Mechanical Property and Microcellular Foamability of iPP/PA11/PP-g-MAH Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Batch Foaming Experiment
2.4. Differential Scanning Calorimetry (DSC) Analysis
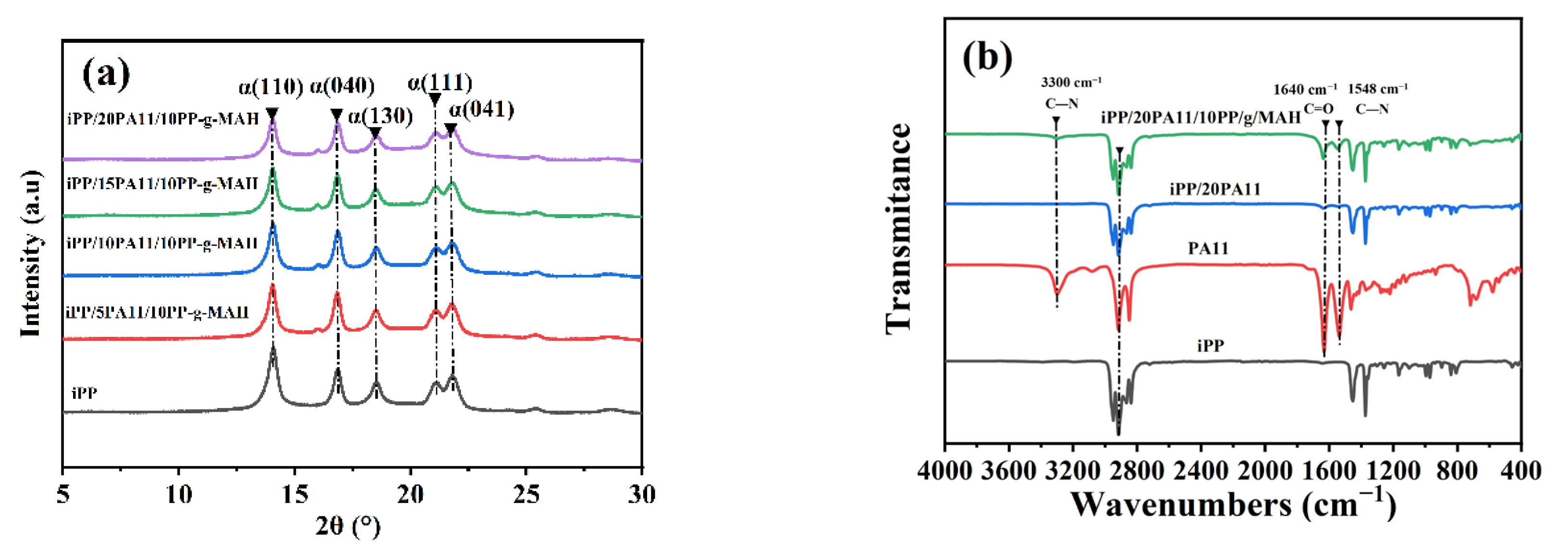
2.5. X-Ray Diffraction Test
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Rheological Characterization
2.8. Scanning Electron Microscopy (SEM)
2.9. Mechanical Test
3. Results and Discussion
3.1. Thermal and Rheological Properties of iPP/PA11/PP-g-MAH
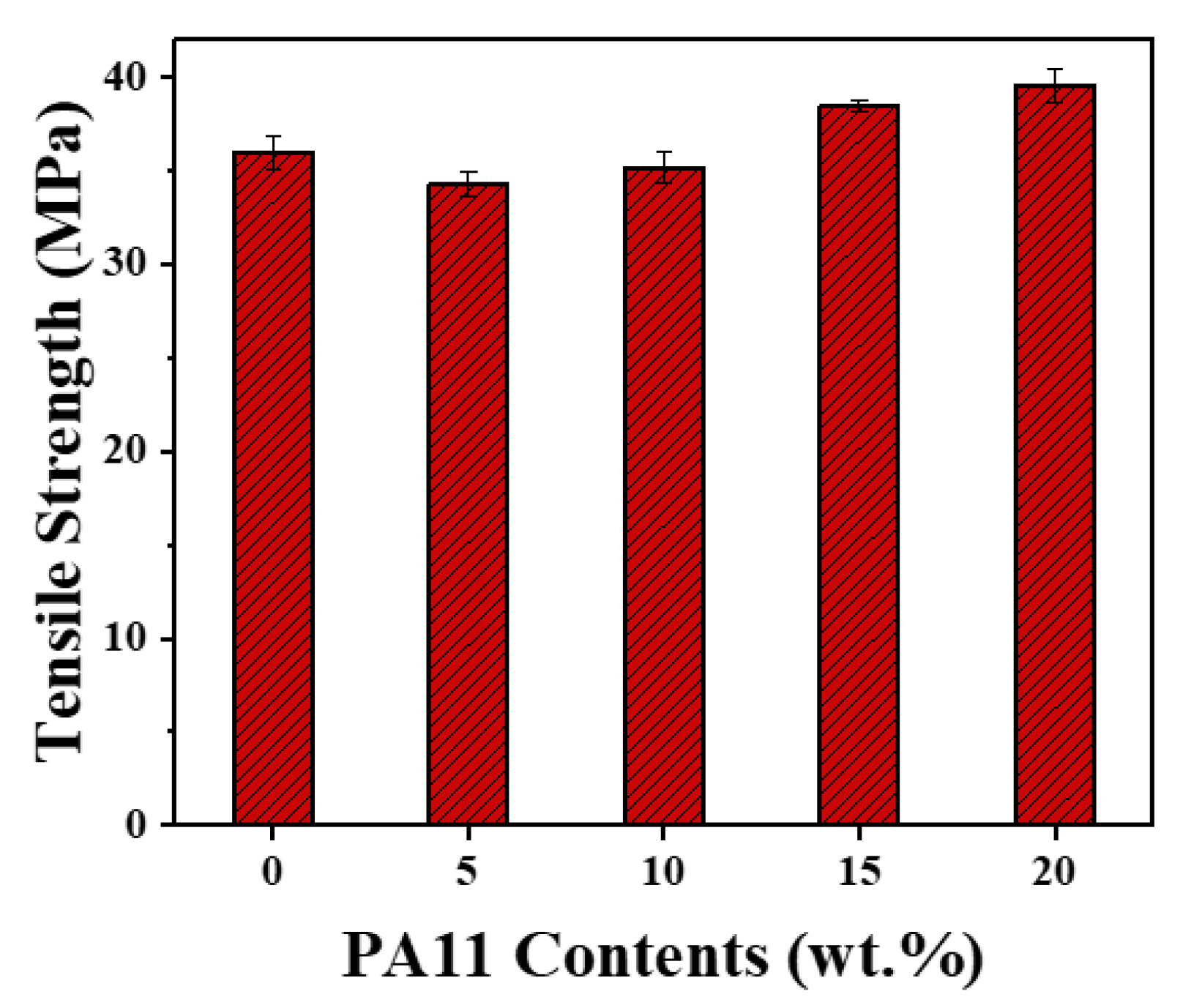
3.2. Morphology and Mechanical Property of iPP/PA11/PP-g-MAH Blends
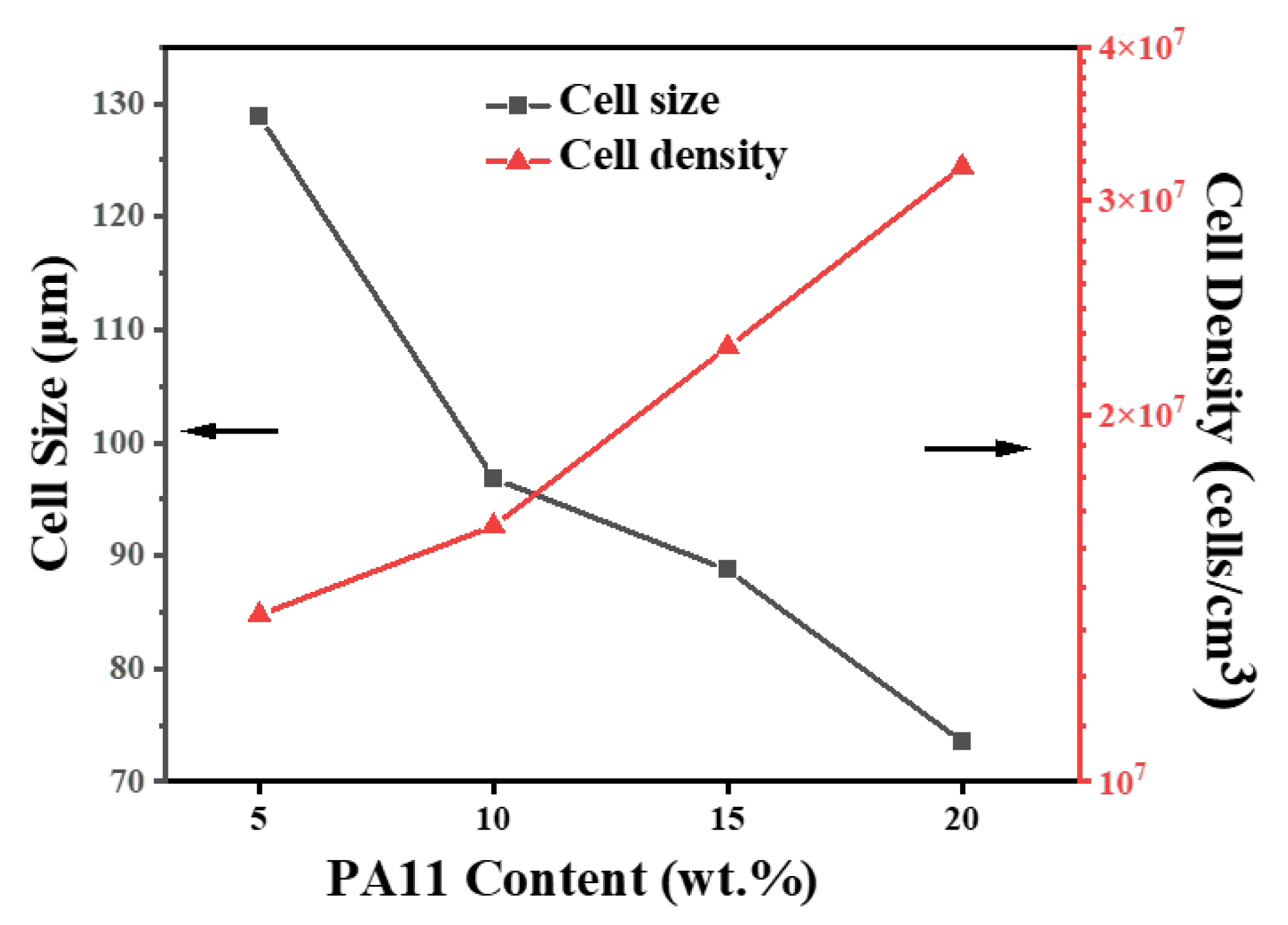
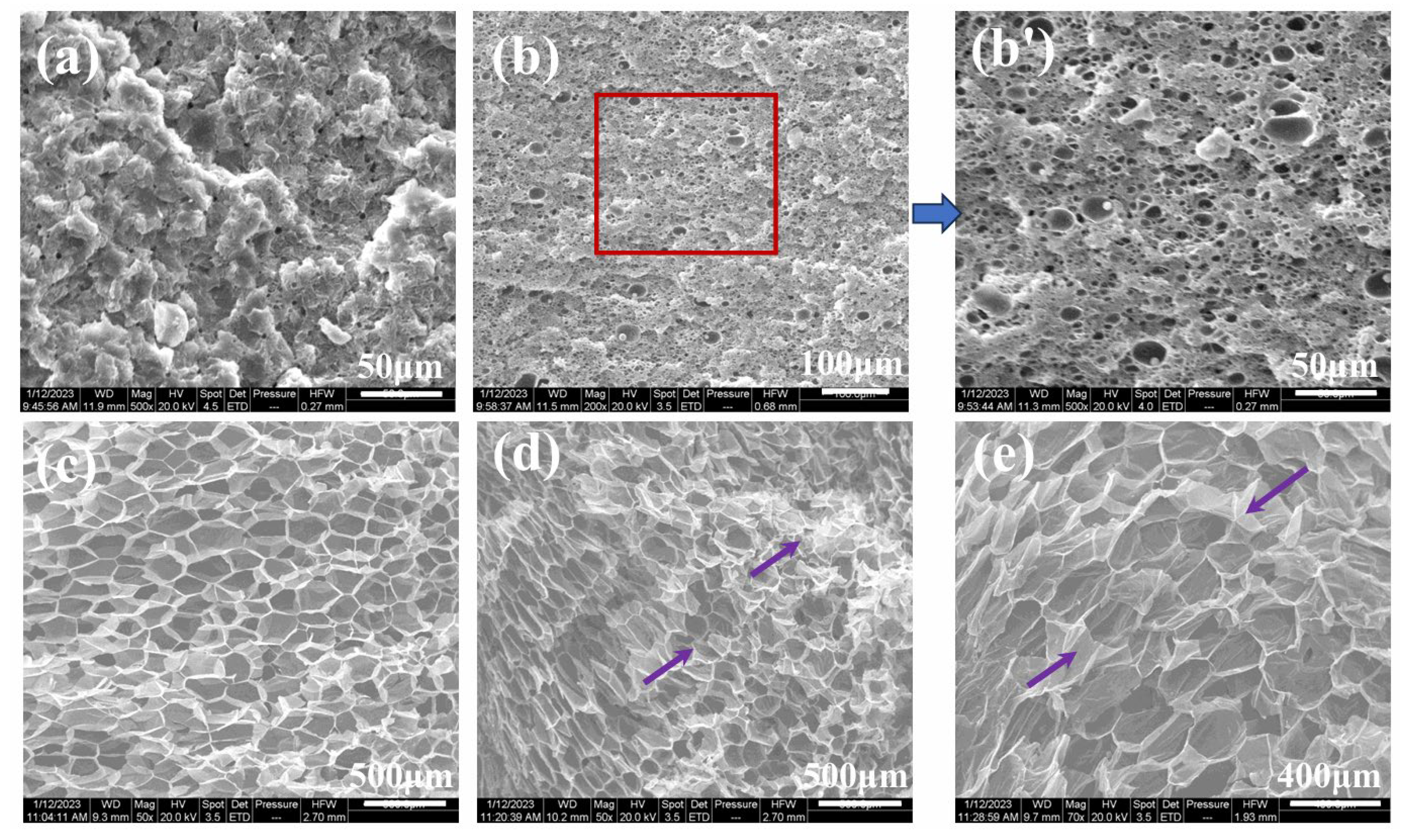
3.3. Microcellular Foaming Behavior of iPP/PA11/PP-g-MAH Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, H.; Song, Y.; Zhang, Q.; Zheng, Q. Contributions of Silica Network and Interfacial Fraction in Reinforcement and Payne Effect of Polypropylene Glycol Nanocomposites. Polymer 2018, 138, 139–145. [Google Scholar] [CrossRef]
- Muñoz-Pascual, S.; Saiz-Arroyo, C.; Vananroye, A.; Moldenaers, P.; Rodriguez-Perez, M.A. Effect on the Impact Properties of Adding Long-Chain Branched Polypropylene in Polypropylene-Polyolefin Elastomer Cellular Polymers Produced by Core-Back Injection Molding. Macromol. Mater. Eng. 2021, 306, 2000728. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Y.; Lu, Y.; Wang, B.; Shen, C.; Chen, J.; Zhang, B. Later Stage Melting of Isotactic Polypropylene. Macromolecules 2020, 53, 2136–2144. [Google Scholar] [CrossRef]
