Structure and Property Evolution of Microinjection Molded PLA/PCL/Bioactive Glass Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Sample Preparation
2.3. Sample Characterization
3. Results and Discussion
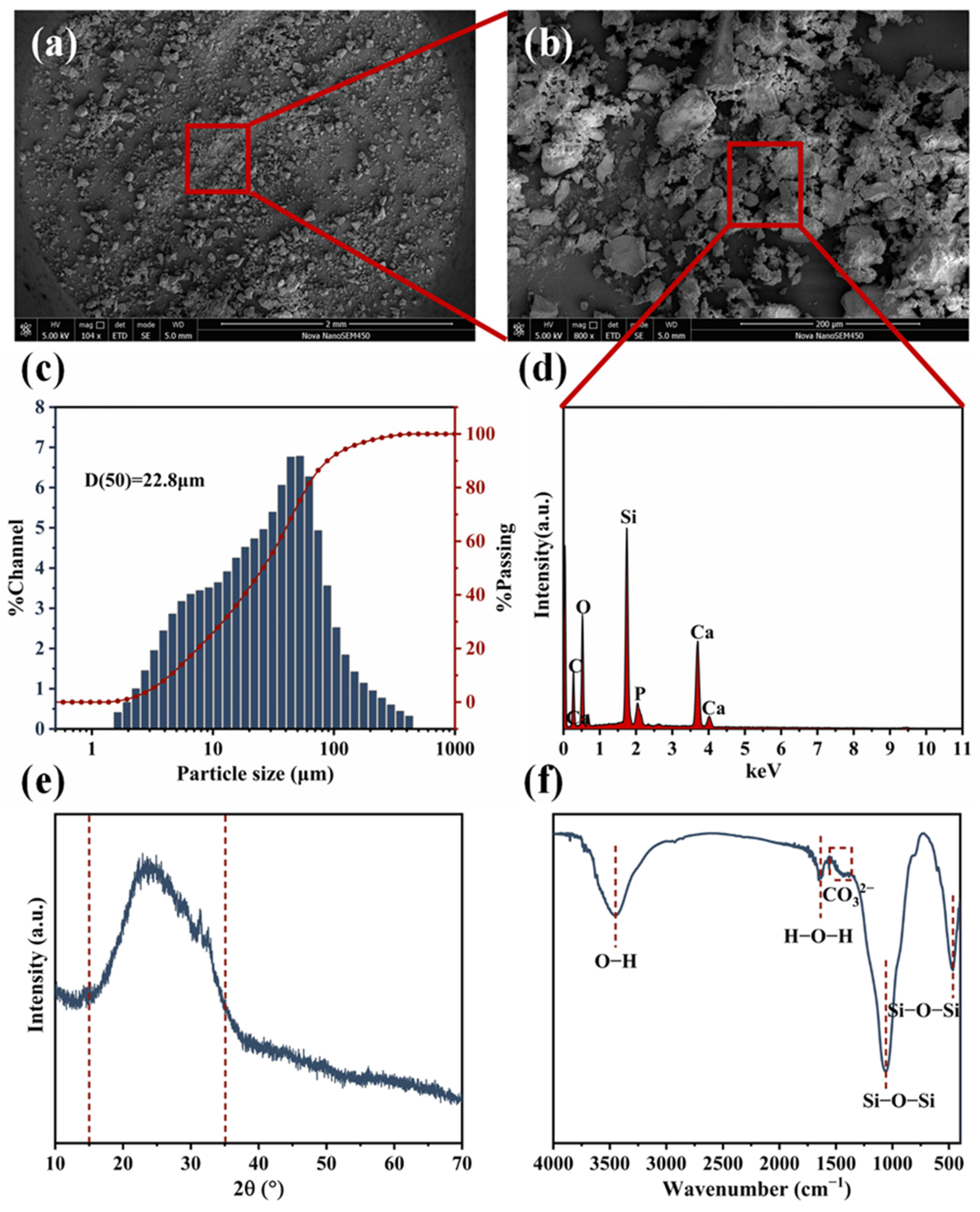
3.1. Characterization of BG Particles
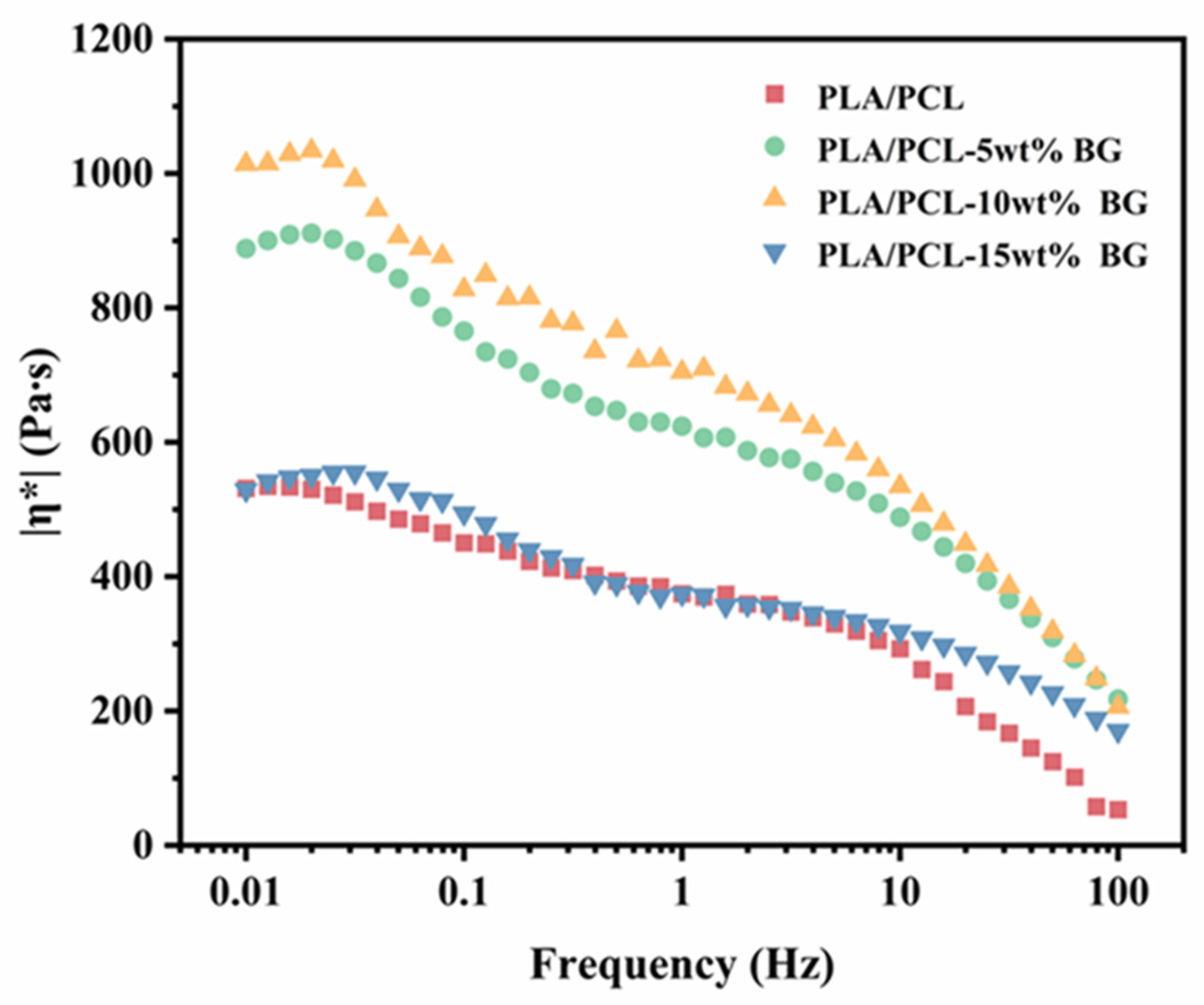
3.2. Rheological Properties
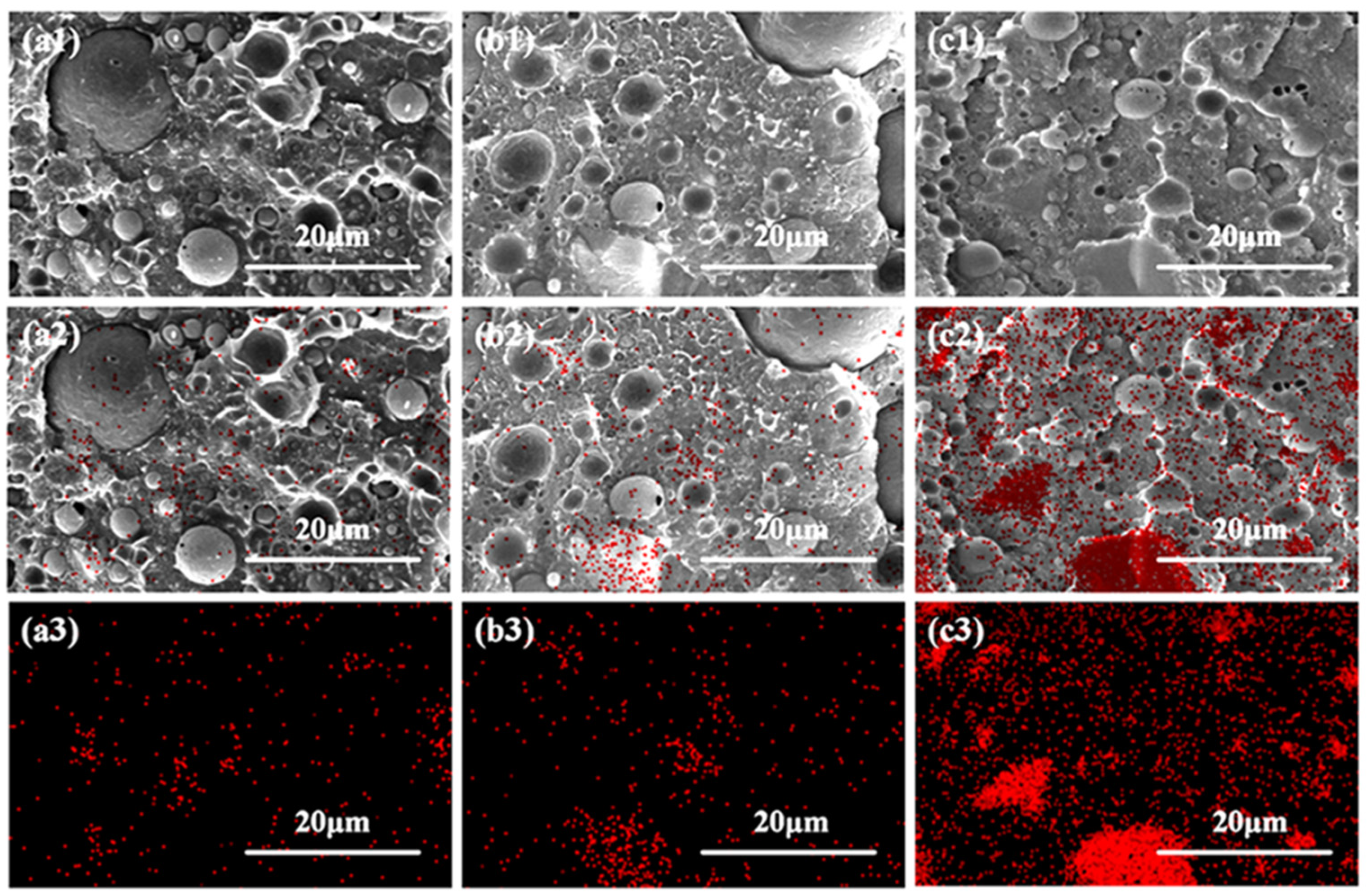
3.3. Morphology Analysis of Dispersed Phases in the Polymer Matrix
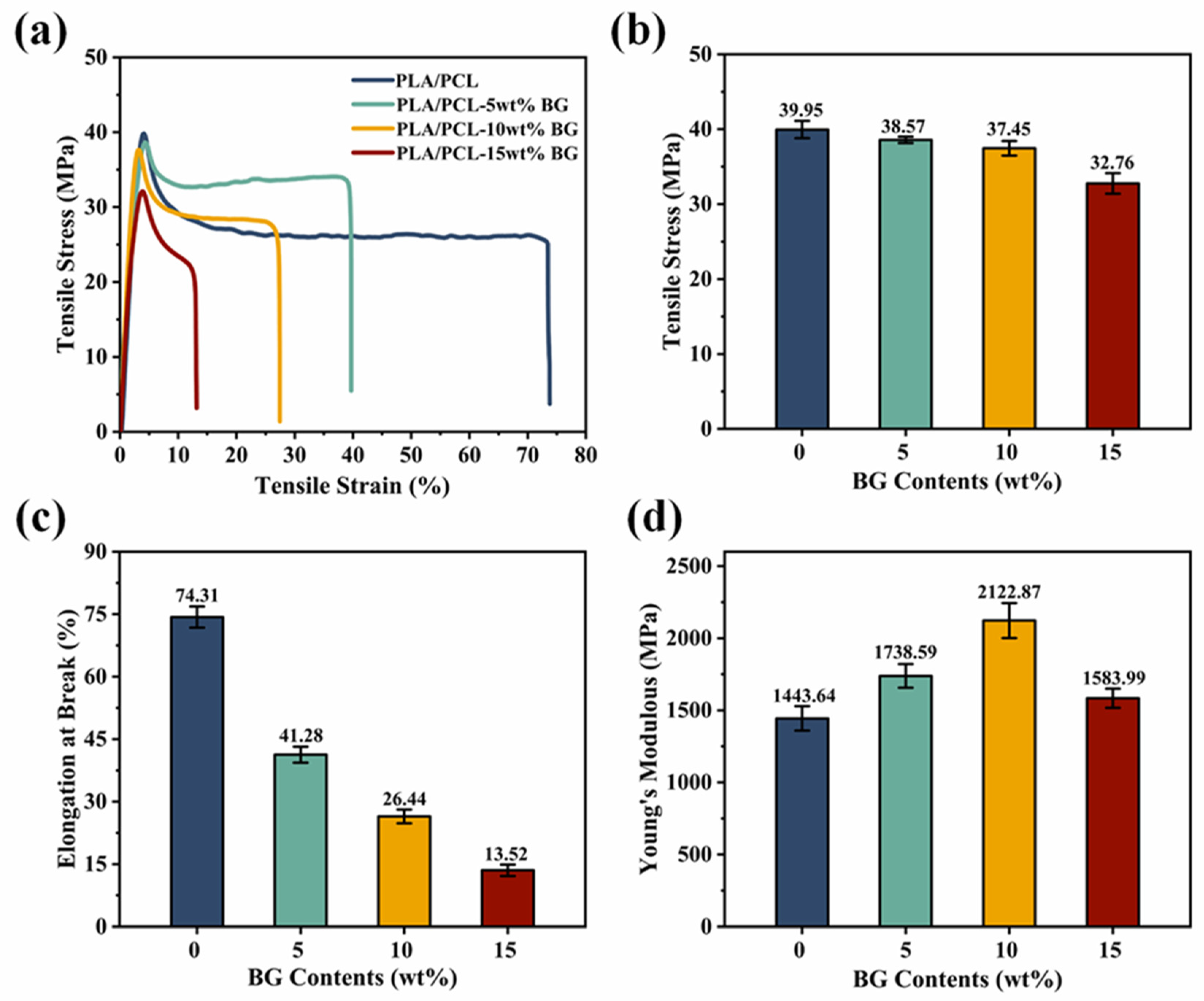
3.4. Mechanical Property Analysis
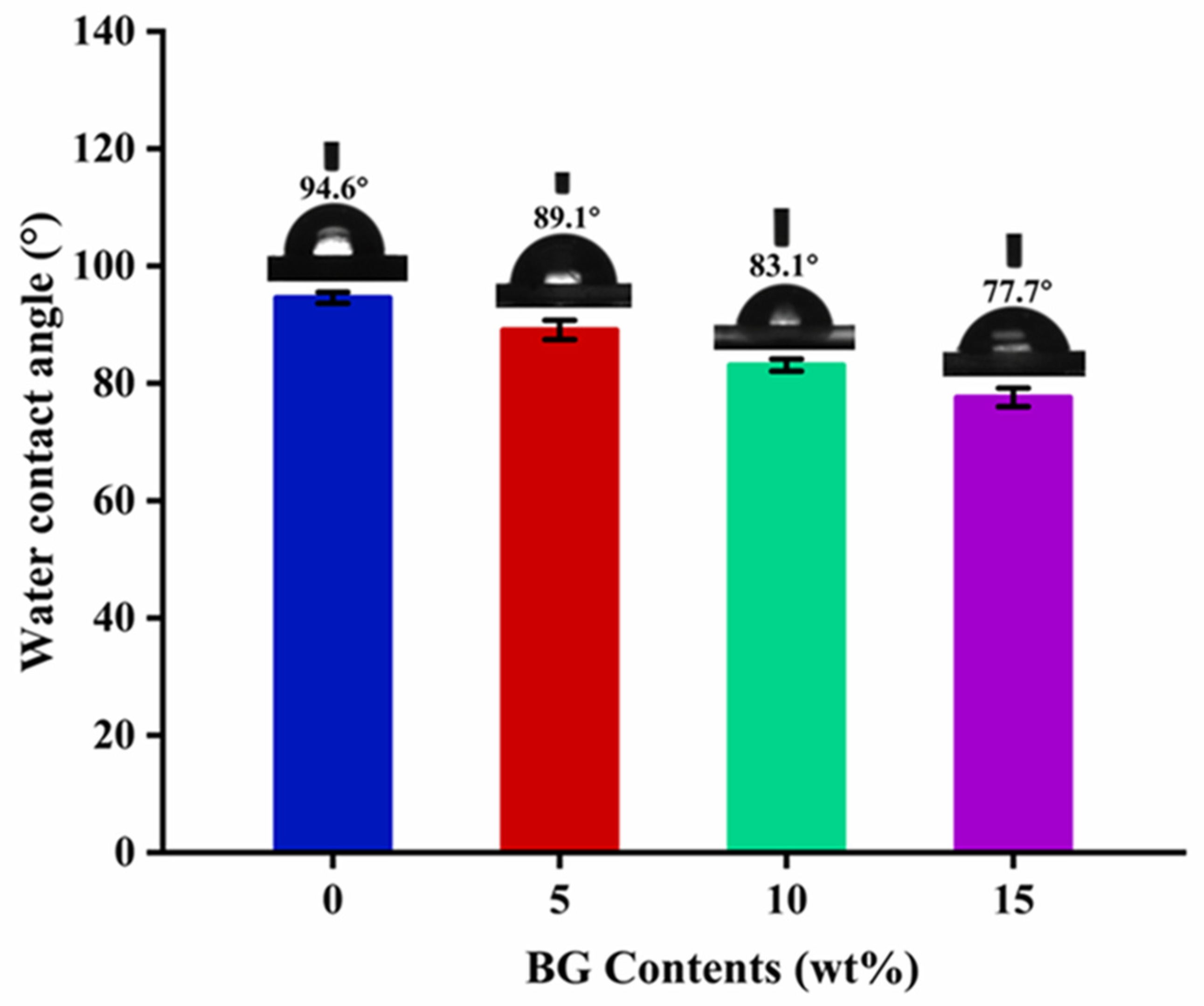
3.5. Hydrophilic Property Analysis
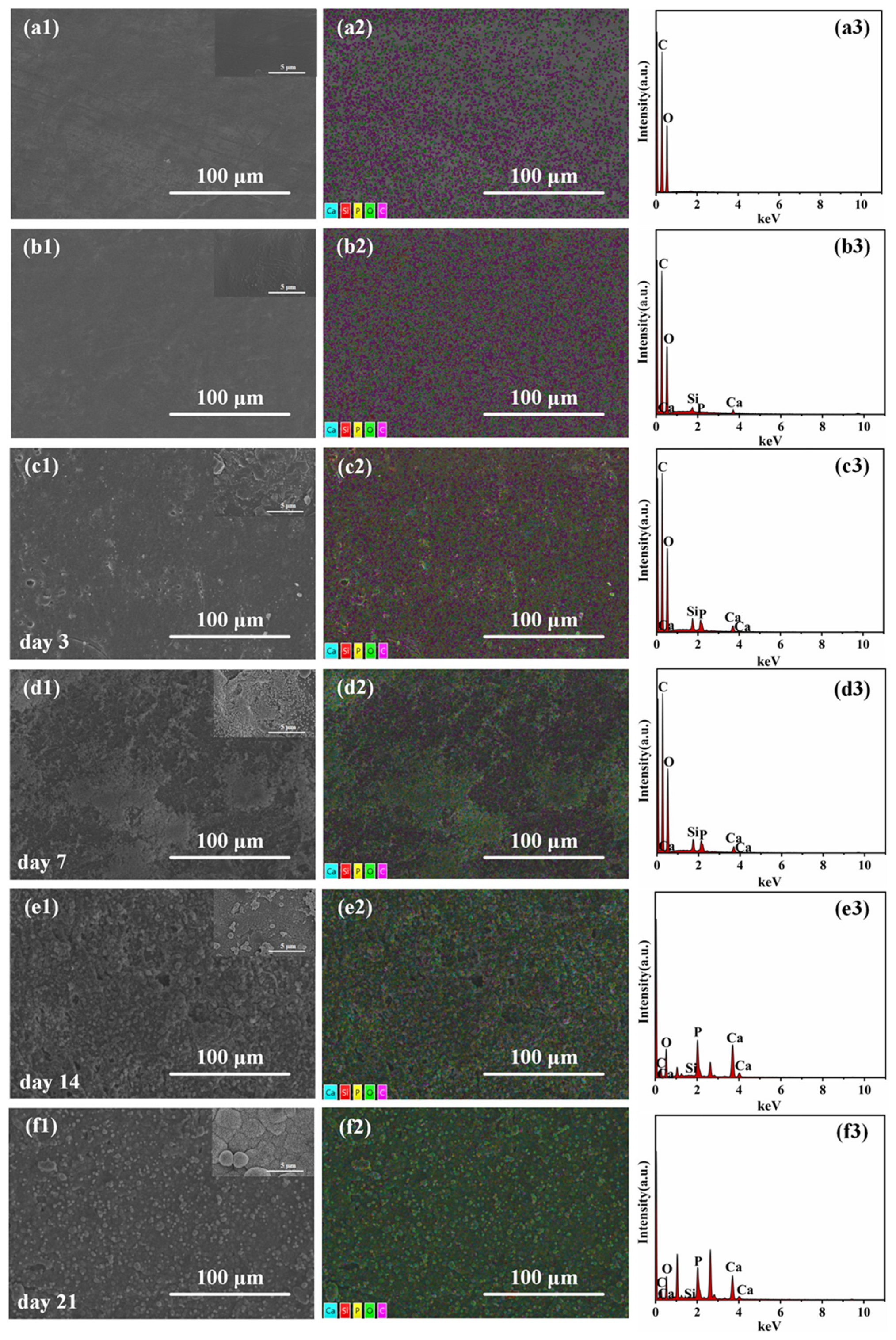
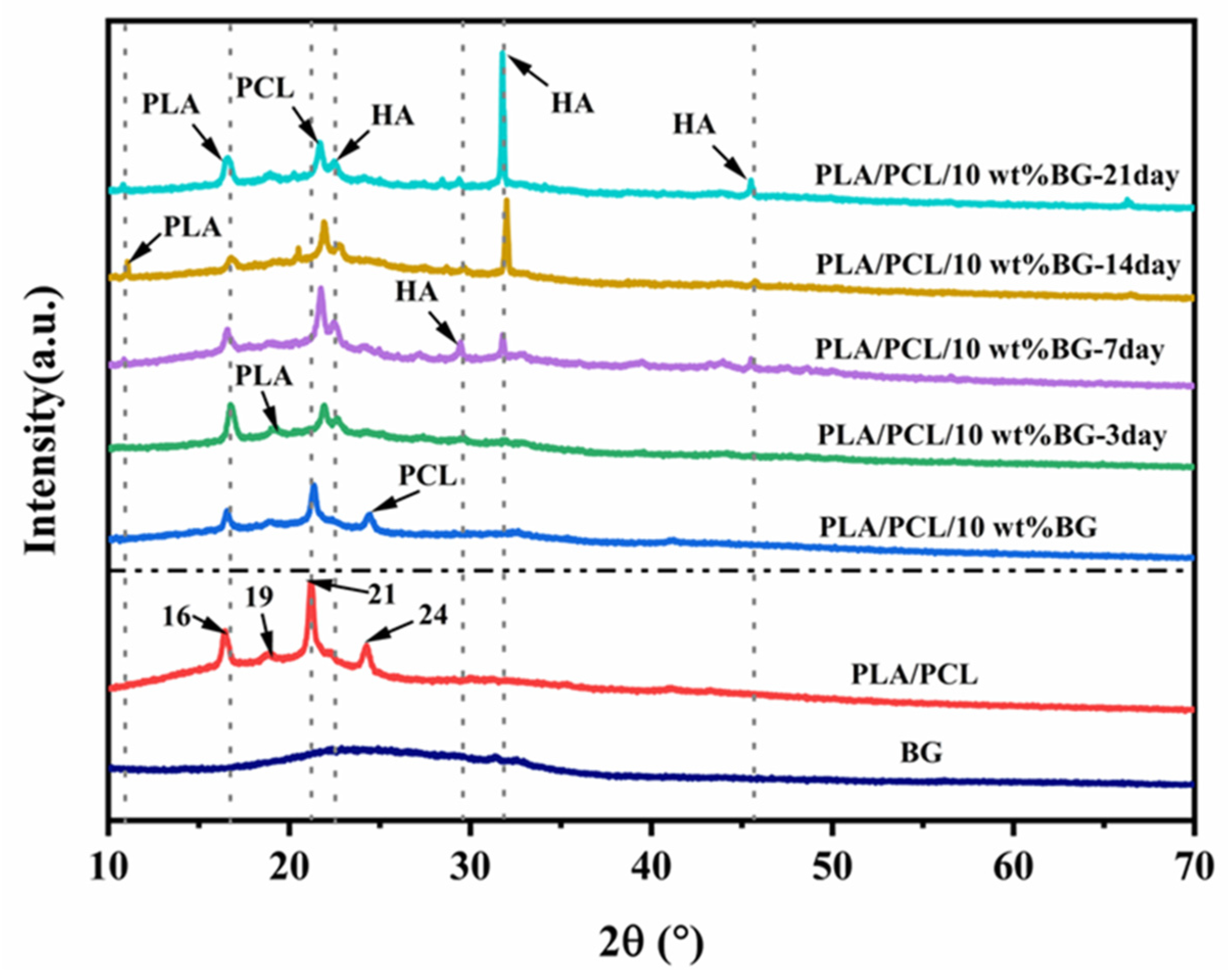
3.6. In Vitro Bioactivity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Datta, S.; Dey, V.; Roy, D.N. Alteration of stainless-steel surface potential by modifying topography inhibits the development of bacterial biofilm. J. Mater. Res. 2024, 39, 1273–1288. [Google Scholar] [CrossRef]
- Kassapidou, M.; Stenport, V.F.; Johansson, C.B.; Syverud, M.; Hammarström Johansson, P.; Börjesson, J.; Hjalmarsson, L. Cobalt chromium alloys in fixed prosthodontics: Investigations of mechanical properties and microstructure. J. Prosthet. Dent. 2023, 130, 255.e1–255.e10. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Wang, L.; Zhang, L.-C. Towards load-bearing biomedical titanium-based alloys: From essential requirements to future developments. Prog. Mater. Sci. 2024, 144, 101277. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Avila, J.D.; Upadhyayula, M.; Bose, S. Porous metal implants: Processing, properties, and challenges. Int. J. Extrem. Manuf. 2023, 5, 032014. [Google Scholar] [CrossRef]
- Chmielewska, A.; Dean, D. The role of stiffness-matching in avoiding stress shielding-induced bone loss and stress concentration-induced skeletal reconstruction device failure. Acta Biomater. 2024, 173, 51–65. [Google Scholar] [CrossRef]
- Mustafa, N.W.N.A.; Ahmad, R.; Ahmad Khushaini, M.A.; Kamar Affendi, N.H.; Ab Ghani, S.M.; Tan, S.K.; Ismail, M.H.; Goo, C.L.; Kassim, M.Z.; Lim, T.W.; et al. Porous NiTi Dental Implant Fabricated by a Metal Injection Molding: An in Vivo Biocompatibility Evaluation in an Animal Model. ACS Biomater. Sci. Eng. 2024, 10, 405–419. [Google Scholar] [CrossRef]
