Cellulose/Aminated Multi-Walled Carbon Nanotube Nanocomposite Aerogels for Oil Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocellulose Aerogel
2.3. Preparation of NC-MWCNT-NH2 Nanocomposite Aerogels
2.4. Characterization of Aerogels
2.5. Adsorption Test
3. Results
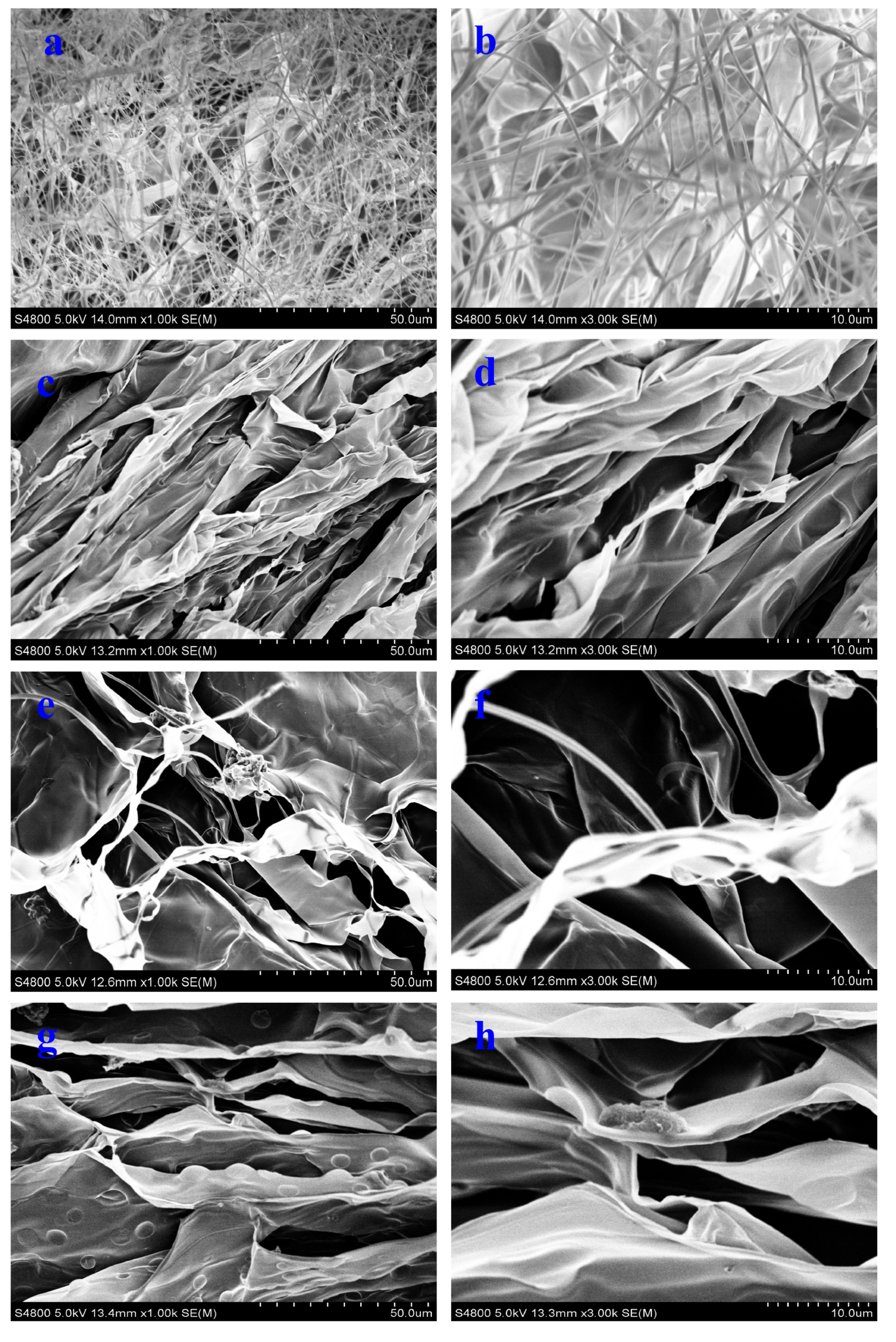
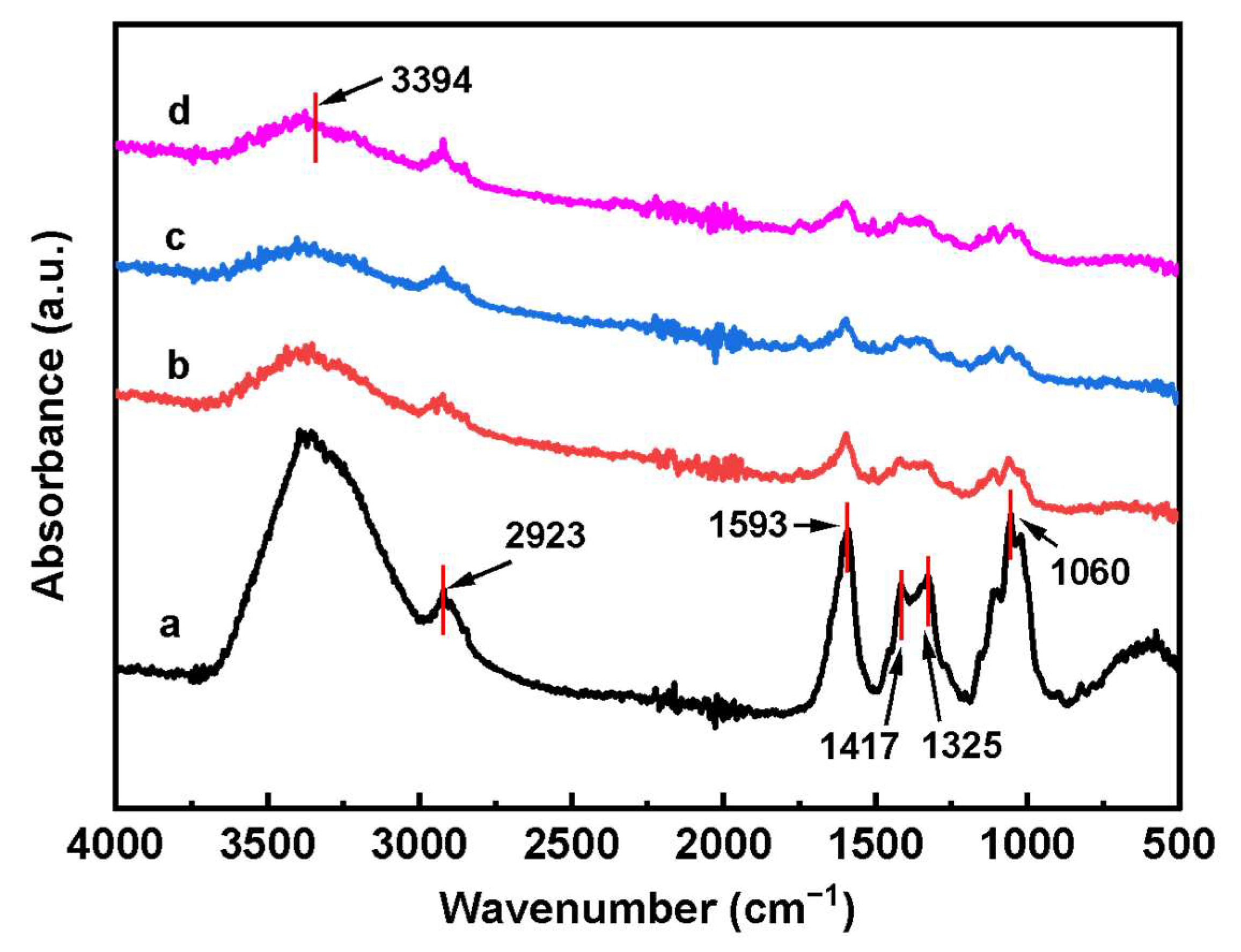
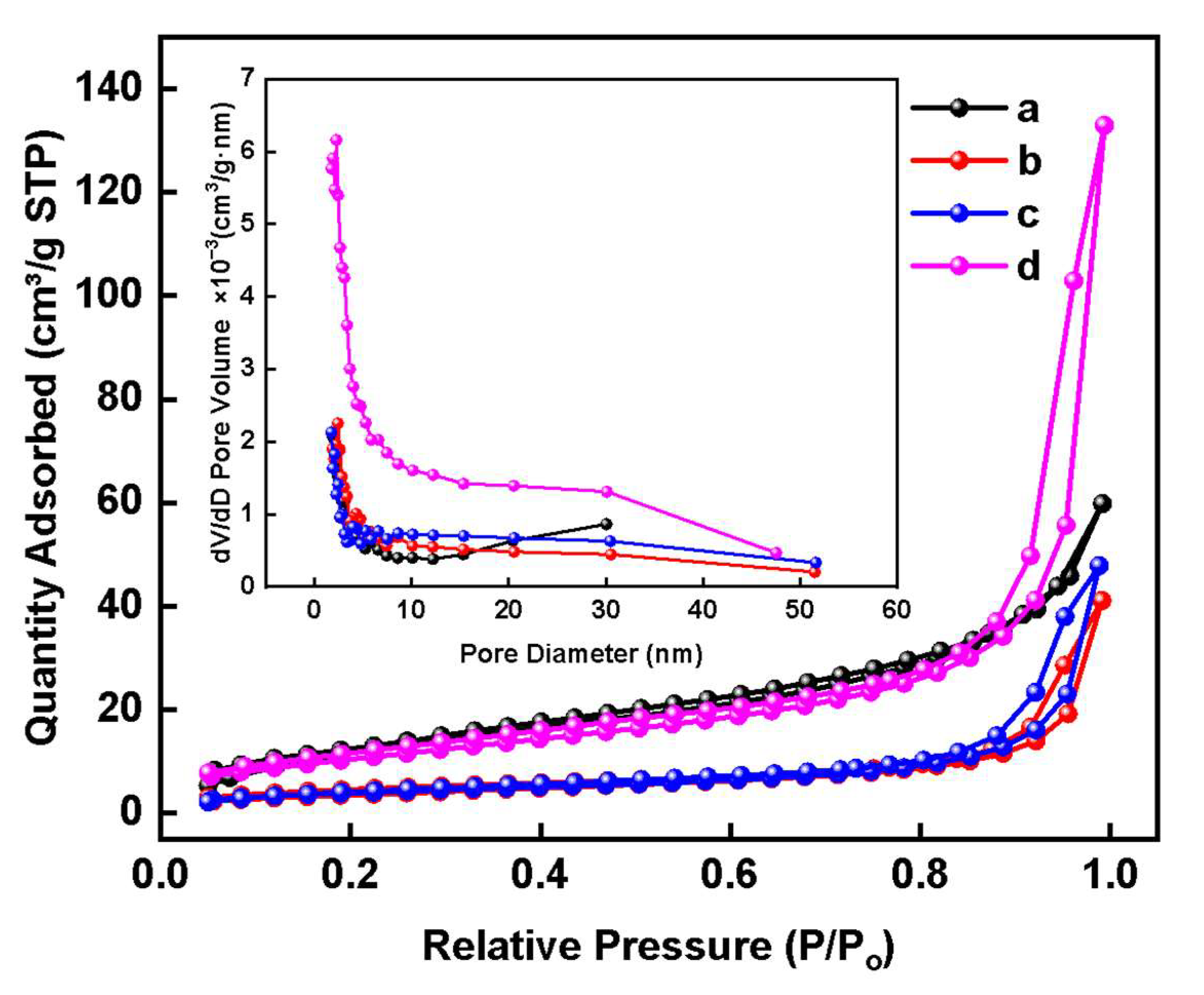
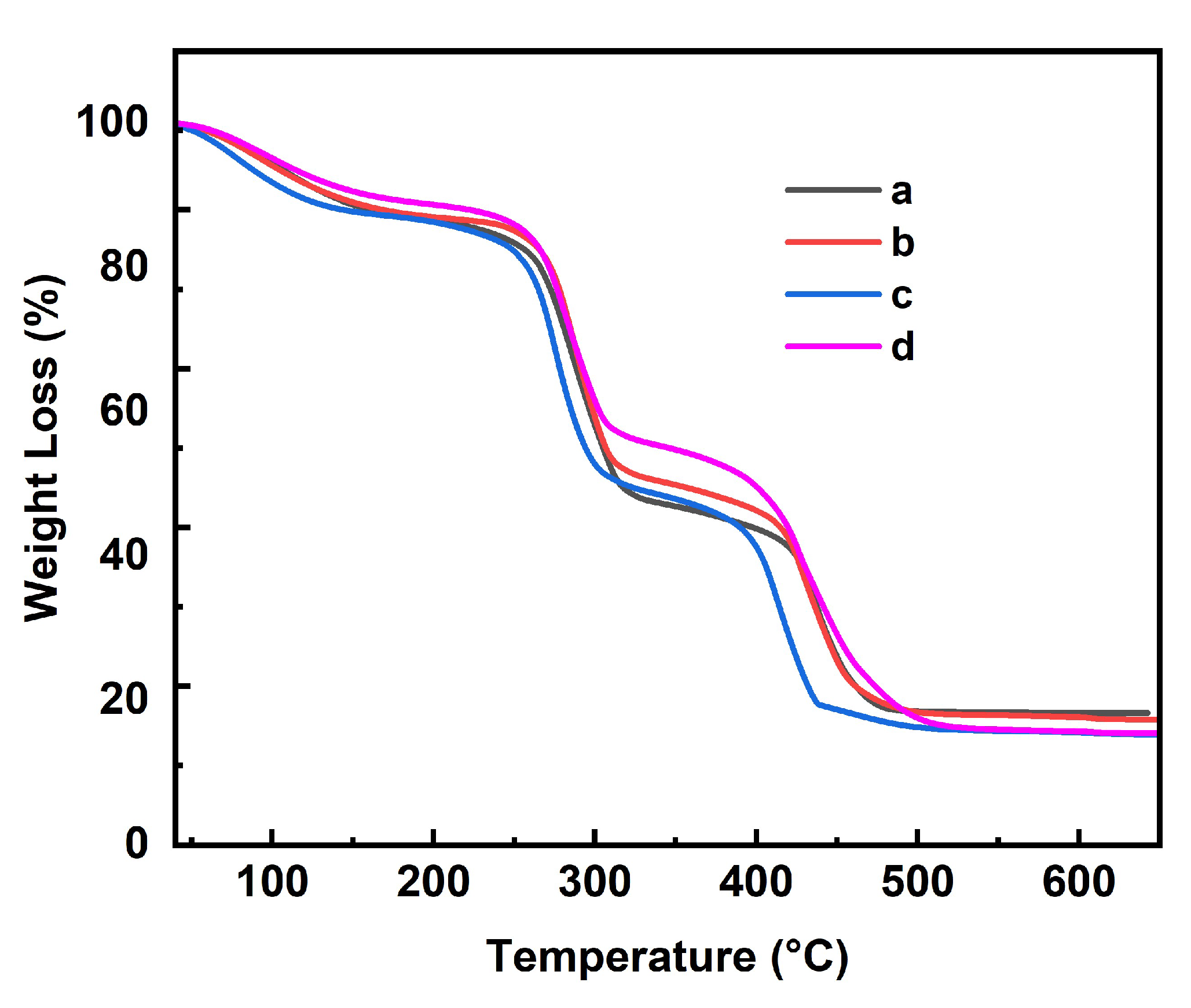
3.1. Structure Characterization
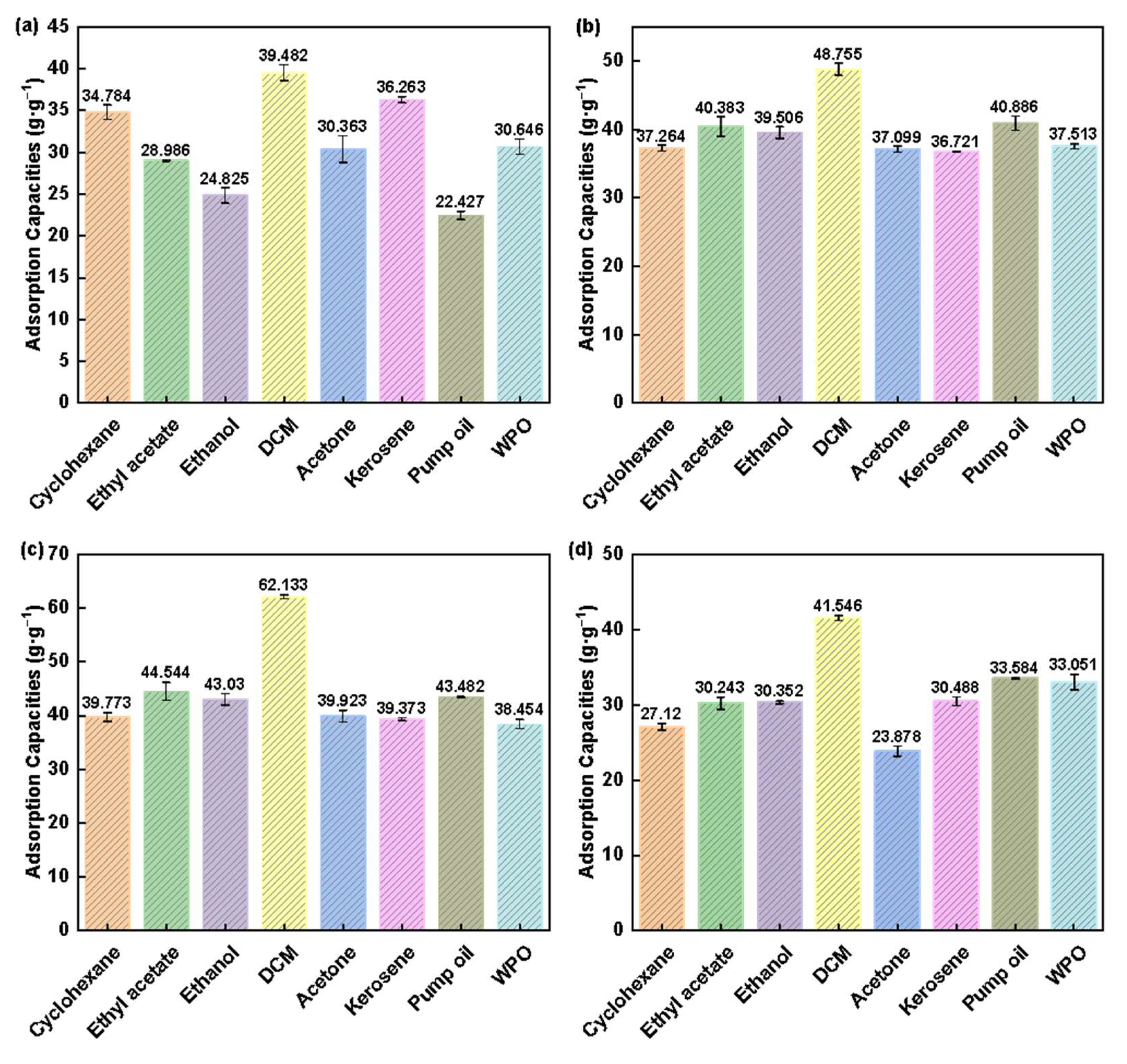
3.2. Adsorption Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Laitinen, O.; Suopajärvi, T.; Österberg, M.; Liimatainen, H. Hydrophobic, Superabsorbing Aerogels from Choline Chloride-Based Deep Eutectic Solvent Pretreated and Silylated Cellulose Nanofibrils for Selective Oil Removal. ACS Appl. Mater. Interfaces 2017, 9, 25029–25037. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Guan, D.; Lin, Z.; Hu, Z.; Ni, M.; Huang, H.; Bhatelia, T.; Jiang, S.P.; Shao, Z. New Nanocomposites Derived from CationNonstoichiometric Bax(Co, Fe, Zr, Y)O3−δ as Efficient Electrocatalysts for Water Oxidation in Alkaline Solution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar] [CrossRef]
