements in flame resistance. The UL-94 results further demonstrated the barrier effect of GNPs in improving flame retardancy.
- The FESEM analysis showed that 1 phr GNPs (GNCS-1) improved the phase dispersion of PA-11 within the RPET matrix. However, at higher concentrations (≥3 phr), agglomeration was evident, reducing the reinforcing efficiency.
- The FTIR spectra indicated that GNPs did not induce chemical modifications but influenced hydrogen bonding interactions within the polymer matrix. The peak intensity variations in the -OH and -NH stretching regions suggested improved interfacial adhesion.
- Investigating finer increments (e.g., 0.5 phr steps) to determine the precise dispersion threshold.
- Exploring combinations of GNPs with other fillers to enhance mechanical, thermal, and flammability properties.
- Examining the electrical performance of RPET/PA-11/GNP nanocomposites, particularly for EMI shielding and electronic applications.
- Using surface functionalization or alternative processing techniques to minimize agglomeration.
- Evaluating melt processing behaviour for better control of flow properties during manufacturing.
- Assessing long-term stability under real-world environmental conditions to validate industrial applicability.
5. Significance of This Study
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhong, M.; Khan, T.K.H.; Devade, K.; Krishna, B.V.; Sura, S.; Eftikhaar, H.K.; Thethi, H.P.; Gupta, N. Review of Composite Materials and Applications. Mater. Today Proc. 2023. In Press, Corrected Proof. [Google Scholar] [CrossRef]
- Ciobanu, R.C.; Schreiner, C.; Caramitu, A.R.; Aradoaei, S.; Aradoaei, M. Sustainability of the Technology for Obtaining Thermoplastic Building Materials from Non-Recyclable Mixed Plastic–Paper Packaging Waste. Sustainability 2024, 16, 3430. [Google Scholar] [CrossRef]
- Khan, Z.I.; Habib, U.; Mohamad, Z.B.; Rahmat, A.R.B.; Abdullah, N.A.S.B. Mechanical and Thermal Properties of Sepiolite Strengthened Thermoplastic Polymer Nanocomposites: A Comprehensive Review. Alex. Eng. J. 2022, 61, 975–990. [Google Scholar] [CrossRef]
- Khan, Z.I.; Mohamad, Z.B.; Rahmat, A.R.B.; Habib, U.; Abdullah, N.A.S.B. A Novel Recycled Polyethylene Terephthalate/Polyamide 11 (RPET/PA11) Thermoplastic Blend. Prog. Rubber Plast. Recycl. Technol. 2021, 37, 233–244. [Google Scholar] [CrossRef]
- Habib, U.; Khan, Z.I.; Mohamad, Z.B. Compatibility and Miscibility of Recycled Polyethylene Terephthalate/Polyamide 11 Blends with and without Joncryl® Compatibilizer: A Comprehensive Study of Mechanical, Thermal, and Thermomechanical Properties. Iran. Polym. J. 2024, 33, 1313–1326. [Google Scholar] [CrossRef]
- Celik, Y.; Shamsuyeva, M.; Endres, H.J. Thermal and Mechanical Properties of the Recycled and Virgin PET—Part I. Polymers 2022, 14, 1326. [Google Scholar] [CrossRef]
- Luo, L.B.; Chen, R.; Lian, Y.X.; Wu, W.J.; Zhang, J.H.; Fu, C.X.; Sun, X.L.; Xiao, L.R. Recycled PET/PA6 Fibers from Waste Textile with Improved Hydrophilicity by In-Situ Reaction-Induced Capacity Enhancement. Polymers 2024, 16, 1052. [Google Scholar] [CrossRef]
- Othman, N.; Mohamad, Z.; Khan, Z.I.; Abdullah, L.C. Rheological Behavior of Recycled Poly(Ethylene Terephthalate) /Poly(Amide) 11 Blends with Chain Extender. Mater. Today Proc. 2023, 110, 87–90. [Google Scholar] [CrossRef]