- Hosin, S.; Vermesan, D.; Prejbeanu, R.; Crisan, D.; Al-Qatawneh, M.; Pop, D.; Mioc, M.; Bratosin, F.; Feciche, B.; Hemaswini, K.; et al. Avoiding the Removal of Syndesmotic Screws after Distal Tibiofibular Diastasis Repair: A Benefit or a Drawback? J. Clin. Med. 2022, 11, 6412. [Google Scholar] [CrossRef]
- Xie, Y.; Xiong, H.; Zheng, Z.; Zhang, L.; Chen, Y. Facile and Scalable Fabrication of High-Performance Polylactide-Based Medical Microparts through Combining the Microinjection Molding Intense Shear Stress Field and Annealing Strategy. Ind. Eng. Chem. Res. 2022, 61, 13886–13897. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, J.; Fang, S.; Li, T.; Chen, Y.; Li, L.; Chen, N. A biodegradable, osteo-regenerative and biomechanically robust polylactide bone screw for clinical orthopedic surgery. Int. J. Biol. Macromol. 2024, 283, 137477. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P.; Sandha, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Shen, Y.; Ao, Q. Synthetic biodegradable polymer materials in the repair of tumor-associated bone defects. Front. Bioeng. Biotechnol. 2023, 11, 1096525. [Google Scholar] [CrossRef]
- Jack, K.S.; Velayudhan, S.; Luckman, P.; Trau, M.; Grøndahl, L.; Cooper-White, J. The fabrication and characterization of biodegradable HA/PHBV nanoparticle-polymer composite scaffolds. Acta Biomater. 2009, 5, 2657–2667. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Wheeler, D.L.; Greenspan, D.C. Molecular control of bioactivity in sol-gel glasses. J. Sol-Gel Sci. Technol. 1998, 13, 245–250. [Google Scholar] [CrossRef]
- Verma, R.; Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. Hydroxyapatite-based composites: Excellent materials for environmental remediation and biomedical applications. Adv. Colloid Interface Sci. 2023, 315, 102890. [Google Scholar] [CrossRef]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Doan, V.H.M.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A journey from biomaterials to advanced functional materials. Adv. Colloid Interface Sci. 2023, 321, 103013. [Google Scholar] [CrossRef]
- Humbert, P.; Kampleitner, C.; De Lima, J.; Brennan, M.Á.; Lodoso-Torrecilla, I.; Sadowska, J.M.; Blanchard, F.; Canal, C.; Ginebra, M.-P.; Hoffmann, O.; et al. Phase composition of calcium phosphate materials affects bone formation by modulating osteoclastogenesis. Acta Biomater. 2024, 176, 417–431. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Ma, Y.; Jiang, Y. Calcium Phosphate-Based Nanomaterials: Preparation, Multifunction, and Application for Bone Tissue Engineering. Molecules 2023, 28, 4790. [Google Scholar] [CrossRef]