- Akhlamadi, G.; Goharshadi, E.K. Sustainable and Superhydrophobic Cellulose Nanocrystal-Based Aerogel Derived from Waste Tissue Paper as a Sorbent for Efficient Oil/Water Separation. Process Saf. Environ. Prot. 2021, 154, 155–167. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive Manufacturing of Silica Aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef]
- Gu, H.; Gao, C.; Zhou, X.; Du, A.; Naik, N.; Guo, Z. Nanocellulose Nanocomposite Aerogel towards Efficient Oil and Organic Solvent Adsorption. Adv. Compos. Hybrid Mater. 2021, 4, 459–468. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Chen, X.; Gan, F.; Teng, C.; Li, X.; Dong, J.; Zhao, X.; Zhang, Q. Continuous and Strong Polyimide Aerogel Fibers Enhanced by Para-Aramid Nanofibers Prepared via a “Reaction Spinning” for Thermal Insulation. Chem. Eng. J. 2024, 486, 150255. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; El-Wakil, N.; Dufresne, A. Current State and New Trends in the Use of Cellulose Nanomaterials for Wastewater Treatment. Biomacromolecules 2019, 20, 573–597. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Wu, H.; Cheng, Y.; Liu, C.; Fang, C. Recent Advances in Cellulose Based Aerogels with Various Dimensions: Design, Functionalization, and Applications. Cellulose 2024, 32, 1–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, Q.; Lai, X.; Li, H.; Zhao, Y.; Li, K.; Jiang, C.; Zeng, X. Multifunctional Cellulose-Based Aerogel for Intelligent Fire Fighting. Carbohyd. Polym. 2023, 316, 121060. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef] [PubMed]
- Idumah, C.I.; Ezika, A.C.; Okpechi, V.U. Emerging Trends in Polymer Aerogel Nanoarchitectures, Surfaces, Interfaces and Applications. Surf. Interfaces 2021, 25, 101258. [Google Scholar] [CrossRef]
- Gu, H.; Zhou, X.; Lyu, S.; Pan, D.; Dong, M.; Wu, S.; Ding, T.; Wei, X.; Seok, I.; Wei, S.; et al. Magnetic nanocellulose-magnetite aerogel for easy oil adsorption. J. Colloid Interface Sci. 2020, 560, 849–856. [Google Scholar]
- Zhang, X.; Wang, H.; Cai, Z.; Yan, N.; Liu, M.; Yu, Y. Highly Compressible and Hydrophobic Anisotropic Aerogels for Selective Oil/Organic Solvent Absorption. ACS Sustain. Chem. Eng. 2019, 7, 332–340. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, M.; Lin, X.; Ren, X.; Huang, T.