- Geyer, B.; Lorenz, G.; Kandelbauer, A. Recycling of Poly(Ethylene Terephthalate)—A Review Focusing on Chemical Methods. Express Polym. Lett. 2016, 10, 559–586. [Google Scholar] [CrossRef]
- Marotta, A.; Causa, A.; Salzano de Luna, M.; Ambrogi, V.; Filippone, G. Tuning the Morphology of HDPE/PP/PET Ternary Blends by Nanoparticles: A Simple Way to Improve the Performance of Mixed Recycled Plastics. Polymers 2022, 14, 5390. [Google Scholar] [CrossRef]
- Schexnaydre, R.J.; Mitchell, B.S. Solid-State Blending of Poly(Ethylene Terephthalate) with Polystyrene: Extent of Compatibilization and Its Dependence on Blend Composition. Polym. Eng. Sci. 2008, 48, 649–655. [Google Scholar] [CrossRef]
- Kit, F.M.; Haq, R.H.A.; Manshoor, B.; Ghafir, M.F.A.; Rahman, M.N.A.; Hoffmann, J.; Marwah, O.M.F.; Khirotdin, R.K. Mechanical Properties of Recycled Polyethylene Terephthalate/Polycarbonate/Methylene Diphenyl Diisocyanate (r-PET/PC/MDI) Composite. J. Adv. Res. Appl. Mech. 2024, 120, 1–13. [Google Scholar] [CrossRef]
- Rosmmi, N.H.M.; Khan, Z.I.; Mohamad, Z.; Majid, R.A.; Othman, N.; Man, S.H.C.; Karim, K.J.A. Impact Strength and Morphology of Sustainably Sourced Recycling Polyethylene Terephthalate Blends. Chem. Eng. Trans. 2021, 83, 265–270. [Google Scholar]
- Romeiro, A.; Teixeira, C.; Costa, H.; Coelho, J.F.J.; Serra, A.C. Recycling Polyethylene/Polyamide Multilayer Films with Poly(Isoprene-g-Maleic Anhydride) Compatibilizer. Polymers 2024, 16, 1079. [Google Scholar] [CrossRef]
- Mousavi, Z.; Heuzey, M.C.; Randall, J.; Carreau, P.J. Enhanced Properties of Polylactide/Polyamide 11 Blends by Reactive Compatibilization. Can. J. Chem. Eng. 2022, 100, 2475–2490. [Google Scholar] [CrossRef]
- Khan, Z.I.; Habib, U.; Mohamad, Z.B.; Tufail, A.; Raji, A.M.; Khan, A.U. Innovative Hybrid Nanocomposites of Recycled Polyethylene Terephthalate/Polyamide 11 Reinforced with Sepiolite and Graphene Nanoplatelets. J. Thermoplast. Compos. Mater. 2025, 38, 1063–1088. [Google Scholar] [CrossRef]
- Costa, A.R.d.M.; Henrique, M.A.; Luna, C.B.B.; de Carvalho, L.H.; de Almeida, Y.M.B. Influence of a Multifunctional Epoxy Additive on the Performance of Polyamide 6 and PET Post-Consumed Blends during Processing. Sustainability 2022, 14, 16658. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, J.; Wang, S.; Yang, S.; Jin, J.; Li, G. Effect of Copoly(Ester-Amide 6)(Pet-Pa6) on Compatibility of Pet/Pa6 Blended Fibers. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2020; Volume 993, MSF; pp. 709–717. [Google Scholar]
- Khan, Z.I.; Habib, U.; Mohamad, Z.B.; Raji, A.M. Enhanced Mechanical Properties of a Novel Compatibilized Recycled Polyethylene Terephthalate/Polyamide 11 (RPET/PA11) Blends. Express Polym. Lett. 2021, 15, 1206–1215. [Google Scholar] [CrossRef]
- Shabafrooz, V.; Bandla, S.; Allahkarami, M.; Hanan, J.C. Graphene/Polyethylene Terephthalate Nanocomposites with Enhanced Mechanical and Thermal Properties. J. Polym. Res. 2018, 25, 256. [Google Scholar] [CrossRef]
- Anas, M.; Nasir, M.A.; Asfar, Z.; Nauman, S.; Akalin, M.; Ahmad, F. Structural Health Monitoring of GFRP Laminates Using Graphene-Based Smart Strain Gauges. J. Brazilian Soc. Mech. Sci. Eng. 2018, 40, 397. [Google Scholar] [CrossRef]
