Investigation into the Synthetic Strategies of Melamine-Based Porous Polymeric Materials: A Bibliometric Analysis
Abstract
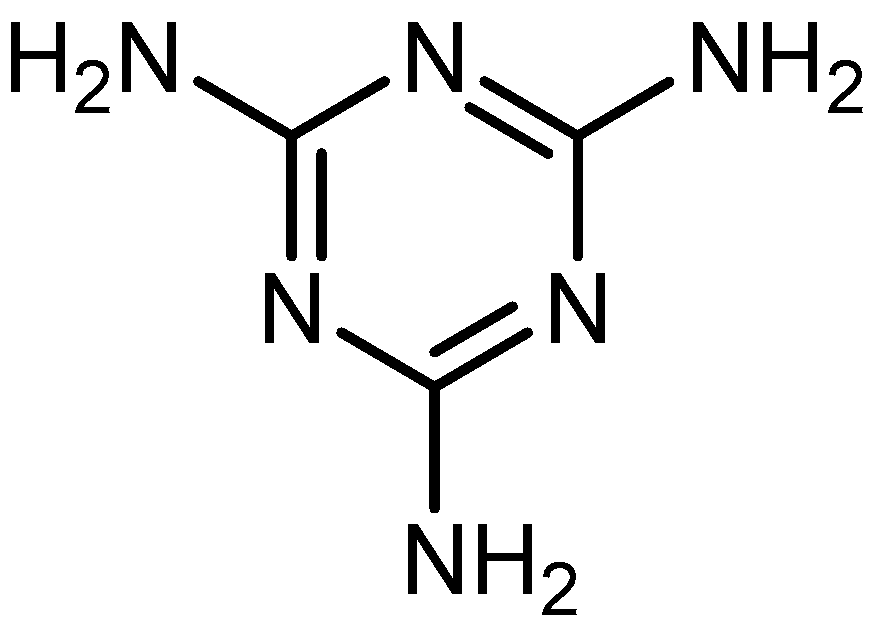
1. Introduction
- Melamine-based POPs are synthesized under various kinds of experimental circumstances, involving reactions conducted at ambient temperatures.
- They are prepared by many molecular precursors that react with melamine to form strong and stable crosslinking networks.
- They have already demonstrated a wide range of promising physical, chemical and thermal properties, allowing them to be employed for various applications.
- Melamine-based POPs have large surface areas with microporous structures, making them suitable for post-modification, i.e., as support for stabilized fine metal nanoparticles.
2. Data Collection
- What is the distribution of research articles by years in the field?
- What are the most relevant authors and journals in the field?
- What are the most productive countries and institutions in the field?
- What are the primary research keywords in the field?
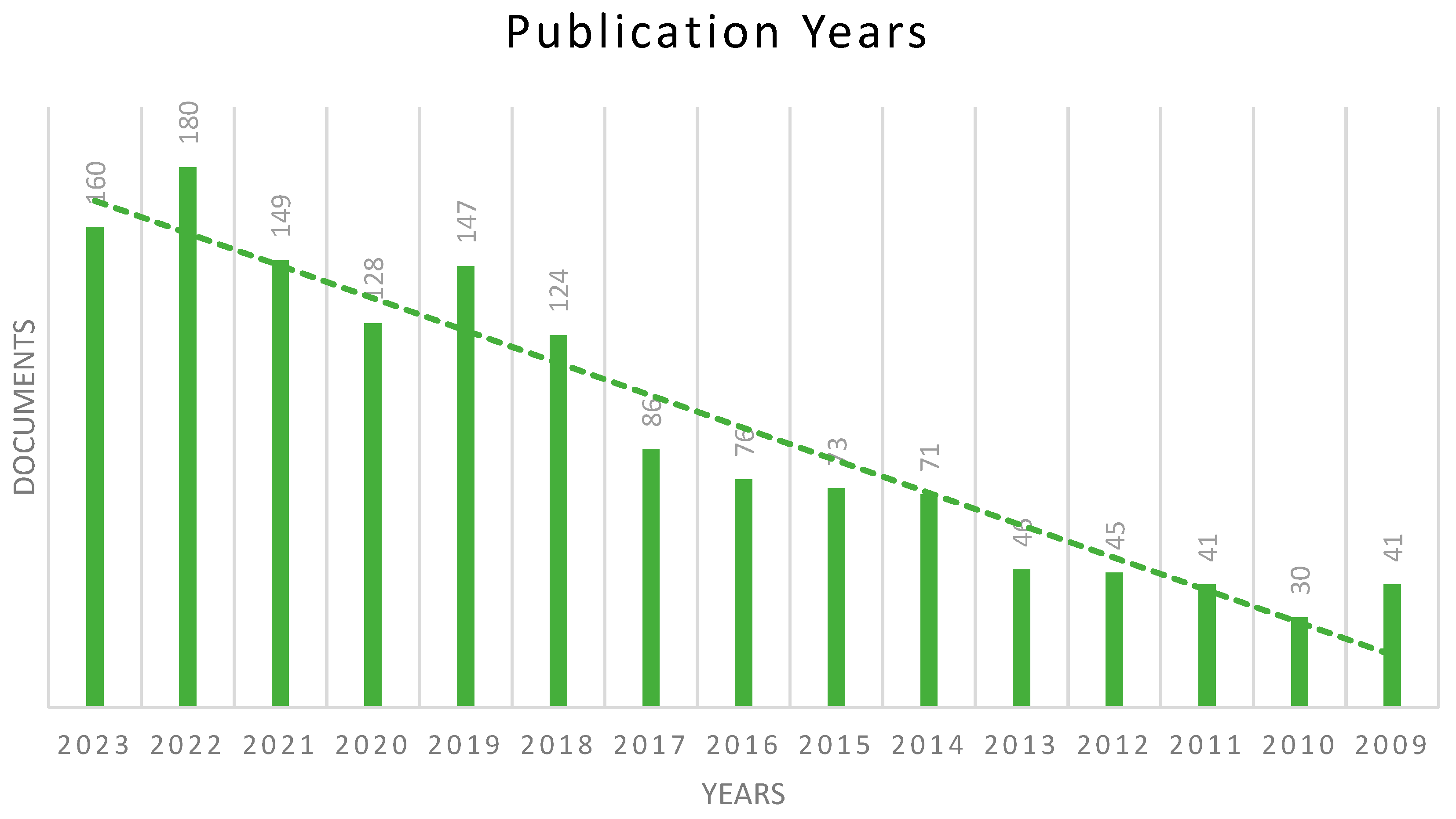
2.1. Publication Years
2.2. Document Types and Categories
2.3. Active Authors
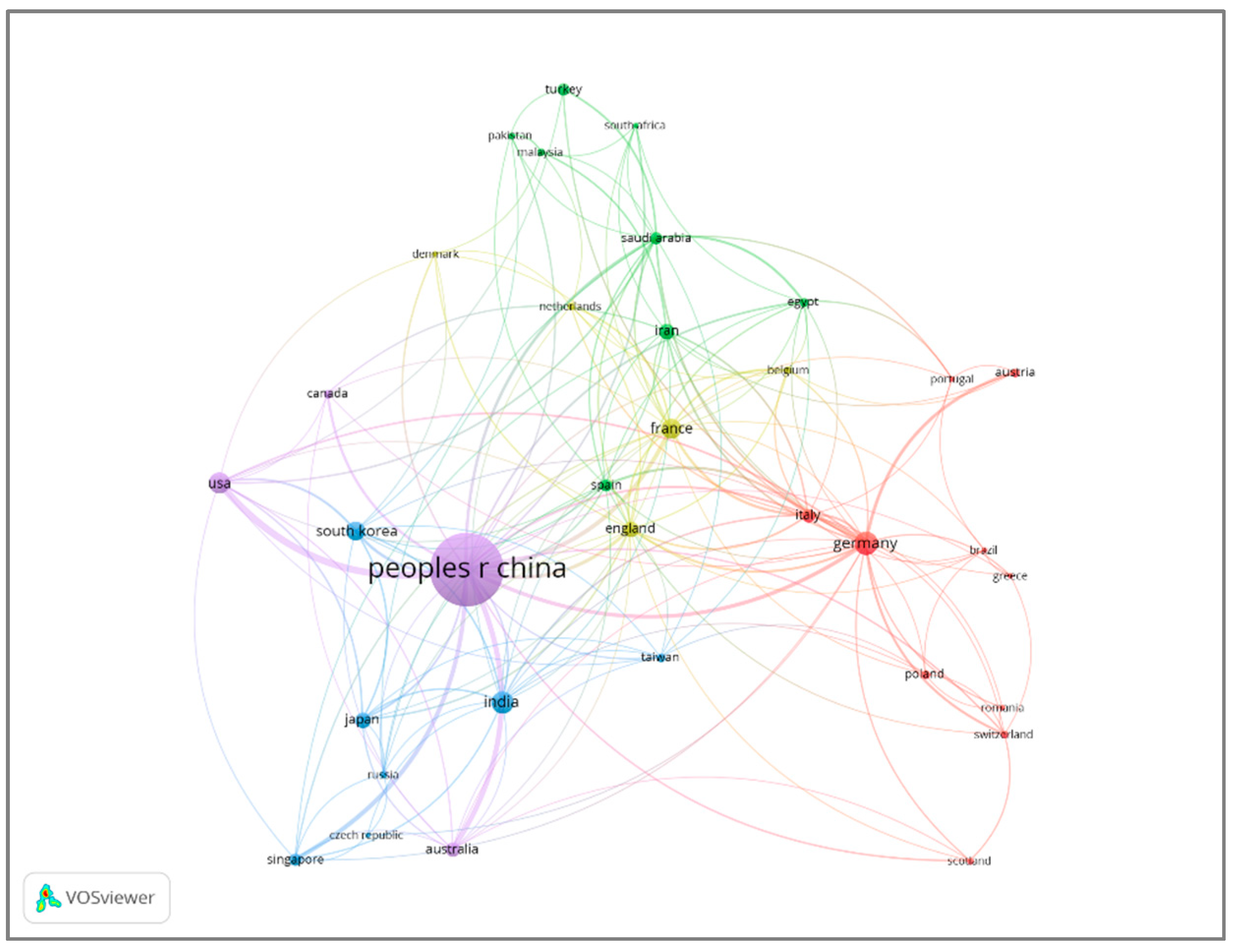
2.4. Active Countries and Institutions
2.5. Active Journals
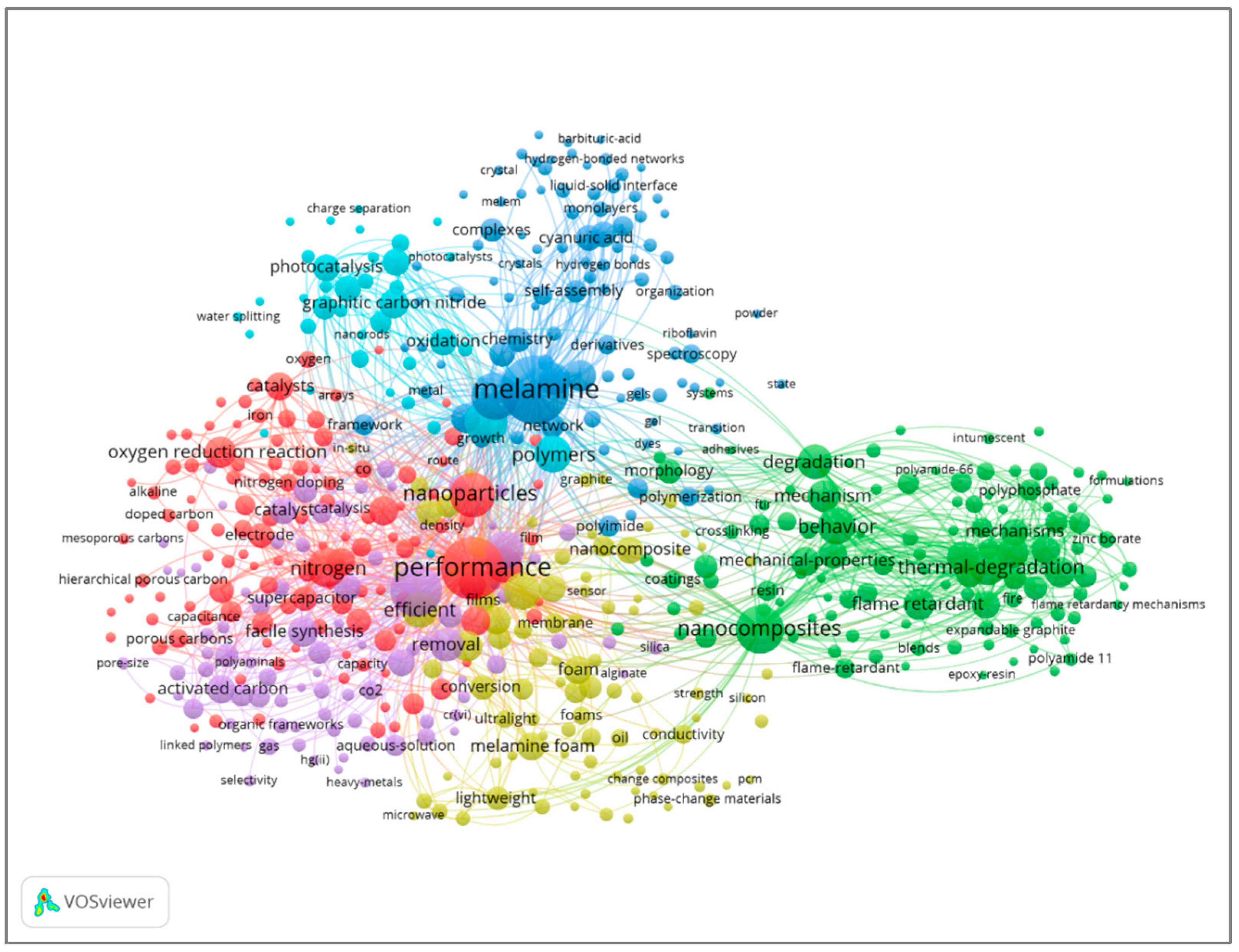
2.6. Keyword Co-Occurrence
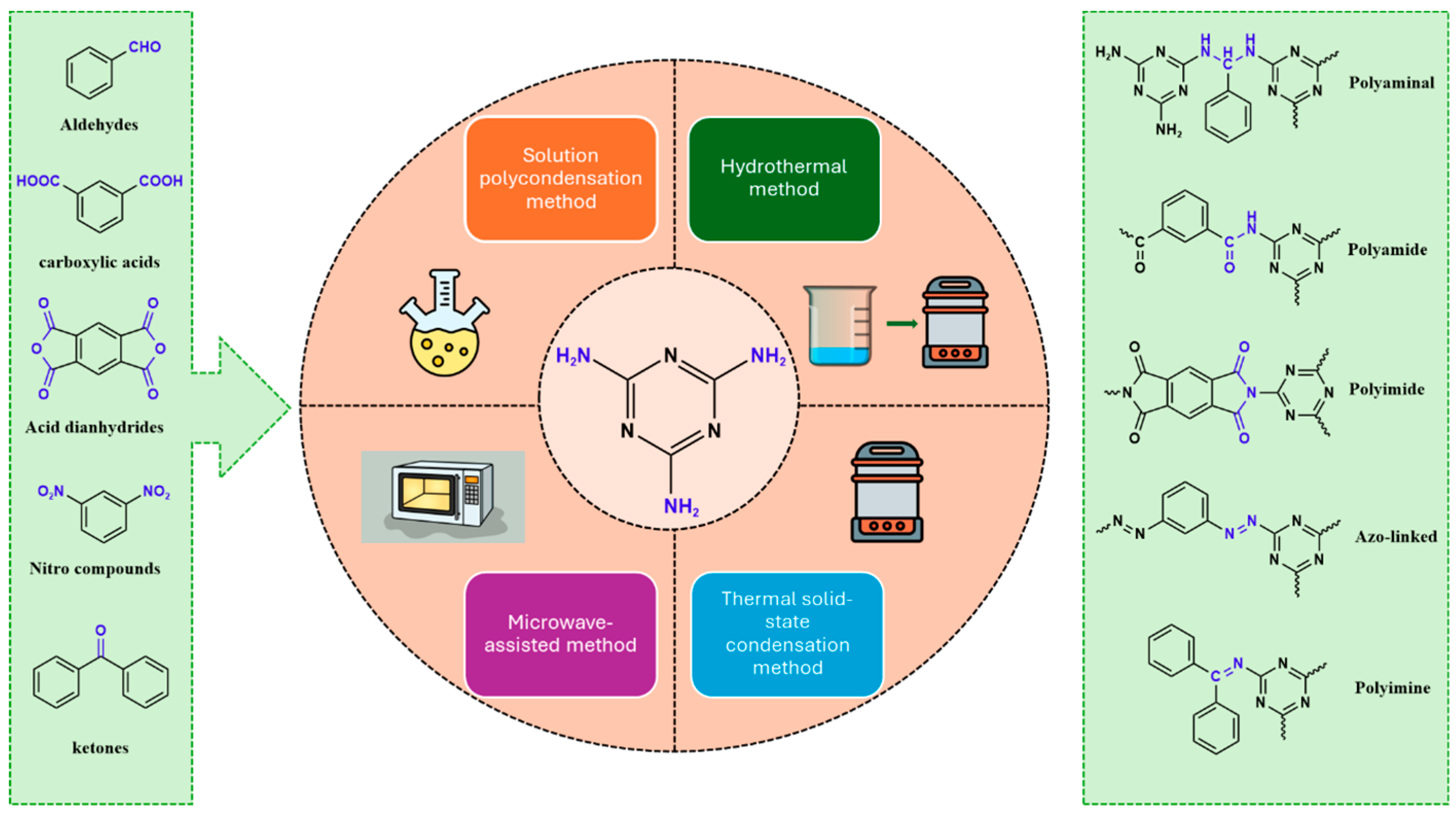
3. Synthesis Strategies of Melamine-Based POPs
3.1. Polyaminal
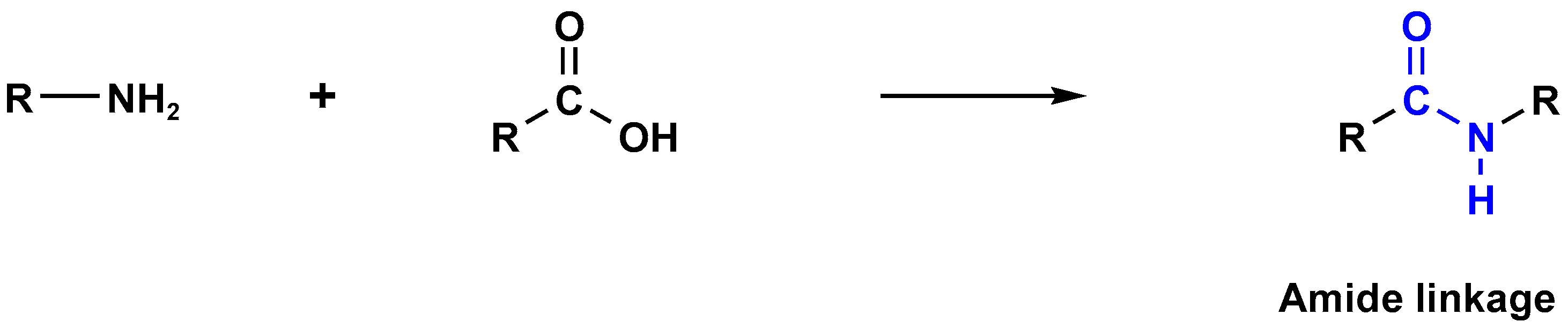
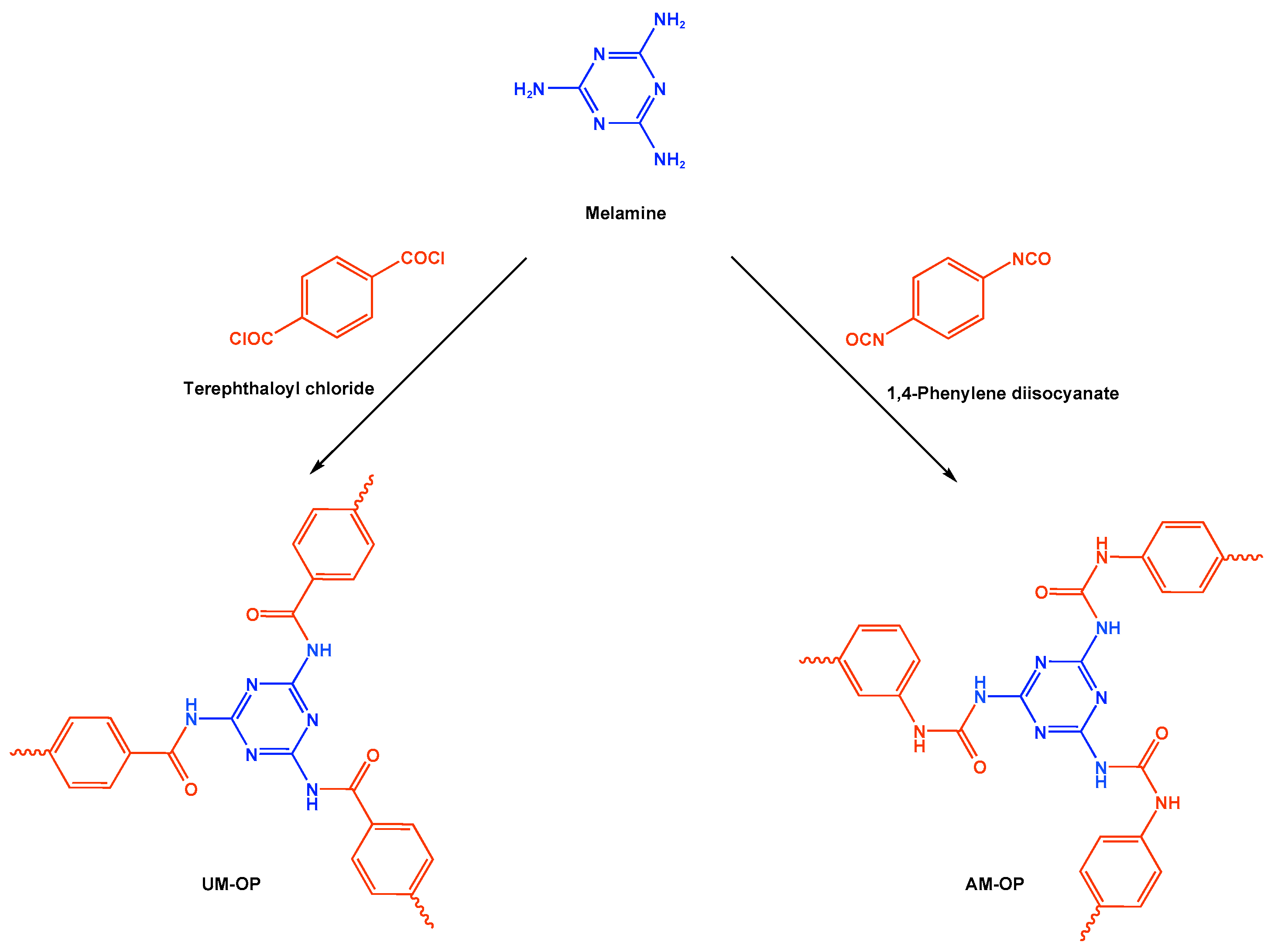
3.2. Polyamide
| Entry | Materials | Building Block | S(BET) .m2/g | Application | Ref. |
|---|---|---|---|---|---|
| 1 | DMAc/NMP | 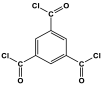 | 84.5 | CO2 uptake of 2.99 cm3/g | [62] |
| 2 | OTPA |  | 83 | 1.83 mmol/g | [69] |
| 3 | MTPA | 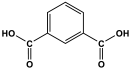 | 481 | 2.87 mmol/g | |
| 4 | TMPA |  | 404 | 2.12 mmol/g | |
| 5 | PTPA-3 |  | 521 | 2.65 mmol/g | |
| 6 | AM-OP |  | 24.72 | CO2 uptake of 3.23 cm3/g | [70] |
| 7 | PA2 | 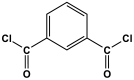 | 20.18 | CO2 uptake of 1.7 cm3/g | [66] |
| 8 | PA6 | 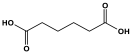 | - | - | [71] |
| 9 | ME-MA/PAN |  | - | For methylene blue removal | [72] |
3.3. Polyimide
3.4. Azo-Linkage
3.5. Polyimine
3.6. Other Melamine-Based POPs
| Method | Porosity and Surface Area | Chemical Stability | Scalability | Advantages | Limitations | References |
|---|---|---|---|---|---|---|
| Conventional condensation | Moderate to high | High | High | Simple, cost-effective | Limited porosity control and long reaction times | [112] |
| Solvothermal synthesis | High | High | Low (batch process) | High porosity, good crystallinity | Long reaction times | [78] |
| Hydrothermal synthesis | Moderate to high | High | Medium | Eco-friendly, good functionalization | High temperature requirement | [78] |
| Microwave-assisted synthesis | Low to moderate | High | High | Fast reaction, energy efficient | Potential heating inhomogeneity | [117] |
4. Applications for Melamine-Based POPs
- CO2 Capture
- 2.
- Water Treatment
- 3.
- Heterogeneous Catalysis
- 4.
- Sensing
5. Conclusions
Funding
Conflicts of Interest
References
- Gao, H.; Li, Q.; Ren, S. Progress on CO2 capture by porous organic polymers. Curr. Opin. Green Sustain. Chem. 2019, 16, 33–38. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, Y.; Zhang, W. Development of organic porous materials through Schiff-base chemistry. CrystEngComm 2013, 15, 1484–1499. [Google Scholar] [CrossRef]
- Zou, L.; Sun, Y.; Che, S.; Yang, X.; Wang, X.; Bosch, M.; Wang, Q.; Li, H.; Smith, M.; Yuan, S.; et al. Porous organic polymers for post-combustion carbon capture. Adv. Mater. 2017, 29, 1700229. [Google Scholar] [CrossRef]
- Chakraborty, J.; Nath, I.; Song, S.; Mohamed, S.; Khan, A.; Heynderickx, P.M.; Verpoort, F. Porous organic polymer composites as surging catalysts for visible-light-driven chemical transformations and pollutant degradation. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100319. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Mellon, N.B.; Rafiq, S.; Babar, M.; Ullah, S.; Muhammad, N.; Khan, A.L.; Ayoub, M.; Ibrahim, M.; et al. A review on CO2 capture via nitrogen-doped porous polymers and catalytic conversion as a feedstock for fuels. J. Clean. Prod. 2020, 277, 123999. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Lee, Y.R.; Zhang, S.; Ahn, W.S. Triazine-based covalent organic polymers: Design, synthesis and applications in heterogeneous catalysis. J. Mater. Chem. A 2016, 4, 16288–16311. [Google Scholar] [CrossRef]
- Modak, A.; Bhanja, P.; Selvaraj, M.; Bhaumik, A. Functionalized porous organic materials as efficient media for the adsorptive removal of Hg(ii) ions. Environ. Sci. Nano 2020, 7, 2887–2923. [Google Scholar] [CrossRef]
- Wu, J.; Xu, F.; Li, S.; Ma, P.; Zhang, X.; Liu, Q.; Fu, R.; Wu, D. Porous Polymers as Multifunctional Material Platforms toward Task-Specific Applications. Adv. Mater. 2019, 31, 1802922. [Google Scholar] [CrossRef]
- Das, S.K.; Wang, X.; Ostwal, M.M.; Lai, Z. A highly stable microporous covalent imine network adsorbent for natural gas upgrading and flue gas CO2 capture. Sep. Purif. Technol. 2016, 170, 68–77. [Google Scholar] [CrossRef]