- Hench, L.L. Bioactive materials: The potential for tissue regeneration. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 41, 511–518. [Google Scholar]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Munakata, M.; Sato, D.; Kataoka, Y.; Kawamata, R. The Effectiveness and Practicality of a Novel Barrier Membrane for the Open Window in Maxillary Sinus Augmentation with a Lateral Approach, with Risk Indicators for Bone Graft Displacement and Bone Height Decrease: A Prospective Study in Humans. Bioengineering 2023, 10, 1110. [Google Scholar] [CrossRef]
- Aminatun; Huriah, R.; Hikmawati, D.; Hadi, S.; Amrillah, T.; Abdullah, C.A.C. Nanofiber Scaffold Based on Polylactic Acid-Polycaprolactone for Anterior Cruciate Ligament Injury. Polymers 2022, 14, 2983. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Li, J.; Wang, R.; Zhang, J.; Lu, Y.; Hu, G.-H.; Wang, Z.; Zhang, L. Current trends in bio-based elastomer materials. SusMat 2022, 2, 2–33. [Google Scholar] [CrossRef]
- Hawes, J.; Gonzalez-Manteiga, A.; Murphy, K.P.; Sanchez-Petidier, M.; Moreno-Manzano, V.; Pathak, B.; Lampe, K.; Lin, C.Y.; Peiro, J.L.; Oria, M. Noggin-Loaded PLA/PCL Patch Inhibits BMP-Initiated Reactive Astrogliosis. Int. J. Mol. Sci. 2024, 25, 11626. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Hu, J.; Dong, Y.; Yu, Z.; Liu, C.; Wang, G.; Chen, S. Advanced biomedical and electronic dual-function skin patch created through microfluidic-regulated 3D bioprinting. Bioact. Mater. 2024, 40, 261–274. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Kang, W.; Liu, X.; Wang, Q. Current advances and future perspectives of additive manufacturing for functional polymeric materials and devices. SusMat 2021, 1, 127–147. [Google Scholar] [CrossRef]
- Álvarez-Carrasco, F.; Varela, P.; Sarabia-Vallejos, M.A.; García-Herrera, C.; Saavedra, M.; Zapata, P.A.; Zárate-Triviño, D.; Martínez, J.J.; Canales, D.A. Development of Bioactive Hybrid Poly(lactic acid)/Poly(methyl methacrylate) (PLA/PMMA) Electrospun Fibers Functionalized with Bioglass Nanoparticles for Bone Tissue Engineering Applications. Int. J. Mol. Sci. 2024, 25, 6843. [Google Scholar] [CrossRef]
- Yon, M.J.Y.; Matinlinna, J.P.; Tsoi, J.K.H.; Vallittu, P.K.; Lassila, L.V.J. Effect of Long-Chain Silane on Mechanical Properties of Experimental Resin Composites. Silicon 2023, 15, 5579–5586. [Google Scholar] [CrossRef]