- Ho, Q.B.; Kontopoulou, M. Stabilization of the Cellular Structure of Polypropylene Foams and Secondary Nucleation Mechanism in the Presence of Graphene Nanoplatelets. Polymer 2020, 198, 122506. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Li, B.; Li, H.; Cao, Z.; Jin, G.; Zhao, L.; Xin, Z. Anti-shrinking foaming of polyethylene with CO2 as blowing agent. J. Supercrit. Fluids 2020, 163, 104883. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, J.; Zhao, F.; Feng, T.; Liu, L. Assessment of Properties of Bamboo Fiber and EPDM Reinforced Polypropylene Microcellular Foam Composites. Polymer 2024, 304, 127142. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Sun, B.; Cheng, H.; Chen, J.; Shen, C.; Park, C.B. Broadened Foaming Scope of iPP Adjusted by Its Self-Enhancement and Nucleating Agent under Compressed CO2. Mater. Today Commun. 2018, 17, 501–510. [Google Scholar] [CrossRef]
- Huang, H.-X.; Xu, H.-F. Preparation of Microcellular Polypropylene/Polystyrene Blend Foams with Tunable Cell Structure. Polym. Adv. Technol. 2011, 22, 822–829. [Google Scholar] [CrossRef]
- Velasco, J.I.; Antunes, M.; Realinho, V.; Ardanuy, M. Characterization of Rigid Polypropylene-Based Microcellular Foams Produced by Batch Foaming Processes. Polym. Eng. Sci. 2011, 51, 2120–2128. [Google Scholar] [CrossRef]
- Heidari, A.; Fasihi, M. Cell Structure-Impact Property Relationship of Polypropylene/Thermoplastic Elastomer Blend Foams. Express Polym. Lett. 2019, 13, 429–442. [Google Scholar] [CrossRef]
- Qiang, W.; Zhao, L.; Gao, X.; Liu, T.; Liu, Z.; Yuan, W.-K.; Hu, D. Dual Role of PDMS on Improving Supercritical CO2 Foaming of Polypropylene: CO2-Philic Additive and Crystallization Nucleating Agent. J. Supercrit. Fluids 2020, 163, 104888. [Google Scholar] [CrossRef]