- Jiangfei, G.; Lizhi, W.; Zhang, D.; Huang, J. Amino-Functionalized Porphyrin-Based Porous Organic Polymers for CO2Capture and Hg2+Removal. Energy Fuels 2020, 34, 9771–9778. [Google Scholar] [CrossRef]
- Krupadam, R.J.; Rayalu, S.S. Melamine-based resins and their carbons for CO2 capture: A review. Emergent Mater. 2021, 4, 545–563. [Google Scholar] [CrossRef]
- Roy, B.; Bairi, P.; Nandi, A.K. Supramolecular assembly of melamine and its derivatives: Nanostructures to functional materials. RSC Adv. 2014, 4, 1708–1734. [Google Scholar] [CrossRef]
- Bretterbauer, K.; Schwarzinger, C.; Cyanuric, K. Melamine derivative—A review on synthesis and application. Curr. Org. Synth. 2012, 9, 342–356. [Google Scholar]
- Li, G.; Zhang, B.; Yan, J.; Wang, Z. The cost-effective synthesis of furan- and thienyl-based microporous polyaminals for adsorption of gases and organic vapors. Chem. Commun. 2016, 52, 1143–1146. [Google Scholar] [CrossRef]
- Liu, Y.; Jian, J.H.; Luan, J.; Zhang, X.S.; Sun, Z.B.; Liu, H.X.; Yang, W.; Li, W.Z. Fabrication of a family of porous azo polymers with multifunctional adsorption and separation performances. J. Environ. Chem. Eng. 2024, 12, 113653. [Google Scholar] [CrossRef]
- Jaleel, A.; Kim, S.H.; Natarajan, P.; Gunasekar, G.H.; Park, K.; Yoon, S.; Jung, K.D. Hydrogenation of CO2 to formates on ruthenium(III) coordinated on melamine polymer network. J. CO2 Util. 2020, 35, 245–255. [Google Scholar] [CrossRef]
- Passas, I. Bibliometric analysis: The main steps. Encyclopedia 2024, 4, 1014–1025. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Naseer, M.N.; Zaidi, A.A.; Dutta, K.; Wahab, Y.A.; Jaafar, J.; Nusrat, R.; Ullah, I.; Kim, B. Past, present and future of materials’ applications for CO2 capture: A bibliometric analysis. Energy Rep. 2022, 8, 4252–4264. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Liu, Y.; Song, Y.; Zeng, P.; Zhang, Y. The research trends of metal-organic frameworks in environmental science: A review based on bibliometric analysis. Environ. Sci. Pollut. Res. 2020, 27, 19265–19284. [Google Scholar] [CrossRef]
- Niu, L.; Zhao, X.; Wu, F.; Tang, Z.; Lv, H.; Wang, J.; Fang, M.; Giesy, J.P. Hotpots and trends of covalent organic frameworks (COFs) in the environmental and energy field: Bibliometric analysis. Sci. Total Environ. 2021, 783, 146838. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Gong, W.; Sun, T.; Wang, Z.; Ning, G. Pillar[5]arene-Derived Microporous Polyaminal Networks with Enhanced Uptake Performance for CO2 and Iodine. Ind. Eng. Chem. Res. 2020, 59, 3269–3278. [Google Scholar] [CrossRef]
- Tan, M.X.; Zhang, Y.; Ying, J.Y. Mesoporous poly(melamine-formaldehyde) solid sorbent for carbon dioxide capture. ChemSusChem 2013, 6, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.X.; Sum, Y.N.; Ying, J.Y.; Zhang, Y. A mesoporous poly-melamine-formaldehyde polymer as a solid sorbent for toxic metal removal. Energy Environ. Sci. 2013, 6, 3254–3259. [Google Scholar] [CrossRef]
- Shunmughanathan, M.; Puthiaraj, P.; Pitchumani, K. Melamine-based microporous network polymer supported palladium nanoparticles: A stable and efficient catalyst for the sonogashira coupling reaction in water. ChemCatChem 2015, 7, 666–673. [Google Scholar] [CrossRef]
- Molla, R.A.; Iqubal, M.A.; Ghosh, K.; Kamaluddin; Islam, S. M. Nitrogen enriched mesoporous organic polymer anchored copper(ii) material: An efficient and reusable catalyst for the synthesis of esters and amides from aromatic systems. Dalt. Trans. 2015, 44, 6546–6559. [Google Scholar] [CrossRef]
- Yang, D.; Liu, P.; Zhang, N.; Wei, W.; Yue, M.; You, J.; Wang, H. Mesoporous poly(melamine-formaldehyde): A green and recyclable heterogeneous organocatalyst for the synthesis of benzoxazoles and benzothiazoles using dioxygen as oxidant. ChemCatChem 2014, 6, 3434–3439. [Google Scholar] [CrossRef]
- Dey, D.; Chandra Murmu, N.; Banerjee, P. Tailor-made synthesis of an melamine-based aminal hydrophobic polymer for selective adsorption of toxic organic pollutants: An initiative towards wastewater purification. RSC Adv. 2019, 9, 7469–7478. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Yan, J.; Wang, Z. Tetraphenyladamantane-based polyaminals for highly efficient captures of CO2 and organic vapors. Macromolecules 2014, 47, 6664–6670. [Google Scholar] [CrossRef]
- Taskin, O.S.; Dadashi-Silab, S.; Kiskan, B.; Weber, J.; Yagci, Y. Highly Efficient and Reusable Microporous Schiff Base Network Polymer as a Heterogeneous Catalyst for CuAAC Click Reaction. Macromol. Chem. Phys. 2015, 216, 1746–1753. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, J.; Shang, Y.; Wang, Z. Synthesis of fluorescent micro- and mesoporous polyaminals for detection of toxic pesticides. Macromolecules 2018, 51, 1769–1776. [Google Scholar] [CrossRef]
- Sobarzo, P.A.; Tundidor, A.; Sanz-Perez, E.S.; Terraza, C.A.; Maya, E.M. Effect of thiophene, furan moieties and zinc ions on melamine-based porous polyaminals properties and catalytic activity on CO2 cycloaddition reaction. Eur. Polym. J. 2022, 177, 111444. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S.J. Highlighting the relative effects of surface characteristics and porosity on CO2 capture by adsorbents templated from melamine-based polyaminals. J. Solid State Chem. 2018, 258, 573–581. [Google Scholar] [CrossRef]