- Canales, D.; Saavedra, M.; Flores, M.T.; Bejarano, J.; Ortiz, J.A.; Orihuela, P.; Alfaro, A.; Pabón, E.; Palza, H.; Zapata, P.A. Effect of bioglass nanoparticles on the properties and bioactivity of poly(lactic acid) films. J. Biomed. Mater. Res. A 2020, 108, 2032–2043. [Google Scholar] [CrossRef]
- Ji, L.; Wang, W.; Jin, D.; Zhou, S.; Song, X. In vitro bioactivity and mechanical properties of bioactive glass nanoparticles/polycaprolactone composites. Mater. Sci. Eng. C 2015, 46, 1–9. [Google Scholar] [CrossRef]
- Giboz, J.; Copponnex, T.; Mélé, P. Microinjection molding of thermoplastic polymers: Morphological comparison with conventional injection molding. J. Micromech. Microeng. 2009, 19, 025023. [Google Scholar] [CrossRef]
- Tan, J.; Li, T.; Xie, Y.; Chen, M.; Li, L.; Zhang, C.; Chen, Y.; Pang, L.; Zhang, C.; Li, Y.; et al. Fabricating high-performance biomedical PLLA/PVDF blend micro bone screws through in situ structuring of oriented PVDF submicron fibers in microinjection molding. Compos. Part B Eng. 2024, 281, 111567. [Google Scholar] [CrossRef]
- Zhang, L.F.; Chen, Y.H.; Tan, J.Y.; Feng, S.; Xie, Y.P.; Li, L. Performance Enhancement of PLA-Based Blend Microneedle Arrays through Shish-Kebab Structuring Strategy in Microinjection Molding. Polymers 2023, 15, 2234. [Google Scholar] [CrossRef]
- Lei, X.; Gong, X.; Li, J.; Shi, Y.; Liang, M.; Zou, H.; Zhou, S. Fabrication of electrically conductive microparts by constructing carbon black-rich network under high shear conditions in microinjection molding. Front. Mater. 2024, 11, 1415283. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Ding, W.; Zhang, C. Filling behavior, morphology evolution and crystallization behavior of microinjection molded poly(lactic acid)/hydroxyapatite nanocomposites. Compos. Part A Appl. Sci. Manuf. 2015, 72, 85–95. [Google Scholar] [CrossRef]
- Giboz, J.; Copponnex, T.; Mélé, P. Microinjection molding of thermoplastic polymers: A review. J. Micromech. Microeng. 2007, 17, R96–R109. [Google Scholar] [CrossRef]
- Piotter, V.; Mueller, K.; Plewa, K.; Ruprecht, R.; Hausselt, J. Performance and simulation of thermoplastic micro injection molding. Microsyst. Technol. 2002, 8, 387–390. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Saberi, A.; Behnamghader, A.; Aghabarari, B.; Yousefi, A.; Majda, D.; Huerta, M.V.M.; Mozafari, M. 3D direct printing of composite bone scaffolds containing polylactic acid and spray dried mesoporous bioactive glass-ceramic microparticles. Int. J. Biol. Macromol. 2022, 207, 9–22. [Google Scholar] [CrossRef]