- Wang, B.; Cavallo, D.; Zhang, X.; Zhang, B.; Chen, J. Evolution of Chain Entanglements under Large Amplitude Oscillatory Shear Flow and Its Effect on Crystallization of Isotactic Polypropylene. Polymer 2020, 186, 121899. [Google Scholar] [CrossRef]
- Moo-Tun, N.M.; Iñiguez-Covarrubias, G.; Valadez-Gonzalez, A. Assessing the Effect of PLA, Cellulose Microfibers and CaCO3 on the Properties of Starch-Based Foams Using a Factorial Design. Polym. Test. 2020, 86, 106482. [Google Scholar] [CrossRef]
- Ahn, S.; Shim, E.; Kim, Y.; Bae, Y.-S.; Eom, H. Air Filtration Performance Enhancement of PTFE Foam-Coated Filters at High Temperatures via Secondary Strongly Adhering PTFE Nanofiber Coatings. Process Saf. Environ. Prot. 2022, 162, 914–922. [Google Scholar] [CrossRef]
- Wang, K.; Pang, Y.; Wu, F.; Zhai, W.; Zheng, W. Cell Nucleation in Dominating Formation of Bimodal Cell Structure in Polypropylene/Polystyrene Blend Foams Prepared via Continuous Extrusion with Supercritical CO2. J. Supercrit. Fluids 2016, 110, 65–74. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Burley, A.; Feng, L.; Yeh, S.-K.; Miyazono, K.; Nirmal-Kumar, S.; Kusaka, I.; Koelling, K. Development of CO2 for Polymer Foam Applications. J. Supercrit. Fluids 2009, 47, 493–499. [Google Scholar] [CrossRef]
- Miyamoto, R.; Yasuhara, S.; Shikuma, H.; Ohshima, M. Preparation of Micro/Nanocellular Polypropylene Foam with Crystal Nucleating Agents. Polym. Eng. Sci. 2014, 54, 2075–2085. [Google Scholar] [CrossRef]
- McCallum, T.J.; Kontopoulou, M.; Park, C.B.; Muliawan, E.B.; Hatzikiriakos, S.G. The Rheological and Physical Properties of Linear and Branched Polypropylene Blends. Polym. Eng. Sci. 2007, 47, 1133–1140. [Google Scholar] [CrossRef]
- Wu, M.-H.; Wang, C.-C.; Chen, C.-Y. Preparation of High Melt Strength Polypropylene by Addition of an Ionically Modified Polypropylene. Polymer 2020, 202, 122743. [Google Scholar] [CrossRef]