- Senthilkumaran, M.; Saravanan, C.; Mareeswaran, P.M. Synthesis and characterization of polyaminals from melamine derivative for carbon dioxide capture studies. Mater. Today Proc. 2021, 40, S117–S119. [Google Scholar] [CrossRef]
- Senthilkumaran, M.; Saravanan, C.; Sethuraman, V.; Puthiaraj, P.; Muthu Mareeswaran, P. Nitrogen-rich polyaminal porous network for CO2 uptake studies and preparation of carbonized materials. Eur. Polym. J. 2020, 124, 109477. [Google Scholar] [CrossRef]
- Rong, M.; Yang, L.; Wang, L.; Yu, J.; Qu, H.; Liu, H. Fabrication of ultramicroporous triphenylamine-based polyaminal networks for low-pressure carbon dioxide capture. J. Colloid Interface Sci. 2019, 548, 265–274. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Zhang, W.; Zhu, Y.; Wang, D.G.; Yu, G.; Kuang, G.C. Nitrogen-rich porous polyaminal network as a platform for iodine adsorption through physical and chemical interaction. J. Appl. Polym. Sci. 2018, 135, 46106. [Google Scholar] [CrossRef]
- Alotaibi, M.M.; Almalki, B.; Tashkandi, N.; Basingab, F.; Abdullah, S.; Alkayal, N.S. Synthesis of silver nanoparticles embedded into melamine polyaminal networks as antibacterial and anticancer active agents. Sci. Rep. 2024, 14, 20008. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Wang, Z. Facile Synthesis of Fluorinated Microporous Polyaminals for Adsorption of Carbon Dioxide and Selectivities over Nitrogen and Methane. Macromolecules 2016, 49, 2575–2581. [Google Scholar] [CrossRef]
- Ibrahim, M.; Tashkandi, N.; Hadjichristidis, N.; Alkayal, N.S. Synthesis of Naphthalene-Based Polyaminal-Linked Porous Polymers for Highly Effective Uptake of CO2 and Heavy Metals. Polymers 2022, 14, 1136–1150. [Google Scholar] [CrossRef]
- Alharthi, A.M.; Alkayal, N.S.; Alotaibi, M.M.; Alrayyani, M.A.; Bahaidarah, E.A.; Alshareef, F.M. Synthesis of anthracene-based polyaminal polymer for CO2 capture and lead (II) removal from water waste. Adsorpt. Sci. Technol. 2024, 42. [Google Scholar] [CrossRef]
- Shen, K.; Wen, M.; Fan, C.G.; Lin, S.H.; Pan, Q.M. Melamine-based porous organic polymers supported Pd(II)-catalyzed addition of arylboronic acids to aromatic aldehydes. Catal. Lett. 2021, 151, 2612–2621. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Chen, Y.; Hu, T.; Pudukudy, M.; Shi, L.; Shan, S.; Zhi, Y. Modified melamine-based porous organic polymers with imidazolium ionic liquids as efficient heterogeneous catalysts for CO2 cycloaddition. J. Colloid Interface Sci. 2023, 652, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.J.; Pudukudy, M.; Lin, L.; Yang, H.G.; Li, M.R.; Shan, S.Y.; Hu, T.D.; Zhi, Y.F. Melamine-based nitrogen-heterocyclic polymer networks as efficient platforms for CO2 adsorption and conversion. Sep. Purif. Technol. 2024, 331, 125645. [Google Scholar] [CrossRef]
- Schwab, M.G.; Fassbender, B.; Spiess, H.W.; Thomas, A.; Feng, X.; Müllen, K. Catalyst-free preparation of melamine-based microporous polymer networks through Schiff base chemistry. J. Am. Chem. Soc. 2009, 131, 7216–7217. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Giordano, C.; Gradzielski, M.; Mehta, S.K. Microwave-assisted synthesis of small Ru nanoparticles and their role in degradation of congo red. J. Colloid Interface Sci. 2013, 411, 173–181. [Google Scholar] [CrossRef]
- Díaz de Greñu, B.; Torres, J.; García-González, J.; Muñoz-Pina, S.; de los Reyes, R.; Costero, A.M.; Amorós, P.; Ros-Lis, J.V. Microwave-assisted synthesis of covalent organic frameworks: A review. ChemSusChem 2021, 14, 208–233. [Google Scholar] [CrossRef]
- Yang, G.; Han, H.; Du, C.; Luo, Z.; Wang, Y. Facile synthesis of melamine-based porous polymer networks and their application for removal of aqueous mercury ions. Polymer 2010, 51, 6193–6202. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, L.G.; Yuan, Y.P.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Microwave-assisted synthesis of highly fluorescent nanoparticles of a melamine-based porous covalent organic framework for trace-level detection of nitroaromatic explosives. J. Hazard. Mater. 2012, 221–222, 147–154. [Google Scholar] [CrossRef]
- Sandín, R.; González-Lucas, M.; Sobarzo, P.A.; Terraza, C.A.; Maya, E.M. Microwave-assisted melamine-based polyaminals and their application for metal cations adsorption. Eur. Polym. J. 2021, 155, 110562. [Google Scholar] [CrossRef]
- Song, K.S.; Fritz, P.W.; Coskun, A. Porous organic polymers for CO2 capture, separation and conversion. Chem. Soc. Rev. 2022, 51, 9831–9852. [Google Scholar] [CrossRef]
- Hu, J.X.; Shang, H.; Wang, J.G.; Luo, L.; Xiao, Q.; Zhong, Y.J.; Zhu, W.D. Highly enhanced selectivity and easy regeneration for the separation of CO2 over N2 on melamine-based microporous organic polymers. Ind. Eng. Chem. Res. 2014, 53, 11828–11837. [Google Scholar] [CrossRef]