- Sam-Daliri, O.; Farahani, M.; Faller, L.M.; Zangl, H. Structural Health Monitoring of Defective Single Lap Adhesive Joints Using Graphene Nanoplatelets. J. Manuf. Process. 2020, 55, 119–130. [Google Scholar] [CrossRef]
- Sam-Daliri, O.; Faller, L.M.; Farahani, M.; Zangl, H. Structural Health Monitoring of Adhesive Joints under Pure Mode I Loading Using the Electrical Impedance Measurement. Eng. Fract. Mech. 2021, 245, 107585. [Google Scholar] [CrossRef]
- Nor Arman, N.S.; Chen, R.S.; Ahmad, S.; Shahdan, D. Mechanical and Physical Characterizations of Compatibilizer-Free Recycled Plastics Blend Composites Modified with Carbon Nanotube and Clay Nanofiller. J. Appl. Polym. Sci. 2022, 139, e52768. [Google Scholar] [CrossRef]
- Costa, U.O.; Garcia Filho, F.d.C.; del Río, T.G.; Rodrigues, J.G.P.; Simonassi, N.T.; Monteiro, S.N.; Nascimento, L.F.C. Mechanical Properties Optimization of Hybrid Aramid and Jute Fabrics-Reinforced Graphene Nanoplatelets in Functionalized HDPE Matrix Nanocomposites. Polymers 2023, 15, 2460. [Google Scholar] [CrossRef]
- Galante, M.; Tkatchenko, A. Anisotropic van Der Waals Dispersion Forces in Polymers: Structural Symmetry Breaking Leads to Enhanced Conformational Search. Phys. Rev. Res. 2023, 5, L012028. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef]
- Tavares, L.; Sousa, L.R.; da Silva, S.M.; Lima, P.S.; Oliveira, J.M. Effect of Incorporation of Graphene Nanoplatelets on Physicochemical, Thermal, Rheological, and Mechanical Properties of Biobased and Biodegradable Blends. Polymers 2023, 15, 3622. [Google Scholar] [CrossRef]
- ASTM D4603-03; Standard Test Method for Determining Inherent Viscosity of Poly(Ethylene Terephthalate) (PET) by Glass Capillary Viscometer. ASTM: West Conshohocken, PA, USA, 2012. [CrossRef]
- ASTM D5630-22; Standard Test Method for Ash Content in Plastics. ASTM: West Conshohocken, PA, USA, 2022. [CrossRef]
- ISO 1183-1; Plastics—Methods for Determining the Density of Non-Cellular Plastics—Part 1: Immersion Method, Liquid Pyknometer Method and Titration Method. ISO: Geneva, Switzerland, 2019. Available online:https://www.iso.org/standard/74990.html (accessed on 30 March 2025).
- ISO 11357-1; Plastics Differential Scanning Calorimetry (DSC)—Part 1: General Principles. ISO: Geneva, Switzerland, 2023. Available online:https://www.iso.org/obp/ui/#iso:std:iso:11357:-1:ed-4:v1:en (accessed on 30 March 2025).
- ISO 294-1; Plastics—Injection Moulding of Test Specimens of Thermoplastic Materials—Part 1: General Principles, and Moulding of Multipurpose and Bar Test Specimens. ISO: Geneva, Switzerland, 2017. Available online:https://www.iso.org/standard/67036.html (accessed on 30 March 2025).
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. ASTM: West Conshohocken, PA, USA, 2014. [CrossRef]
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM: West Conshohocken, PA, USA, 2002. [CrossRef]
- Felix Sahayaraj, A.; Selvan, M.T.; Sasi Kumar, M.; Sathish, S.; Gokulkumar, S.; Jenish, I.; Makeshkumar, M. Fire Retardant Potential of Natural Fiber Reinforced Polymer Composites: A Review. Polym. Technol. Mater. 2024, 63, 771–797. [Google Scholar] [CrossRef]
- Huang, W.; Wang, K.; Tu, C.; Xu, X.; Tian, Q.; Ma, C.; Fu, Q.; Yan, W. Synergistic Effects of DOPO-Based Derivative and Organo-Montmorillonite on Flame Retardancy, Thermal Stability and Mechanical Properties of Polypropylene. Polymers 2022, 14, 2372. [Google Scholar] [CrossRef]