- Fu, L.; Shi, Q.; Ji, Y.; Wang, G.; Zhang, X.; Chen, J.; Shen, C.; Park, C.B. Improved Cell Nucleating Effect of Partially Melted Crystal Structure to Enhance the Microcellular Foaming and Impact Properties of Isotactic Polypropylene. J. Supercrit. Fluids 2020, 160, 104794. [Google Scholar] [CrossRef]
- Larrue, C.; Bounor-Legare, V.; Cassagnau, P. Enhancement of EPDM Crosslinked Elastic Properties by Association of Both Covalent and Ionic Networks. Polymers 2021, 13, 3161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhao, S.; Xin, Z. Preparation and foamability of high melt strength polypropylene based on grafting vinyl polydimethylsiloxane and styrene. Polym. Eng. Sci. 2015, 55, 251–259. [Google Scholar] [CrossRef]
- Zhai, W.; Park, C.B. Effect of Nanoclay Addition on the Foaming Behavior of Linear Polypropylene-Based Soft Thermoplastic Polyolefin Foam Blown in Continuous Extrusion. Polym. Eng. Sci. 2011, 51, 2387–2397. [Google Scholar] [CrossRef]
- Mi, D.; Zhou, M.; Hou, F.; Zhang, J. Effect of High-Temperature Annealing on the Microstructure and Mechanical Properties of Polypropylene with Shish Kebab or Spherulite Structure. J. Appl. Polym. Sci. 2018, 135, 46465. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.-J.; Yang, Y.; Zhou, N. Effect of Dynamic Shear on the Microcellular Foaming of Polypropylene/High-Density Polyethylene Blends. J. Appl. Polym. Sci. 2009, 114, 1320–1328. [Google Scholar] [CrossRef]
- Yu, K.; Jiang, H.; Zhou, H.; Mi, J.; He, Y.; Wang, X. Evolution of Double Crystal Melting Peak in Polypropylene Foam Assisted by β-Nucleating Agent and Supercritical CO2. J. Appl. Polym. Sci. 2018, 135, 46007. [Google Scholar] [CrossRef]