- Zhang, N.; Zou, B.; Yang, G.P.; Yu, B.; Hu, C.W. Melamine-based mesoporous organic polymers as metal-Journal of CO2 UtilizFree heterogeneous catalyst: Effect of hydroxyl on CO2 capture and conversion. Ation 2017, 22, 9–14. [Google Scholar] [CrossRef]
- Alkayal, N.S.; Alotaibi, M.M.; Tashkandi, N.Y.; Alrayyani, M.A. Synthesis and characterization of bipyridine-based polyaminal network for CO2 capture. Polymers 2022, 14, 3746. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, C.; Chen, J.; Liu, L.; Yang, Q. Synthesis of a pyridine–zinc-based porous organic polymer for the Co-catalyst-free cycloaddition of epoxides. Chem. An Asian J. 2017, 12, 1095–1103. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhong, M.; Yang, Q. Hierarchical mesoporous organic polymer with an intercalated metal complex for the efficient synthesis of cyclic carbonates from flue gas. Green Chem. 2016, 18, 6493–6500. [Google Scholar] [CrossRef]
- Tang, F.; Hou, J.; Liang, K.; Huang, J.; Liu, Y.N. Melamine-based metal-chelating porous organic polymers for efficient CO2 capture and conversion. Eur. J. Inorg. Chem. 2018, 2018, 4175–4180. [Google Scholar] [CrossRef]
- Liu, L.; Song, C.; Kong, A. Nitrogen and sulfur-enriched porous bithiophene-melamine covalent organic polymers for effective capture of CO2 and iodine. Mater. Lett. 2020, 277, 128291. [Google Scholar] [CrossRef]
- Liu, J.; Qi, N.; Zhou, B.; Chen, Z. Exceptionally high CO2 capture in amorphous polymer with ultramicropores studied by positron annihilation. ACS Appl. Mater. Interfaces 2019, 11, 30747–30755. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, J.; Li, G.Y.; Wang, Z.G. Carboxyl-, hydroxyl-, and nitro-functionalized porous polyaminals for highly selective CO2 capture. ACS Appl. Polym. Mater. 2019, 1, 1524–1531. [Google Scholar] [CrossRef]
- Seong, H.G.; Ryu, J.; Qian, Y.; So, J.I.; Baeck, S.H.; Shim, S.E. Novel hierarchically porous melamine-vanillin polymer: Synthesis and application for the Pb(II) ion removal in wastewater. Macromol. Res. 2019, 27, 882–887. [Google Scholar] [CrossRef]
- Ryu, J.; Lee, M.Y.; Song, M.G.; Baeck, S.H.; Shim, S.E.; Qian, Y. Highly selective removal of Hg(II) ions from aqueous solution using thiol-modified porous polyaminal-networked polymer. Sep. Purif. Technol. 2020, 250, 117120. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.; Yavuz, C.T. Melamine based porous organic amide polymers for CO2 capture. RSC Adv. 2014, 4, 52263–52269. [Google Scholar] [CrossRef]
- Song, D.; Yang, L.; Yang, J.; Zhai, D.; Sun, L.; Deng, W. In silico design of new nitrogen-rich melamine-based porous polyamides applied to CO2/N2 separation. Chem. Phys. Lett. 2021, 771, 138509. [Google Scholar] [CrossRef]
- Madhad, H.V.; Vasava, D.V. Carboxyl graphene reinforced melamine-based polyamide nanocomposites. J. Thermoplast. Compos. Mater. 2021, 36, 1–15. [Google Scholar] [CrossRef]
- Rehman, A.; Park, S.J. Preparation and characterization of polyamides and nitrogen-doped carbons for enhanced CO2 capture. Bull. Korean Chem. Soc. 2017, 38, 1285–1292. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Li, T.; Zhu, D.J. Shape-controlled synthesis of melamine based polyamide materials and application in suzuki-miyaura coupling reaction. Macromol. Res. 2019, 27, 876–881. [Google Scholar] [CrossRef]
- Peng, R.; Chen, G.; Zhou, F.; Man, R.; Huang, J. Catalyst-free synthesis of triazine-based porous organic polymers for Hg2+ adsorptive removal from aqueous solution. Chem. Eng. J. 2019, 371, 260–266. [Google Scholar] [CrossRef]
- Shao, L.; Liu, M.; Sang, Y.; Huang, J. One-pot synthesis of melamine-based porous polyamides for CO2 capture. Microporous Mesoporous Mater. 2019, 285, 105–111. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Chen, Y.; Li, M.; Chen, Z.; Hu, T.; Shi, L.; Pudukudy, M.; Shan, S.; Zhi, Y. Urea/amide-functionalized melamine-based organic polymers as efficient heterogeneous catalysts for CO2 cycloaddition. Chem. Eng. J. 2023, 474, 145918. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Y.; Sha, K.; Xiao, R. Preparation and characterizations of flame retardant melamine cyanurate/polyamide 6 composite fibers via in situ polymerization. Text. Res. J. 2017, 87, 561–569. [Google Scholar] [CrossRef]
- Alprol, A.E.; Abu-Saied, M.; Thabet, W.M.; Abdelwahab, O.; El-Ghaffar, M.A.A. Melamine-maleic acid polyamide adduct/polyacrylonitrile nanofibers as a novel adsorbent for removal of methyelene blue dye from aqueous solutions. Water. Air. Soil Pollut. 2024, 235, 425. [Google Scholar] [CrossRef]
- Li, C.J.; Zhang, H.Q.; Song, Y.H.; Cai, L.X.; Wu, J.M.; Wu, J.X.; Wang, S.; Xiong, C.A.X. Robust superhydrophobic and porous melamine-formaldehyde based composites for high-performance electromagnetic interference shielding. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 624, 126742. [Google Scholar] [CrossRef]
- Ding, Q.; Li, H. Properties of porous polyimide films based on melamine template. Key Eng. Mater. 2019, 821, 160–166. [Google Scholar] [CrossRef]
- Song, N.; Ma, T.; Wang, T.; Shi, K.; Tian, Y.; Yao, H.; Zhang, Y.; Guan, S. Crosslinked microporous polyimides with polar substituent group for efficient CO2 capture. Microporous Mesoporous Mater. 2020, 293, 109809. [Google Scholar] [CrossRef]