-S.; Kim, I.S. Functional Nanocomposite Aerogels Based on Nanocrystalline Cellulose for Selective Oil/Water Separation and Antibacterial Applications. Chem. Eng. J. 2019, 371, 306–313. [Google Scholar] [CrossRef]
- Sengupta, A.; Gupta, N.K. MWCNTs Based Sorbents for Nuclear Waste Management: A Review. J. Environ. Chem. Eng. 2017, 5, 5099–5114. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, H.; Li, Y.; Kuang, Y.; Xu, X.; Chen, C.; Huang, H.; Jia, C.; Zhao, X.; Hitz, E.; et al. Lightweight, Mesoporous, and Highly Absorptive All-Nanofiber Aerogel for Efficient Solar Steam Generation. ACS Appl. Mater. Interfaces 2018, 10, 1104–1112. [Google Scholar] [CrossRef]
- Zou, J.; Liu, J.; Karakoti, A.S.; Kumar, A.; Joung, D.; Li, Q.; Khondaker, S.I.; Seal, S.; Zhai, L. Ultralight Multiwalled Carbon Nanotube Aerogel. ACS Nano 2010, 4, 7293–7302. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, Q.; Liu, H.; Gu, H.; Guo, Z. Solvent-Free Nanoalumina Loaded Nanocellulose Aerogel for Efficient Oil and Organic Solvent Adsorption. J. Colloid Interface Sci. 2021, 581, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Hsieh, Y.