- Jariya, S.A.I.; Manivannan, N.; Ali, B.M.; Narayanan, T.S.N.S.; Ravichandran, K. Engineering the surface of titanium to improve its bioactivity and antibacterial activity through a multi-functional coating approach. New J. Chem. 2023, 47, 5843–5862. [Google Scholar] [CrossRef]
- Daskalakis, E.; Hassan, M.H.; Omar, A.M.; Cooper, G.; Weightman, A.; Bartolo, P. Rheological behaviour of different composite materials for additive manufacturing of 3D bone scaffolds. J. Mater. Res. Technol. 2023, 24, 3670–3682. [Google Scholar] [CrossRef]
- Blaker, J.J.; Bismarck, A.; Boccaccini, A.R.; Young, A.M.; Nazhat, S.N. Premature degradation of poly(α-hydroxyesters) during thermal processing of Bioglass®-containing composites. Acta Biomater. 2010, 6, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.; Sarasua, J.-R. Effect of bioactive glass particles on the thermal degradation behaviour of medical polyesters. Polym. Degrad. Stab. 2013, 98, 751–758. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Liu, W.; Yang, Y.; Huang, L.; Huo, E.; Ma, Z. New strategy for reinforcing polylactic acid composites: Towards the insight into the effect of biochar microspheres. Int. J. Biol. Macromol. 2023, 245, 125487. [Google Scholar] [CrossRef] [PubMed]
- Kuklin, V.; Karandashov, S.; Bobina, E.; Drobyshev, S.; Smirnova, A.; Morozov, O.; Danilaev, M. Analysis of Aluminum Oxides Submicron Particle Agglomeration in Polymethyl Methacrylate Composites. Int. J. Mol. Sci. 2023, 24, 2515. [Google Scholar] [CrossRef]
- Ding, W.; Chen, Y.; Liu, Z.; Yang, S.J.R.a. In situ nano-fibrillation of microinjection molded poly (lactic acid)/poly (ε-caprolactone) blends and comparison with conventional injection molding. RSC Adv. 2015, 5, 92905–92917. [Google Scholar] [CrossRef]
- Gönen, S.Ö.; Erol Taygun, M.; Küçükbayrak, S. Fabrication of bioactive glass containing nanocomposite fiber mats for bone tissue engineering applications. Compos. Struct. 2016, 138, 96–106. [Google Scholar] [CrossRef]