- Ding, J.; Shangguan, J.; Ma, W.; Zhong, Q. Foaming Behavior of Microcellular Foam Polypropylene/Modified Nano Calcium Carbonate Composites. J. Appl. Polym. Sci. 2013, 128, 3639–3651. [Google Scholar] [CrossRef]
- Zhao, C.; Mark, L.H.; Chang, E.; Chu, R.K.M.; Lee, P.C.; Park, C.B. Highly Expanded, Highly Insulating Polypropylene/Polybutylene Terephthalate Composite Foams Manufactured by Nano-fibrillation Technology. Mater. Des. 2020, 188, 108450. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Liu, L.-C.; Liang, W.-C.; Liu, H.-C.; Chen, C.-M. Preparation of Polypropylene (PP) Composite Foams with High Impact Strengths by Supercritical Carbon Dioxide and Their Feasible Evaluation for Electronic Packages. Compos. Part B 2015, 79, 1–5. [Google Scholar] [CrossRef]
- Rizvi, A.; Park, C.B.; Favis, B.D. Tuning Viscoelastic and Crystallization Properties of Polypropylene Containing in-Situ Generated High Aspect Ratio Polyethylene Terephthalate Fibrils. Polymer 2015, 68, 83–91. [Google Scholar] [CrossRef]
- Dehghan, P.; Simiari, M.; Gholampour, M.; Aghvami-Panah, M.; Amirkiai, A. Tuning the Electromagnetic Interference Shielding Performance of Polypropylene Cellular Nanocomposites: Role of Hybrid Nanofillers of MXene and Reduced Graphene Oxide. Polym. Test. 2023, 126, 108162. [Google Scholar] [CrossRef]
- Yang, C.; Xing, Z.; Zhang, M.; Zhao, Q.; Wang, M.; Wu, G. Supercritical CO2 Foaming of Radiation Crosslinked Polypropylene/High-Density Polyethylene Blend: Cell Structure and Tensile Property. Radiat. Phys. Chem. 2017, 141, 276–283. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Wang, X.; Li, K.; Wang, G.; Chen, J.; Park, C.B. Modification of iPP Microcellular Foaming Behavior by Thermal History Control and Nucleating Agent at Compressed CO2. J. Supercrit. Fluids 2018, 133, 383–392. [Google Scholar] [CrossRef]
