Phosphorous-Based, Halogen-Free Flame Retardants for Thin, Flexible Polyurethane Artificial Leathers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
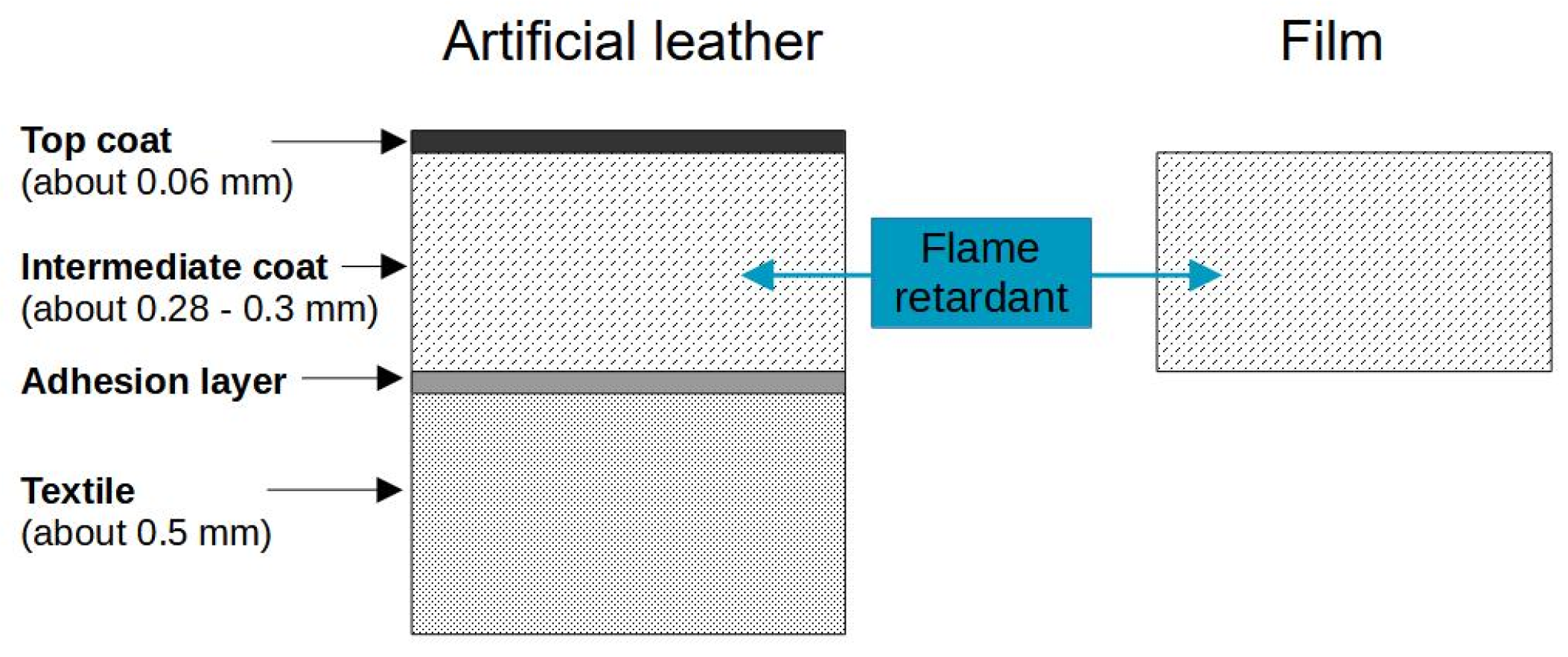
2.2. Preparation of Films and Artificial Leathers
2.3. Characterization Methods