- Canales, D.A.; Piñones, N.; Saavedra, M.; Loyo, C.; Palza, H.; Peponi, L.; Leonés, A.; Baier, R.V.; Boccaccini, A.R.; Grünelwald, A.; et al. Fabrication and assessment of bifunctional electrospun poly(l-lactic acid) scaffolds with bioglass and zinc oxide nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2023, 228, 78–88. [Google Scholar] [CrossRef]
- Backes, E.H.; Pires, L.d.N.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Analysis of the Degradation During Melt Processing of PLA/Biosilicate® Composites. J. Compos. Sci. 2019, 3, 52. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Hui, D. Influences of nanoparticles aggregation/agglomeration on the interfacial/interphase and tensile properties of nanocomposites. Compos. Part B Eng. 2017, 122, 41–46. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Kozlov, M.; McCarthy, T.J. Adsorption of Poly(Vinyl Alcohol) from Water to a Hydrophobic Surface: Effects of Molecular Weight, Degree of Hydrolysis, Salt, and Temperature. Langmuir 2004, 20, 9170–9176. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Huang, S.; Linghu, X.; Chen, W.C.; Wang, Y.; Li, J.J.; Yin, H.N.; Zhang, H.; Xu, W.K.; Wa, Q.D. 3D-Printed mesoporous bioglass/polycaprolactone scaffolds induce macrophage polarization toward M2 phenotype and immunomodulates osteogenic differentiation of BMSCs. Int. J. Bioprinting 2024, 10, 320–339. [Google Scholar] [CrossRef]
- Mousavi Nejad, Z.; Zamanian, A.; Saeidifar, M.; Vanaei, H.R.; Salar Amoli, M. 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers 2021, 13, 4442. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Tzetzis, D.; Bikiaris, D. Composite Membranes of Poly(?-caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications. Molecules 2019, 24, 3067. [Google Scholar] [CrossRef]
- Vafa, E.; Tayebi, L.; Abbasi, M.; Azizli, M.J.; Bazargan-Lari, R.; Talaiekhozani, A.; Zareshahrabadi, Z.; Vaez, A.; Amani, A.M.; Kamyab, H.; et al. A better roadmap for designing novel bioactive glasses: Effective approaches for the development of innovative revolutionary bioglasses for future biomedical applications. Environ. Sci. Pollut. Res. Int. 2023, 30, 116960–116983. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Fathi, M.H.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Chandra Bose, A.; Rameshbabu, N. X-ray peak broadening studies of nanocrystalline hydroxyapatite by Williamson–Hall analysis. Phys. B Condens. Matter 2010, 405, 4256–4261. [Google Scholar] [CrossRef]