- Xiao, J.D.; Qiu, L.G.; Yuan, Y.P.; Jiang, X.; Xie, A.J.; Shen, Y.H. Ultrafast microwave-enhanced ionothermal synthesis of luminescent crystalline polyimide nanosheets for highly selective sensing of chromium ions. Inorg. Chem. Commun. 2013, 29, 128–130. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, J.; Lee, J.; Jo, S.I.; Chang, J.Y. A hierarchically porous polyimide composite prepared by one-step condensation reaction inside a sponge for heterogeneous catalysis. Macromol. Res. 2017, 25, 629–634. [Google Scholar] [CrossRef]
- Chu, S.; Pan, Y.; Wang, Y.; Zhang, H.; Xiao, R.; Zou, Z. Polyimide-based photocatalysts: Rational design for energy and environmental applications. J. Mater. Chem. A 2020, 8, 14441–14462. [Google Scholar] [CrossRef]
- Ding, G.; Wang, Q.; Liu, F.; Dan, Y.; Jiang, L. A novel iron-chelating polyimide network as a visible-light-driven catalyst for photoinduced radical polymerization. Chin. J. Catal. 2021, 42, 141–151. [Google Scholar] [CrossRef]
- Liao, Y.Z.; Weber, J.; Faul, C.F.J. Fluorescent microporous polyimides based on perylene and triazine for highly CO2-selective carbon materials. Macromolecules 2015, 48, 2064–2073. [Google Scholar] [CrossRef]
- Li, Z.T.; Zhou, J.Y.; Xu, R.F.; Liu, S.P.; Wang, Y.K.; Li, P.; Wu, W.T.; Wu, M.B. Synthesis of three-dimensional extended conjugated polyimide and application as sodium-ion battery anode. Chem. Eng. J. 2016, 287, 516–522. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Cui, Z.; Hu, Y.; Hao, X.; Wang, Y.; Zou, Z. Ultrathin conjugated polymer nanosheets as highly efficient photocatalyst for visible light driven oxygen activation. Appl. Catal. B Environ. 2020, 277, 119228. [Google Scholar] [CrossRef]
- Kong, P.; Tan, H.; Lei, T.; Wang, J.; Yan, W.; Wang, R.; Waclawik, E.R.; Zheng, Z.; Li, Z. Oxygen vacancies confined in conjugated polyimide for promoted visible-light photocatalytic oxidative coupling of amines. Appl. Catal. B Environ. 2020, 272, 118964. [Google Scholar] [CrossRef]
- Liu, S.; Chen, W.W.; Zhou, X.M. Polyimide aerogels using melamine as an economical yet effective crosslinker. J. Porous Mater. 2021, 28, 1155–1165. [Google Scholar] [CrossRef]
- Han, S.; Liu, C.; Li, N.; Zhang, S.; Song, Y.; Chen, L.; Xi, M.; Yu, X.; Wang, W.; Kong, M.; et al. One-step fabrication of nitrogen-doped laser-induced graphene derived from melamine/polyimide for enhanced flexible supercapacitors. CrystEngComm 2022, 24, 1866–1876. [Google Scholar] [CrossRef]
- Chen, W.; Liu, S.; Sun, Y.; Zhou, X.; Zhou, F. Melamine-Crosslinked Polyimide Aerogels from Supercritical Ethanol Drying with Improved In-Use Shape Stability Against Shrinking. Macromol. Mater. Eng. 2022, 307, 2100645. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J. Facile synthesis of azo-linked porous organic frameworks via reductive homocoupling for selective CO2 capture. J. Mater. Chem. A 2014, 2, 13831–13834. [Google Scholar] [CrossRef]
- Patel, H.A.; Hyun Je, S.; Park, J.; Chen, D.P.; Jung, Y.; Yavuz, C.T.; Coskun, A. Unprecedented high-temperature CO2 selectivity in N2-phobic nanoporous covalent organic polymers. Nat. Commun. 2013, 4, 1357. [Google Scholar] [CrossRef]
- Arab, P.; Parrish, E.; İslamoğlu, T.; El-Kaderi, H.M. Synthesis and evaluation of porous azo-linked polymers for carbon dioxide capture and separation. J. Mater. Chem. A 2015, 3, 20586–20594. [Google Scholar] [CrossRef]
- Arab, P.; Rabbani, M.G.; Sekizkardes, A.K.; Islamoǧlu, T.; El-Kaderi, H.M. Copper(I)-catalyzed synthesis of nanoporous azo-linked polymers: Impact of textural properties on gas storage and selective carbon dioxide capture. Chem. Mater. 2014, 26, 1385–1392. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, H.; Yu, B.; Zhao, Y.; Ma, Z.; Ji, G.; Han, B.; Liu, Z. Azo-functionalized microporous organic polymers: Synthesis and applications in CO2 capture and conversion. Chem. Commun. 2015, 51, 11576–11579. [Google Scholar] [CrossRef]
- Maroufi, P.; Najafi Moghadam, P.; Vahabi, H. New nitrogen-rich flame retardant based on conductive poly(aniline-co-melamine). React. Funct. Polym. 2020, 150, 104548. [Google Scholar] [CrossRef]
- Prakash, D.; Murali, A.; Jaisankar, S.N. Synthesis and characterization of nitrogen-rich azo linked conjugated polymer materials. Int. J. Polym. Anal. Charact. 2017, 22, 455–462. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Z.; Zhang, H.; Liu, Z. Nitrogen-doped microporous carbon materials with uniform pore diameters: Design and applications in CO2 and H2 adsorption. Microporous Mesoporous Mater. 2020, 296, 109992. [Google Scholar] [CrossRef]
- Youm, K.; Choi, Y.; Byun, H.; Kumar, S.; Cho, Y.; Hsan, N.; Koh, J. A highly efficient porphyrin-based azo-porous organic polymer for selective CO2 capture and conversion. J. CO2 Util. 2024, 84, 102854. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Li, H.S.; Sun, Z.B.; Wan, J.Q.; Xin, Y.; Li, W.Z.; Luan, J.; Liu, Y. Fabrication of carbonized derivatives from a novel azo polymer as a precursor for the layered adsorption of iodine and bromine. Carbon 2024, 226, 119232. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Bhaumik, A. Porous organic polymers for CO2 storage and conversion reactions. ChemCatChem 2019, 11, 244–257. [Google Scholar] [CrossRef]