- Dargahi, A.; Duncan, M.; Runka, J.; Hammami, A.; Wen, T.; Wang, X.; Chen, W.; Naguib, H.E. Low-Concentration Graphene Nanoplatelet/HDPE Nanocomposites with Enhanced Dispersion and Interfacial Bonding for Improved CO2 Barrier and Mechanical Performance at Elevated Temperatures. Ind. Eng. Chem. Res. 2025, 64, 5359–5371. [Google Scholar] [CrossRef]
- Aria, A.I.; Kidambi, P.R.; Weatherup, R.S.; Xiao, L.; Williams, J.A.; Hofmann, S. Time Evolution of the Wettability of Supported Graphene under Ambient Air Exposure. J. Phys. Chem. C 2016, 120, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Kadela, K.; Grzybek, G.; Kotarba, A.; Stelmachowski, P. Enhancing Graphene Nanoplatelet Reactivity through Low-Temperature Plasma Modification. ACS Appl. Mater. Interfaces 2024, 16, 19771–19779. [Google Scholar] [CrossRef] [PubMed]
- Golec, B.; Sałdyka, M.; Mielke, Z. Complexes of Formaldehyde and α-Dicarbonyls with Hydroxylamine: FTIR Matrix Isolation and Theoretical Study. Molecules 2021, 26, 1144. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, P.; Sun, L.; Wang, L. The Effect of Graphene Nanofiller on the Surface Structure and Performance of Epoxy Resin–Polyhedral Oligomeric Silsesquioxane (EP-POSS). Nanomaterials 2021, 11, 841. [Google Scholar] [CrossRef]
- Hansen, P.E.; Vakili, M.; Kamounah, F.S.; Spanget-Larsen, J. NH Stretching Frequencies of Intramolecularly Hydrogen-Bonded Systems: An Experimental and Theoretical Study. Molecules 2021, 26, 7651. [Google Scholar] [CrossRef]
- Eayal Awwad, K.Y.; Yousif, B.F.; Fallahnezhad, K.; Saleh, K.; Zeng, X. Influence of Graphene Nanoplatelets on Mechanical Properties and Adhesive Wear Performance of Epoxy-Based Composites. Friction 2021, 9, 856–875. [Google Scholar] [CrossRef]
- Ahmad, K.H.; Mohamad, Z.; Khan, Z.I. Influence of Graphene Nanoplatelets and Post-Curing Conditions on the Mechanical and Viscoelastic Properties of Stereolithography 3D-Printed Nanocomposites. Polymers 2024, 16, 2721. [Google Scholar] [CrossRef]
- Rahmat, N.F.; Sajab, M.S.; Afdzaluddin, A.M.; Ding, G.; Chia, C.H. Thermoplastic Sugar Palm Starch Reinforced Graphene Nanoplatelets for Sustainable Biocomposite Films. BioResources 2024, 19, 1526–1541. [Google Scholar] [CrossRef]
- Akter, M.; Ozdemir, H.; Bilisik, K. Epoxy/Graphene Nanoplatelet (GNP) Nanocomposites: An Experimental Study on Tensile, Compressive, and Thermal Properties. Polymers 2024, 16, 1483. [Google Scholar] [CrossRef]
- Kolev, M.; Lazarova, R.; Petkov, V.; Mourdjeva, Y.; Nihtianova, D. Investigating the Effects of Graphene Nanoplatelets and Al4C3 on the Tribological Performance of Aluminum-Based Nanocomposites. Metals 2023, 13, 943. [Google Scholar] [CrossRef]
- Lin, B.; Li, Z.T.; Yang, Y.; Li, Y.; Lin, J.C.; Zheng, X.M.; He, F.A.; Lam, K.H. Enhanced Dielectric Permittivity in Surface-Modified Graphene/PVDF Composites Prepared by an Electrospinning-Hot Pressing Method. Compos. Sci. Technol. 2019, 172, 58–65. [Google Scholar] [CrossRef]
- Ge, Z.; Qin, J.; Sun, R.; Guan, Y.; Zhang, H.; Wang, Z. The Effect of the Addition of Graphene Nanoplatelets on the Selected Properties of Cementitious Composites. Front. Built Environ. 2021, 7, 673346. [Google Scholar] [CrossRef]