- Vasconcelos, E.V.; da Luz, F.B.; da Paz, S.P.A.; dos Reis, M.A.L.; da Silva, A.C.R.; Passos, M.F.; Barboza, C.A.G.; Monteiro, S.N.; Candido, V.S. Nanostructured 3D bioprinting of PLA with bioglass-CNT scaffolds for osseus tissue graft manufacturing. J. Mater. Res. Technol. 2023, 23, 5923–5938. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chueh, J.-Y.; Tseng, H.; Huang, H.-M.; Lee, S.-Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- de Moura, N.K.; Martins, E.F.; Oliveira, R.L.M.S.; de Brito Siqueira, I.A.W.; Machado, J.P.B.; Esposito, E.; Amaral, S.S.; de Vasconcellos, L.M.R.; Passador, F.R.; de Sousa Trichês, E. Synergistic effect of adding bioglass and carbon nanotubes on poly (lactic acid) porous membranes for guided bone regeneration. Mater. Sci. Eng. C 2020, 117, 111327. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Thomas, S.; Loganathan, S.; Valapa, R.B. 3D bioprinted poly(lactic acid)/mesoporous bioactive glass based biomimetic scaffold with rapid apatite crystallization and in-vitro Cytocompatability for bone tissue engineering. Int. J. Biol. Macromol. 2022, 217, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Canales, D.A.; Reyes, F.; Saavedra, M.; Peponi, L.; Leonés, A.; Palza, H.; Boccaccini, A.R.; Grünewald, A.; Zapata, P.A. Electrospun fibers of poly (lactic acid) containing bioactive glass and magnesium oxide nanoparticles for bone tissue regeneration. Int. J. Biol. Macromol. 2022, 210, 324–336. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M. Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Li, Z.; Li, Y.; Wang, X.; Jiang, J.; Li, Q. Enhancing interfacial interaction of immiscible PCL/PLA blends by in-situ crosslinking to improve the foamability. Polym. Test. 2023, 124, 108063. [Google Scholar] [CrossRef]
- Mahović Poljaček, S.; Priselac, D.; Tomašegović, T.; Leskovac, M.; Šoster, A.; Stanković Elesini, U. Quantitative Analysis of Morphology and Surface Properties of Poly(lactic acid)/Poly(ε-caprolactone)/Hydrophilic Nano-Silica Blends. Polymers 2024, 16, 1739. [Google Scholar] [CrossRef]
- Vignoles, M.; Bonel, G.; Holcomb, D.W.; Young, R.A. Influence of preparation conditions on the composition of type B carbonated hydroxyapatite and on the localization of the carbonate ions. Calcif. Tissue Int. 1988, 43, 33–40. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Sroka-Bartnicka, A.; Borkowski, L.; Ginalska, G.; Ślósarczyk, A.; Kazarian, S.G. Structural transformation of synthetic hydroxyapatite under simulated in vivo conditions studied with ATR-FTIR spectroscopic imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 155–161. [Google Scholar] [CrossRef]