- Chu, F.; Hu, Y.; Zhang, K.; Li, X.; Zhao, G.; Huang, X.; Wang, G. Salt-templated porous melamine-based conjugated polymers for selective oxidation of amines into imines under visible light. J. Colloid Interface Sci. 2023, 634, 159–168. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, Y.; Wu, X.; Wang, S.; Li, Z.; Zhao, S. Efficient capture of mercury ions by a novel organic melamine polymer. Mater. Today Commun. 2023, 35, 106230. [Google Scholar] [CrossRef]
- Lin, X.; Peng, P.; Guo, J.; Xiang, Z. Reaction milling for scalable synthesis of N, P-codoped covalent organic polymers for metal-free bifunctional electrocatalysts. Chem. Eng. J. 2019, 358, 427–434. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, Q.; Zhang, D.; Kuang, W.; Huang, J.; Liu, Y.N. Postfunctionalization of porous organic polymers based on friedel-crafts acylation for CO2 and Hg2+ Capture. ACS Appl. Mater. Interfaces 2020, 12, 36652–36659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, Y.; Wang, J.; Wang, Y.; Cao, Y.; Li, J.; Zhou, F.; Huang, J.; Liu, Y.N. Melamine-functionalization of the carbonyl-rich polymers for iodine vapor and Hg2+ capture. Chem. Eng. J. 2023, 460, 141669. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.; Xiao, Q.; Zhang, D.; Sang, Y.; Huang, J. Bifunctional porous organic polymers based on postfunctionalization of the ketone-based polymers. Ind. Eng. Chem. Res. 2020, 59, 19117–19125. [Google Scholar] [CrossRef]
- Wang, L.; Guo, J.; Xiang, X.; Sang, Y.; Huang, J. Melamine-supported porous organic polymers for efficient CO2 capture and Hg2+ removal. Chem. Eng. J. 2020, 387, 124070. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, J.J.; Wang, Y.; Cao, Y.W.; Li, J.W.; Zhou, F.; Huang, J.H.; Liu, Y.N. Aromatic ketone-based melamine-knitted polymers for CO2 capture and Hg2+ removal. Chem. Eng. J. 2023, 452, 139275. [Google Scholar] [CrossRef]
- Kamaci, M.; Kaya, I. Melamine-based poly(azomethine) hydrogels: Mechanical, biodegradability, drug loading and antibacterial properties. Eur. Polym. J. 2018, 108, 107–115. [Google Scholar] [CrossRef]
- Kaya, I.; Yildirim, M. Synthesis and characterization of graft copolymers of melamine: Thermal stability, electrical conductivity, and optical properties. Synth. Met. 2009, 159, 1572–1582. [Google Scholar] [CrossRef]
- Kaya, S.; Yildirim, M.; Kamaci, M. A new kind of optical Mn(II) sensor with high selectivity: Melamine based poly(azomethine–urethane). Synth. Met. 2011, 161, 2036–2040. [Google Scholar] [CrossRef]
- Ravi, S.; Choi, Y.; Bae, Y.S. Melamine-functionalized aromatic carbonyl-based polymer with high surface area for efficient CO2 capture. Sep. Purif. Technol. 2023, 317, 123828. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Sun, M.; Wang, Y.; Zhao, R.; Zhang, X.; Zhao, Y. Novel melamine-based porous organic polymers: Synthesis, characterizations, morphology modifications, and their applications in lithium–sulfur batteries. Nanotechnology 2021, 33, 085704. [Google Scholar] [CrossRef]
- Ghanbari, R.; Marandi, A.; Zare, E.N. Development of melamine-based covalent organic framework-MOF pearl-like heterostructure integrated poly(ether-block-amide) for CO2/CH4 separation. J. Environ. Chem. Eng. 2023, 11, 109269. [Google Scholar] [CrossRef]
- Tian, P.; Ai, Z.; Hu, H.; Wang, M.; Li, Y.; Gao, X.; Qian, J.; Su, X.; Xiao, S.; Xu, H.; et al. Synthesis of electron-rich porous organic polymers via schiff-base chemistry for efficient iodine capture. Molecules 2022, 27, 5161. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carríllo, C.; Benítez, M.; El Haskouri, J.; Amorós, P.; Ros-Lis, J.V. Novel Microwave-Assisted Synthesis of COFs: 2020–2022. Molecules 2023, 28, 3112. [Google Scholar] [CrossRef]
- Tighadouini, S.; Roby, O.; Radi, S.; Lakbaibi, Z.; Saddik, R.; Mabkhot, Y.N.; Almarhoon, Z.M.; Garcia, Y. A highly efficient environmental-friendly adsorbent based on schiff base for removal of Cu(II) from aqueous solutions: A Combined Experimental and Theoretical Study. Molecules 2021, 26, 5164. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Guo, J.; Huang, Q.; He, J.; Fu, Y.; Kuang, G.; Pan, C.; Yu, G. 1,3,5-Triazine-based microporous polymers with tunable porosities for CO2 capture and fluorescent Sensing. Macromolecules 2017, 50, 8512–8520. [Google Scholar] [CrossRef]
- Alkayal, N.S.; Ibrahim, M.; Tashkandi, N.; Alotaibi, M.M. Efficient reduction in methylene blue using palladium nanoparticles supported by melamine-based polymer. Materials 2023, 16, 5887. [Google Scholar] [CrossRef]
- Alloush, A.M.; Abdulghani, H.; Amasha, H.A.; Saleh, T.A.; Al Hamouz, O.C.S. Microwave-assisted synthesis of novel porous organic polymers for effective selective capture of CO2. J. Ind. Eng. Chem. 2022, 113, 215–225. [Google Scholar] [CrossRef]
- Sadhasivam, V.; Balasaravanan, R.; Chithiraikumar, C.; Siva, A. Palladium nanoparticles supported on nitrogen-rich containing melamine-based microporous covalent triazine polymers as efficient heterogeneous catalyst for C-Se coupling reactions. ChemCatChem 2018, 10, 3833–3844. [Google Scholar] [CrossRef]




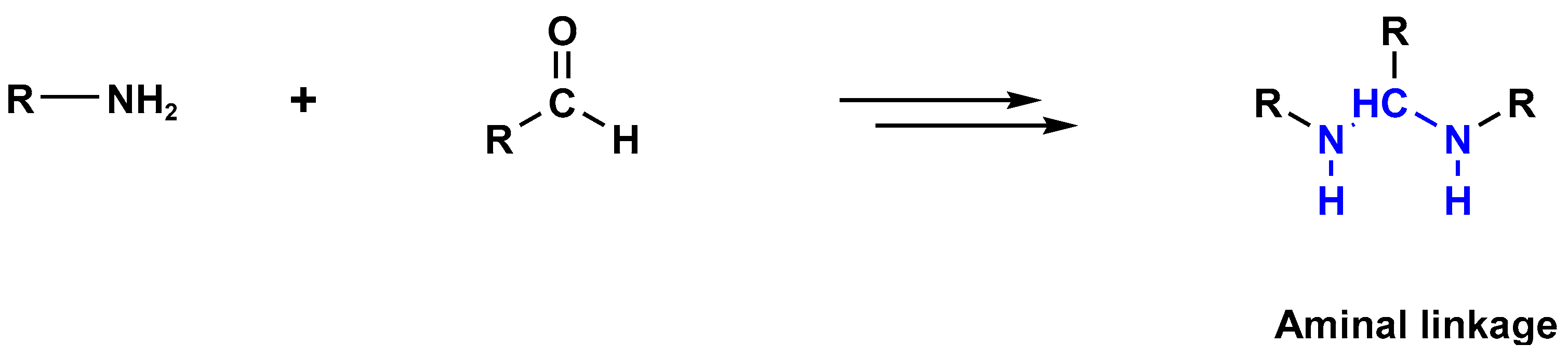