- Zuo, K.; Xu, J.; Xie, S.; Zhang, S.; Hou, J.; Yang, Y.; Zhang, X.; Chen, J. Microcellular Foaming and Mechanical Properties of iPPF Reinforced PPR Composites. J. Supercrit. Fluids 2021, 170, 105161. [Google Scholar] [CrossRef]
- Zuo, K.; Li, K.; Yun, Z.; He, G.; Islam, S.R.; Yang, Y.; Zhang, X.; Chen, J. Microcellular Foaming and Mechanical Properties of iPP-iPPF Using Supercritical CO2. J. Supercrit. Fluids 2022, 190, 105754. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, X.; Wang, L.; Liu, G.; Liu, X.; Tuinea-Bobe, C.; Whiteside, B.; Coates, P.; Wang, D.; Han, C.C. Flow-Induced Crystallization of Long Chain Aliphatic Polyamides Under a Complex Flow Field: Inverted Anisotropic Structure and Formation Mechanism. Polymer 2015, 73, 91–101. [Google Scholar] [CrossRef]
- Ville, J.; Médéric, P.; Huitric, J.; Aubry, T. Structural and Rheological Investigation of Interphase in Polyethylene/Polyamide/Nanoclay Ternary Blends. Polymer 2012, 53, 1733–1740. [Google Scholar] [CrossRef]
- Fu, D.; Chen, F.; Peng, X.; Kuag, T. Polyamide 6 Modified Polypropylene with Remarkably Enhanced Mechanical Performance, Thermal Properties, and Foaming Ability via Pressure-flow Processing Approach. Adv. Polym. Technol. 2018, 37, 2721–2729. [Google Scholar] [CrossRef]
- Li, D.-C.; Liu, T.; Zhao, L.; Yuan, W.-K. Foaming of Linear Isotactic Polypropylene Based on Its Non-Isothermal Crystallization Behaviors under Compressed CO2. J. Supercrit. Fluids 2011, 60, 89–97. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.; Ma, S.; Pan, K.; Deng, J. Polyamide Foams Prepared by Solution Foaming Approach and their Adsortion Property towards Bisphenol A. Micropor. Mesopor. Mat. 2022, 330, 111626. [Google Scholar] [CrossRef]