-L. Cellulose Nanofibril Aerogels: Synergistic Improvement of Hydrophobicity, Strength, and Thermal Stability via Cross-Linking with Diisocyanate. ACS Appl. Mater. Interfaces 2017, 9, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, C.M.; Zhou, Q.; Gan, Y.; Bao, Q.L. Functionalized Multi-Walled Carbon Nanotubes as Affinity Ligands. Nanotechnology 2007, 18, 115614. [Google Scholar] [CrossRef]
- Bora, D.K.; Bavdane, P.P.; Bhatt, B.; Nikumbe, D.Y.; Sethia, G.; Nagarale, R.K. Ruthenium-Anchored Aminated MWCNTs/Polyaniline Membrane Electrode Assembly for Alkaline Water Splitting. J. Electroanal. Chem. 2024, 954, 118027. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Ni, L.; Huang, Z.; Liu, L.; Ke, Q.; Xu, H. An elastic MOF/graphene aerogel with high photothermal efficiency for rapid removal of crude oil. J. Hazard. Mater. 2023, 443, 130339. [Google Scholar] [CrossRef]
- Zhai, Y.; Yuan, X. One-Pot Fabrication of Hydrophobic, Superelastic, Harakeke-Derived Nanocellulose Aerogels with Excellent Shape Recovery for Oil Adsorption and Water-in-Oil Emulsion Separation. Inter. J. Biol. Macromol. 2024, 280, 135489. [Google Scholar] [CrossRef]