| Rank | Author | Country | Documents | Documents Contribution | Citations | H-Index |
|---|---|---|---|---|---|---|
| 1 | Li Qiang | China | 32 | 2.291% | 756 | 17 |
| 2 | Wang Qi | China | 27 | 1.933% | 575 | 15 |
| 3 | Wang Yong | China | 25 | 1.790% | 1020 | 17 |
| 4 | Wang Lei | China | 18 | 1.288% | 515 | 10 |
| 5 | Hu Yuan | China | 16 | 1.145% | 598 | 14 |
| 6 | Zhang Sheng | China | 15 | 1.074% | 476 | 11 |
| 7 | Zhang Xiao | China | 15 | 1.074% | 466 | 10 |
| 8 | Zhang Yong | China | 15 | 1.074% | 418 | 10 |
| 9 | Li Yue | China | 14 | 1.002% | 584 | 10 |
| 10 | Bourbigot Serge | France | 13 | 0.931% | 480 | 11 |
| Rank | Country | Documents | Document Contribution | Citations | H-Index |
|---|---|---|---|---|---|
| 1 | China | 863 | 61.78% | 31,305 | 85 |
| 2 | Germany | 92 | 6.586% | 3848 | 30 |
| 3 | India | 80 | 5.727% | 1746 | 23 |
| 4 | USA | 78 | 5.583% | 4306 | 33 |
| 5 | France | 66 | 4.724% | 1894 | 22 |
| 6 | South Korea | 61 | 4.366% | 2388 | 24 |
| 7 | Iran | 44 | 3.150% | 728 | 18 |
| 8 | England | 42 | 3.006% | 1577 | 22 |
| 9 | Japan | 40 | 2.863% | 1065 | 18 |
| 10 | Australia | 35 | 2.505% | 1795 | 20 |
| Rank | Institutions | Country | Documents | Document Contribution | Citations | H-Index |
|---|---|---|---|---|---|---|
| 1 | Chinese Academy of Sciences | China | 108 | 7.731% | 4665 | 35 |
| 2 | Sichuan University | China | 60 | 4.295% | 1902 | 22 |
| 3 | Centre National De La Recherche Scientifique CNRS | France | 42 | 3.006% | 1357 | 18 |
| 4 | Qingdao University of Science Technology | China | 31 | 2.219% | 1070 | 18 |
| 5 | University Of Chinese Academy of Sciences Cas | China | 31 | 2.219% | 1857 | 21 |
| 6 | Beijing University of Chemical Technology | China | 28 | 2.004% | 1864 | 20 |
| 7 | University Of Science Technology of China Cas | China | 26 | 1.861% | 1467 | 17 |
| 8 | Donghua University | China | 23 | 1.646% | 791 | 15 |
| 9 | Central South University | China | 22 | 1.575% | 790 | 13 |
| 10 | Universite De Lille | France | 22 | 1.575% | 591 | 14 |
| Rank | Journal | Documents | Documents Contribution | Citations | H-Index | Publisher Name |
|---|---|---|---|---|---|---|
| 1 | Journal of Applied Polymer Science | 58 | 4.152% | 1137 | 21 | Wiley |
| 2 | Polymer Degradation and Stability | 46 | 3.293% | 1926 | 28 | Elsevier |
| 3 | RSC Advances | 45 | 3.221% | 919 | 22 | RSC |
| 4 | ACS Applied Materials Interfaces | 38 | 2.720% | 2166 | 23 | ACS |
| 5 | Chemical Engineering Journal | 38 | 2.720% | 1920 | 27 | Elsevier |
| 6 | Polymers | 35 | 2.505% | 499 | 12 | MDPI |
| 7 | Journal of Materials Chemistry A | 34 | 2.434% | 2051 | 27 | RSC |
| 8 | Journal of Physical Chemistry C | 25 | 1.790% | 582 | 16 | ACS |
| 9 | Carbon | 24 | 1.718% | 1687 | 18 | Elsevier |
| 10 | Polymers for Advanced Technologies | 23 | 1.646% | 386 | 14 | Wiley |
| Rank | Keywords | Occurrences |
|---|---|---|
| 1 | Melamine | 236 |
| 2 | Performance | 201 |
| 3 | Adsorption | 132 |
| 4 | Nanocomposite | 119 |
| 5 | Networks | 114 |
| 6 | Nanoparticles | 104 |
| 7 | Water | 91 |
| 8 | Nitrogen | 80 |
| 9 | Thermal degradation | 69 |
| 10 | Polymer | 70 |
| Entry | Materials | Building Block | S(BET). m2/g | CO2 Capture | Ref. |
|---|---|---|---|---|---|
| 1 | PAN-NA | 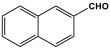 | 607.5 | 67.9 cm3/g | [40] |
| 2 | PAN-AN | 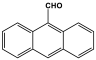 | 230.4 | 30.1 cm3/g | [41] |
| 3 | PAN-PY | 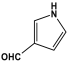 | 321.0 | 039. cm3/g | [44] |
| 4 | PAN-PZ |  | 557.4 | 62.7 cm3/g | |
| 5 | PAN-IM | 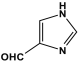 | 506.5 | 50.9 cm3/g | |
| 6 | MA-Py | 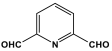 | 424 | - | [50] |
| 7 | MBMOP-1 | 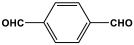 | 747 | 57.3 cm3/g | [52] |
| 8 | MPA-1 |  | 30 | 38.9 cm3/g | [33] |
| 9 | MPA-2 | 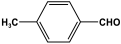 | 33.5 | 21.9 cm3/g | |
| 10 | MOP-1 | 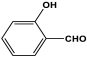 | 82 | 33.2 cm3/g | [53] |
| 11 | MOP-2 | 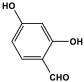 | 77 | 29.2 cm3/g | |
| 12 | MOP-3 | 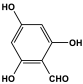 | 36 | 58.2 cm3/g | |
| 13 | PA-M |  | 114.29 | 13.6 cm3/g | [35] |
| 14 | Bipy-PAN | 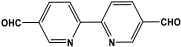 | 160.7 | 22.8 cm3/g | [54] |
| 15 | Py@MA |  | 729 | 43.5 cm3/g | [55] |
| 16 | Py-Zn@MA |  | 207 | 29.6 cm3/g | |
| 17 | Bp-Zn@MA |  | 445 | 26.7 cm3/g | [56] |
| 18 | PAN-F | 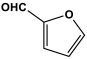 | 702 | 75.33 cm3/g | [14] |
| 19 | PAN-T |  | 795 | 50.93 cm3/g | |
| 20 | PAN-FPP5 |  | 788 | 63.62 cm3/g | [22] |
| 21 | PAN-TPDA |  | 752 | 58.55 cm3/g | |
| 22 | TPAMP | 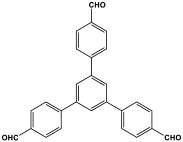 | 557 | 40.21 cm3/g | [57] |
| 23 | TPBMP |  | 873 | 85.01 cm3/g | |
| 24 | TPFM | 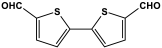 | 611.3 | 82.46 cm3/g | [58] |
| 25 | SNW-2 | 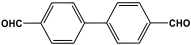 | 665 | 75 cm3/g | [59] |
| 26 | SNW-3 | 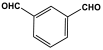 | 703 | 93 cm3/g | |
| 27 | PAN-FP |  | 703 | 66.7 cm3/g | [35] |
| 28 | PAN-MP | 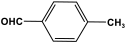 | 615 | 59.1 cm3/g | |
| 29 | PAN-FMP |  | 907 | 74.3 cm3/g | |