| Materials | Tm (PP) °C | Tm (PA11) °C | ΔHm (PP) (J/g) | ΔHm (PA11) (J/g) | Xc (PP) % | Xc (PA11) % |
|---|---|---|---|---|---|---|
| iPP | 169.2 | - | 76.46 | - | 36.6 | - |
| iPP/20PA11 | 169.1 | 190.3 | 56.53 | 5.23 | 27.0 | 2.7 |
| iPP/20PA11/5PP-g-MAH | 168.9 | 189.4 | 48.30 | 7.55 | 23.11 | 3.9 |
| iPP/20PA11/10PP-g-MAH | 167.8 | 188.7 | 44.75 | 11.26 | 21.41 | 5.8 |
| Materials | Elongation at Break/% |
|---|---|
| iPP | 202.41 |
| iPP/5PA11/10 PP-g-MAH | 318.28 |
| iPP/10PA11/10 PP-g-MAH | 373.78 |
| iPP/15PA11/10 PP-g-MAH | 444.77 |
| iPP/20PA11/10 PP-g-MAH | 465.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Wang, Y.; Pei, J.; Fan, Q.; Li, K.; Li, L.; Zhang, X. Mechanical Property and Microcellular Foamability of iPP/PA11/PP-g-MAH Blends. Polymers 2025, 17, 1952. https://doi.org/10.3390/polym17141952
Liu B, Wang Y, Pei J, Fan Q, Li K, Li L, Zhang X. Mechanical Property and Microcellular Foamability of iPP/PA11/PP-g-MAH Blends. Polymers. 2025; 17(14):1952. https://doi.org/10.3390/polym17141952
Chicago/Turabian StyleLiu, Bosi, Yangzheng Wang, Jingke Pei, Qiongdan Fan, Kun Li, Lele Li, and Xiaoli Zhang. 2025. "Mechanical Property and Microcellular Foamability of iPP/PA11/PP-g-MAH Blends" Polymers 17, no. 14: 1952. https://doi.org/10.3390/polym17141952
APA StyleLiu, B., Wang, Y., Pei, J., Fan, Q., Li, K., Li, L., & Zhang, X. (2025). Mechanical Property and Microcellular Foamability of iPP/PA11/PP-g-MAH Blends. Polymers, 17(14), 1952. https://doi.org/10.3390/polym17141952