- Khan, Z.I.; Habib, U.; Mohamad, Z.B.; Khan, I. Mechanical and Thermal Properties of a Newly Developed Sepiolite Filler-Filled RPET/PA11 Thermoplastic Nanocomposites. Results Eng. 2024, 21, 101731. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Following the Morphological and Thermal Properties of PLA/PEO Blends Containing Carbon Nanotubes (CNTs) during Hydrolytic Degradation. Compos. Part B Eng. 2019, 175, 107132. [Google Scholar] [CrossRef]
- Van De Voorde, B.; Katalagarianakis, A.; Huysman, S.; Toncheva, A.; Raquez, J.; Duretek, I.; Holzer, C.; Cardon, L.; Bernaerts, K.V.; Hemelrijck, D.V.; et al. Effect of Extrusion and Fused Filament Fabrication Processing Parameters of Recycled Poly (Ethylene Terephthalate) on the Crystallinity and Mechanical Properties. Addit. Manuf. 2022, 50, 102518. [Google Scholar] [CrossRef]
- Barra, G.; Guadagno, L.; Raimondo, M.; Santonicola, M.G.; Toto, E.; Vecchio Ciprioti, S. A Comprehensive Review on the Thermal Stability Assessment of Polymers and Composites for Aeronautics and Space Applications. Polymers 2023, 15, 3786. [Google Scholar] [CrossRef]
- Rehman, S.U.; Javaid, S.; Shahid, M.; Gul, I.H.; Rashid, B.; Szczepanski, C.R.; Naveed, M.; Curley, S.J. Polystyrene-Sepiolite Clay Nanocomposites with Enhanced Mechanical and Thermal Properties. Polymers 2022, 14, 3576. [Google Scholar] [CrossRef]
- Alvaredo, Á.; Martín, M.I.; Castell, P.; de Villoria, R.G.; Fernández-Blázquez, J.P. Non-Isothermal Crystallization Behavior of PEEK/Graphene Nanoplatelets Composites from Melt and Glass States. Polymers 2019, 11, 124. [Google Scholar] [CrossRef]
- Drobny, J.G. Applications of Thermoplastic Elastomers. In Handbook of Thermoplastic Elastomers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 301–337. [Google Scholar]
- Wiese, M.; Thiede, S.; Herrmann, C. Rapid Manufacturing of Automotive Polymer Series Parts: A Systematic Review of Processes, Materials and Challenges. Addit. Manuf. 2020, 36, 101582. [Google Scholar] [CrossRef]
- Wong, S.; Lee, J.W.S.; Naguib, H.E.; Park, C.B. Effect of Processing Parameters on the Mechanical Properties of Injection Molded Thermoplastic Polyolefin (TPO) Cellular Foams. Macromol. Mater. Eng. 2008, 293, 605–613. [Google Scholar] [CrossRef]






| Formulation (%) | RPET/PA-11 (wt%) | Joncryl® (phr) | GNPs (phr) |
|---|---|---|---|
| GNCS-0 | 80:20 | 2 | 0 |
| GNCS-1 | 80:20 | 2 | 1 |
| GNCS-2 | 80:20 | 2 | 2 |
| GNCS-3 | 80:20 | 2 | 3 |
| GNCS-4 | 80:20 | 2 | 4 |
| Formulations | Tensile Strength (MPa) | Tensile Strain (%) | Young’s Modulus (MPa) | Flexural Strength (MPa) | Flexural Strain (%) | Flexural Modulus (MPa) | Impact Strength (J/m) |
|---|---|---|---|---|---|---|---|
| GNCS-0 | 44.825 ± 8.31 | 4.1 ± 0.69 | 785.75 ± 171.5 | 62.9 ± 3.10 | 2.3 ± 0.18 | 3090.4 ± 273.4 | 225.12 ± 72.45 |
| GNCS-1 | 37.85 ± 2.13 | 2.2 ± 0.12 | 1036.6 ± 159.9 | 64.78 ± 5.26 | 1.9 ± 0.24 | 3580 ± 237.06 | 221.0225 ± 52.08 |
| GNCS-2 | 36.94 ± 1.21 | 2.14 ± 0.09 | 1210.6 ± 183.3 | 64.82 ± 2.23 | 1.9 ± 0.08 | 3590 ± 103.6 | 195.725 ± 11.63 |
| GNCS-3 | 36.54 ± 1.93 | 2.14 ± 0.11 | 1165.2 ± 148.6 | 64.84 ± 4.77 | 1.78 ± 0.16 | 3820 ± 157.3 | 189.005 ± 6.78 |
| GNCS-4 | 36.3 ± 0.66 | 2 ± 0.07 | 1137.5 ± 115.32 | 65.88 ± 4.02 | 1.62 ± 0.17 | 4170 ± 217.79 | 185.552 ± 20.53 |