| 30 | PAN-CP | 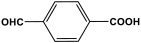 | 988 | 67.7 cm3/g | [60] |
| 31 | PAN-HP | 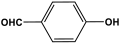 | 782 | 68.8 cm3/g | |
| 32 | PAN-NP | 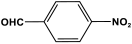 | 581 | 52.4 cm3/g | |
| 33 | TMP-1 | 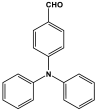 | 452 | 52.4 cm3/g | [36] |
| 34 | TMP-2 |  | 613 | 69.2 cm3/g | |
| 35 | MVP | 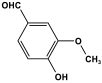 | 745 | - | [61] |
| 36 | 4A-MBP | 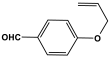 | 335 | - | [62] |
| 37 | PA-T1 |  | 336 | 18.5 cm3/g | [32] |
| 38 | PA-T3 | 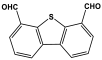 | 183 | 7.23 cm3/g | |
| 39 | PA-F3 | 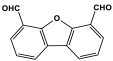 | 283 | 9.53 cm3/g | |
| 40 | PA-T4 | 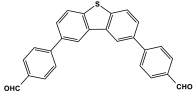 | 135 | 25.8 cm3/g | |
| 41 | PA-F4 | 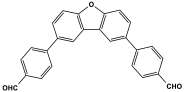 | 23.8 | 5.04 cm3/g | |
| 42 | POPa |  | 213 | - |
| Entry | Materials | Building Block | S(BET) .m2/g | Application | Ref. |
|---|---|---|---|---|---|
| 1 | PI-1 | 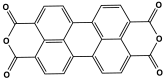 | 19 | CO2 uptake of 15.3 cm3/g | [80] |
| 2 | MPI |  | 30.6 | Photocatalyst for radical polymerization of poly(methyl methacrylate) with molecular weight up to 31.3 × 104 g/mol | [79] |
| 3 | R-PA |  | Photocatalyst for selective benzylamine oxidation reactions with % conversion up to 95.2% | [83] | |
| 4 | MPI-40-10 |  | 303 | - | [84] |
| 5 | PI-COF | 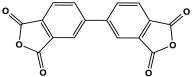 | 103.50 | For O2/N2 Separation | [86] |
| Entry | Materials | Building Block | S(BET) .m2/g | Application | Ref. |
|---|---|---|---|---|---|
| 1 | PANI | 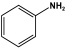 | - | Flame retardants | [92] |
| 2 | TNPP-M | 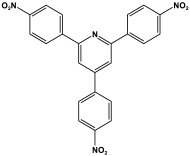 | - | For solar fuel cell uses | [93] |
| 3 | N-MC-3 |  | 843 | Gas adsorption: CO2 uptake of 97 cm3/g H2 uptake of 7.5 cm3/g | [94] |
| 4 | PBPOP1 |  | 630.76 | For CO2 capture and conversion | [95] |
| 5 | AZO-TPA | 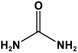 | - | For adsorption of iodine and bromine | [96] |
| Entry | Materials | Building Block | S(BET) .m2/g | Application | Ref. |
|---|---|---|---|---|---|
| 1 | MMP |  | 231.8 | Hg2+ capability of 2018.1 mg/g | [99] |
| 2 | Py-TMC-MA | 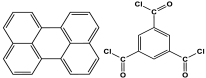 | 555 | CO2 uptake of 71.8 cm3/g | [101] |
| 3 | TPE-OAC-MA |  | CO2 uptake of 73.8 cm3/g | CO2 uptake of 73.8 cm3/g | |
| 4 | TPP-TMC-MA | 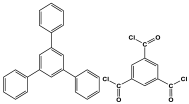 | 448 | CO2 uptake of 52.4 cm3/g | |
| 5 | TPA-TMC-MA | 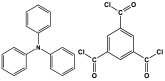 | 492 | CO2 uptake of 62.6 cm3/g | |
| 6 | Py-TC-MA | 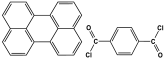 | 357 | CO2 uptake of 47.3 cm3/g | |
| 7 | TPP-TC-MA | 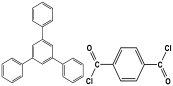 | 364 | CO2 uptake of 45.5 cm3/g | |
| 8 | TPE-TC-MA |  | 512 | CO2 uptake of 71.8 cm3/g | |
| 9 | TPA-TC-MA |  | 454 | CO2 uptake of 64.7 cm3/g | |
| 10 | TPE−PMDA−MA |  | 703 | CO2 uptake of 98.3 cm3/g | [103] |
| 11 | TPE−PTCDA−MA | 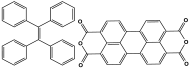 | 612 | CO2 uptake of 77.4 cm3/g | |
| 12 | TPA−PTCDA−MA |  | 555 | CO2 uptake of 66.2 cm3/g | |
| 13 | TPA−PMDA−MA |  | 557 | CO2 uptake of 68.2 cm3/g | |
| 14 | Py−PTCDA−MA | 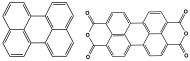 | 561 | CO2 uptake of 73.3 cm3/g | |
| 15 | Py−PMDA−MA |  | 611 | CO2 uptake of 84 cm3/g | |
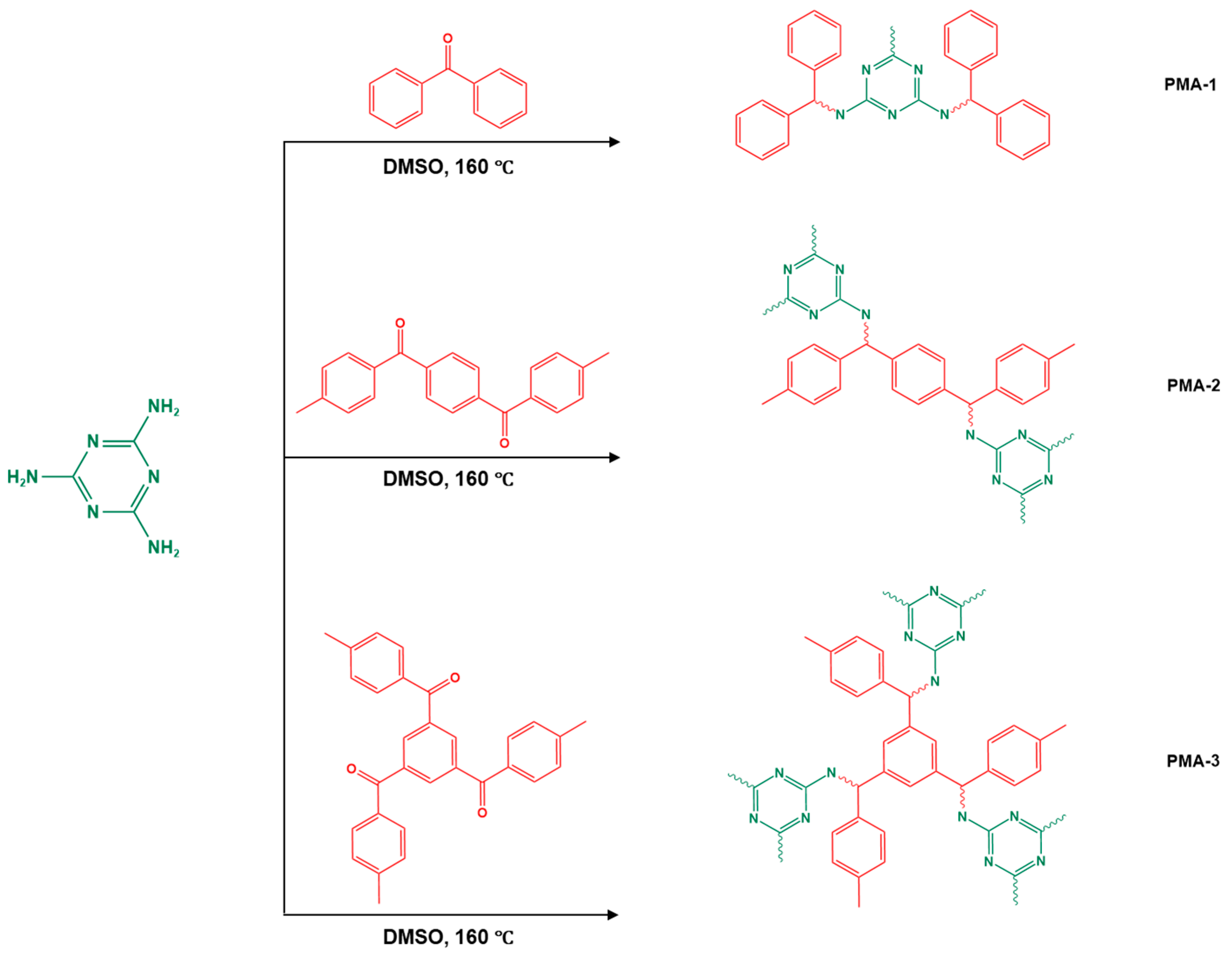
| 16 | PMA-1–8 | 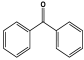 | 85 | Hg2+ capability of 462 mg/g | [105] |
| 17 | PMA-2–8 | 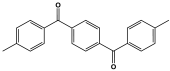 | 213 | Hg2+ capability of 501 mg/g | |
| 18 | PMA-3–8 |  | 772 | Hg2+ capability of 702 mg/g | |
| 19 | P-MEL-BUT gel | 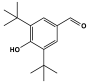 | - | Biomaterials for the various biomedical applications | [106] |
| 20 | P-MEL-MET gel | 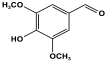 | - | ||
| 21 | PHTCZ-1-MA | PHTCZ-1 | 613 | CO2 uptake of 91.6 cm3/g | [106] |
| 22 | P-2-HPMTT |  | - | - | [107] |
| 23 | P-3-HPMTT | 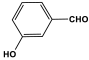 | - | - | |
| 24 | O-4-HPMTT | 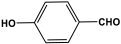 | - | - | |
| 25 | TDI-co-2-HNMTT | 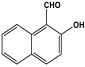 | - | Optical Mn(II) sensor | [108] |
| 26 | ACP-MEL | 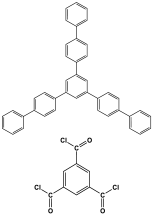 | 1082 | CO2 uptake of 134.1 mg/g | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkayal, N.S. Investigation into the Synthetic Strategies of Melamine-Based Porous Polymeric Materials: A Bibliometric Analysis. Polymers 2025, 17, 868. https://doi.org/10.3390/polym17070868
Alkayal NS. Investigation into the Synthetic Strategies of Melamine-Based Porous Polymeric Materials: A Bibliometric Analysis. Polymers. 2025; 17(7):868. https://doi.org/10.3390/polym17070868
Chicago/Turabian StyleAlkayal, Nazeeha S. 2025. "Investigation into the Synthetic Strategies of Melamine-Based Porous Polymeric Materials: A Bibliometric Analysis" Polymers 17, no. 7: 868. https://doi.org/10.3390/polym17070868
APA StyleAlkayal, N. S. (2025). Investigation into the Synthetic Strategies of Melamine-Based Porous Polymeric Materials: A Bibliometric Analysis. Polymers, 17(7), 868. https://doi.org/10.3390/polym17070868