- Sam, E.K.; Sam, D.K.; Chen, J.; Lv, X.; Liu, J. Hydrophobic Porous BN/SiO2@PU as Ternary Adsorbents for Efficient Oil/Water Separation. J. Porous Mater. 2020, 27, 1149–1158. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, L.; Lai, X.; Li, H.; Zeng, X. Highly Hydrophobic F-rGO@wood Sponge for Efficient Clean-up of Viscous Crude Oil. Chem. Eng. J. 2020, 386, 123994. [Google Scholar] [CrossRef]
- Huang, J.; Yan, Z. Adsorption Mechanism of Oil by Resilient Graphene Aerogels from Oil–Water Emulsion. Langmuir 2018, 34, 1890–1898. [Google Scholar] [CrossRef]
- Wang, D.; McLaughlin, E.; Pfeffer, R.; Lin, Y.S. Adsorption of Oils from Pure Liquid and Oil–Water Emulsion on Hydrophobic Silica Aerogels. Separ. Purif. Technol. 2012, 99, 28–35. [Google Scholar] [CrossRef]
| NO | MWCNT-NH2 Content (wt.%) | Cellulose Content (wt.%) | Mass Ratio of MWCNT-NH2 and Cellulose |
|---|---|---|---|
| A1 | 0.05 | 0.4 | 1:8 |
| A2 | 0.1 | 0.4 | 2:8 |
| A3 | 0.15 | 0.4 | 3:8 |
| A0 | 0 | 0.4 | 0 |
| Materials | Oil Type | Adsorption Capacity (g·g−1) | Literature |
|---|---|---|---|
| BN/SiO2@PU | Chloroform | 3 | [26] |
| F-rGO@WS | Pump oil | 3.63 | [27] |