| Formulations | Glass Transition Tg (°C) | Cold Crystallization Temperature Tc (°C) | Melting of Cold Crystallization Temperature Tm1 (°C) | Melting Temperature Tm2 (°C) | Crystallinity Xc (%) |
|---|---|---|---|---|---|
| GNCS-0 | 64.45 | 113.19 | 189.24 | 253.53 | 4.19 |
| GNCS-1 | 65.57 | 117.08 | 189.94 | 252.26 | 25.73 |
| GNCS-2 | 64.52 | 108.41 | 188.90 | 253.66 | 31.28 |
| GNCS-3 | 65.24 | 119.44 | 191.00 | 255.13 | 30.90 |
| GNCS-4 | 66.41 | 118.08 | 191.28 | 255.14 | 27.19 |
| Formulations | Onset of Decomposition Temperature Tonset (°C) | Maximum Degradation Temperature Tmax (°C) |
|---|---|---|
| GNCS-0 | 386.38 | 410.38 |
| GNCS-1 | 398.08 | 430.06 |
| GNCS-2 | 390.48 | 420.47 |
| GNCS-3 | 391.35 | 420.82 |
| GNCS-4 | 383.89 | 420.01 |
| Sample | O2 Flow Rate (mL/min) | N2 Flow Rate (mL/min) | LOI | Burning Behaviour |
|---|---|---|---|---|
| GNCS-0 | 60.5 | 116.5 | 19 | Burned easily; poor flame resistance. |
| GNCS-1 | 66.0 | 113.5 | 21 | Ignited in 30 s; not fully consumed in 180 s. |
| GNCS-2 | 66.0 | 113.5 | 21 | Did not ignite in 30 s. |
| 69.0 | 112.0 | 22 | Ignited in 30 s; extinguished in 60 s. | |
| 71.5 | 110.5 | 23 | Ignited in 30 s; extinguished in 80 s. | |
| 74.5 | 109.0 | 24 | Ignited in 30 s; consumed fully in 120 s. | |
| GNCS-3 | 74.5 | 109.0 | 24 | Ignited in 30 s; consumed fully in 180 s. |
| GNCS-4 | 74.5 | 109.0 | 24 | Ignited in 30 s; consumed fully in 150 s. |
| 71.5 | 110.5 | 23 | Ignited in 30 s; consumed fully in 140 s. |
| Sample | Ignition Time (s) | Burning Duration After Flame Removal (s) | Dripping | Cotton Ignition | Classification |
|---|---|---|---|---|---|
| GNCS-0 | 10 | Prolonged | Yes | Yes | Not Classified |
| GNCS-1 | 10 | 5 | Yes | Yes | V-2 |
| GNCS-2 | 10 | 10 | Yes | Yes | V-2 |
| GNCS-3 | 7–8 | 7–8 | Yes | Yes | V-2 |
| GNCS-4 | 10 | 10 | Yes | Yes | V-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, U.; Mohsin, M.E.A.; Khan, Z.I.; Mohamad, Z.; Othman, N.; Mousa, S.; Hossain, S.S.; Ali, S.S. Mechanical, Thermal, and Flammability Properties of Eco-Friendly Nanocomposites from Recycled PET/PA-11 Blends Reinforced with Graphene Nanoplatelets. Polymers 2025, 17, 1038. https://doi.org/10.3390/polym17081038
Habib U, Mohsin MEA, Khan ZI, Mohamad Z, Othman N, Mousa S, Hossain SS, Ali SS. Mechanical, Thermal, and Flammability Properties of Eco-Friendly Nanocomposites from Recycled PET/PA-11 Blends Reinforced with Graphene Nanoplatelets. Polymers. 2025; 17(8):1038. https://doi.org/10.3390/polym17081038
Chicago/Turabian StyleHabib, Unsia, Mohammed E. Ali Mohsin, Zahid Iqbal Khan, Zurina Mohamad, Norhayani Othman, Suleiman Mousa, SK Safdar Hossain, and Syed Sadiq Ali. 2025. "Mechanical, Thermal, and Flammability Properties of Eco-Friendly Nanocomposites from Recycled PET/PA-11 Blends Reinforced with Graphene Nanoplatelets" Polymers 17, no. 8: 1038. https://doi.org/10.3390/polym17081038
APA StyleHabib, U., Mohsin, M. E. A., Khan, Z. I., Mohamad, Z., Othman, N., Mousa, S., Hossain, S. S., & Ali, S. S. (2025). Mechanical, Thermal, and Flammability Properties of Eco-Friendly Nanocomposites from Recycled PET/PA-11 Blends Reinforced with Graphene Nanoplatelets. Polymers, 17(8), 1038. https://doi.org/10.3390/polym17081038