- Liu, X.; Khor, S.; Petinakis, E.; Yu, L.; Simon, G.; Dean, K.; Bateman, S. Effects of hydrophilic fillers on the thermal degradation of poly(lactic acid). Thermochim. Acta 2010, 509, 147–151. [Google Scholar] [CrossRef]
- Sivalingam, G.; Madras, G. Thermal degradation of binary physical mixtures and copolymers of poly(ε-caprolactone), poly(d, l-lactide), poly(glycolide). Polym. Degrad. Stab. 2004, 84, 393–398. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.J.L.U. Thermal Degradation of Polymeric Materials; Rapra Technology: Shropshire, UK, 2005; pp. 80–100. [Google Scholar]
- Abdullah, N.; Kamarazaman, Z. Copper (II) Mixed Carboxylates as Metal-Containing Ionic Liquids. In Proceedings of the Nanoscience and Nanotechnology: International Conference on Nanoscience and Nanotechnology-2008; American Institute of Physics (AIP): Melville, NY, USA, 2009; Volume 1136, pp. 361–365. [Google Scholar] [CrossRef]
- Seesanong, S.; Wongchompoo, Y.; Boonchom, B.; Sronsri, C.; Laohavisuti, N.; Chaiseeda, K.; Boonmee, W. Economical and Environmentally Friendly Track of Biowaste Recycling of Scallop Shells to Calcium Lactate. ACS Omega 2022, 7, 14756–14764. [Google Scholar] [CrossRef]
- Landoll, M.P.; Holtzapple, M.T. Kinetics study of thermal decomposition of sodium carboxylate salts. Biomass Bioenergy 2012, 45, 195–202. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Chen, Y.; He, H.; Zhou, X.; Chen, N. Structure and Property Evolution of Microinjection Molded PLA/PCL/Bioactive Glass Composite. Polymers 2025, 17, 991. https://doi.org/10.3390/polym17070991
Chen M, Chen Y, He H, Zhou X, Chen N. Structure and Property Evolution of Microinjection Molded PLA/PCL/Bioactive Glass Composite. Polymers. 2025; 17(7):991. https://doi.org/10.3390/polym17070991
Chicago/Turabian StyleChen, Meiqiong, Yinghong Chen, Haihao He, Xinwen Zhou, and Ning Chen. 2025. "Structure and Property Evolution of Microinjection Molded PLA/PCL/Bioactive Glass Composite" Polymers 17, no. 7: 991. https://doi.org/10.3390/polym17070991
APA StyleChen, M., Chen, Y., He, H., Zhou, X., & Chen, N. (2025). Structure and Property Evolution of Microinjection Molded PLA/PCL/Bioactive Glass Composite. Polymers, 17(7), 991. https://doi.org/10.3390/polym17070991