| Graphene aerogels | Diesel oil | 25 | [28] |
| Silica aerogels | Motor oil | 15.1 | [29] |
| Nanocellulose aerogels | Hexane | 80 | [30] |
| NC-MWCNT-NH2 aerogel | Pump oil | 43.48 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, R.; Liu, Z.; Feng, F.; Su, S.; Dong, G.; Liu, X.; Gu, H. Cellulose/Aminated Multi-Walled Carbon Nanotube Nanocomposite Aerogels for Oil Adsorption. Polymers 2025, 17, 869. https://doi.org/10.3390/polym17070869
Han R, Liu Z, Feng F, Su S, Dong G, Liu X, Gu H. Cellulose/Aminated Multi-Walled Carbon Nanotube Nanocomposite Aerogels for Oil Adsorption. Polymers. 2025; 17(7):869. https://doi.org/10.3390/polym17070869
Chicago/Turabian StyleHan, Runlin, Zihan Liu, Faxiang Feng, Shi Su, Guilin Dong, Xiaobing Liu, and Hongbo Gu. 2025. "Cellulose/Aminated Multi-Walled Carbon Nanotube Nanocomposite Aerogels for Oil Adsorption" Polymers 17, no. 7: 869. https://doi.org/10.3390/polym17070869
APA StyleHan, R., Liu, Z., Feng, F., Su, S., Dong, G., Liu, X., & Gu, H. (2025). Cellulose/Aminated Multi-Walled Carbon Nanotube Nanocomposite Aerogels for Oil Adsorption. Polymers, 17(7), 869. https://doi.org/10.3390/polym17070869