2.3.1. Cone Calorimetry
2.3.2. Limiting Oxygen Index
2.3.3. Horizontal Burning Behavior
2.3.4. Thermogravimetric Analysis
2.3.5. Mechanical Characterization
3. Results and Discussion
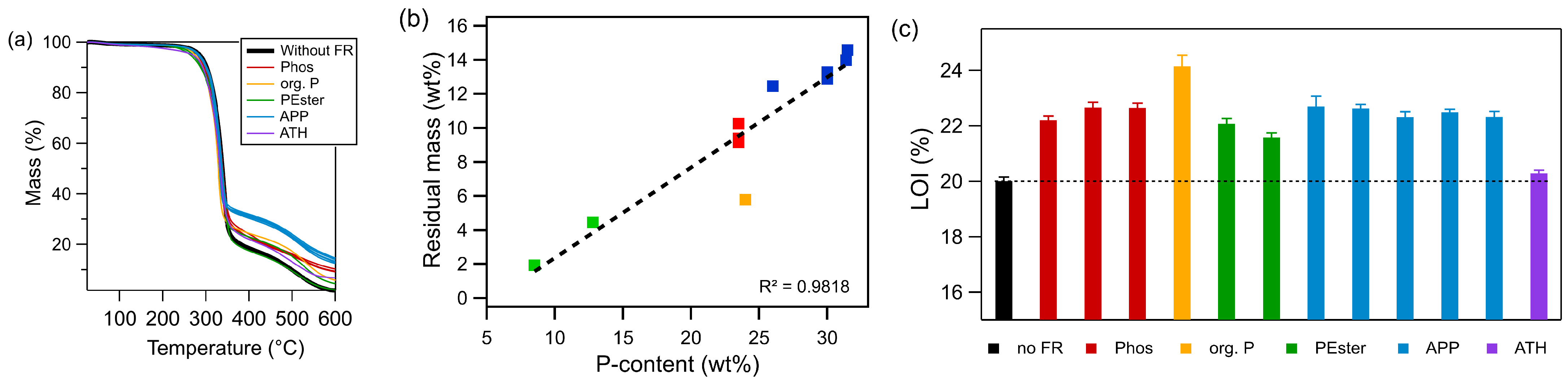
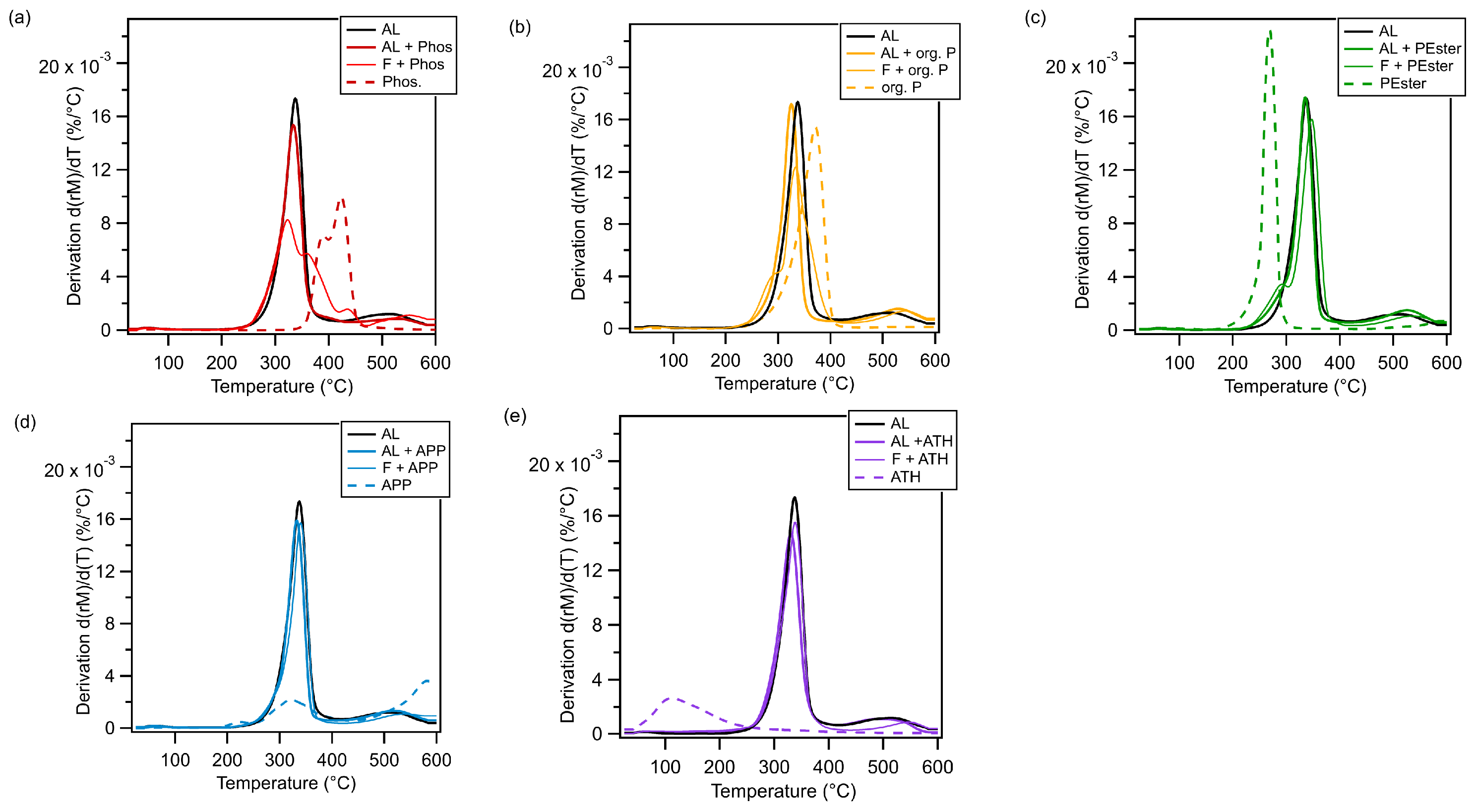
3.1. Flame Retardant Comparison
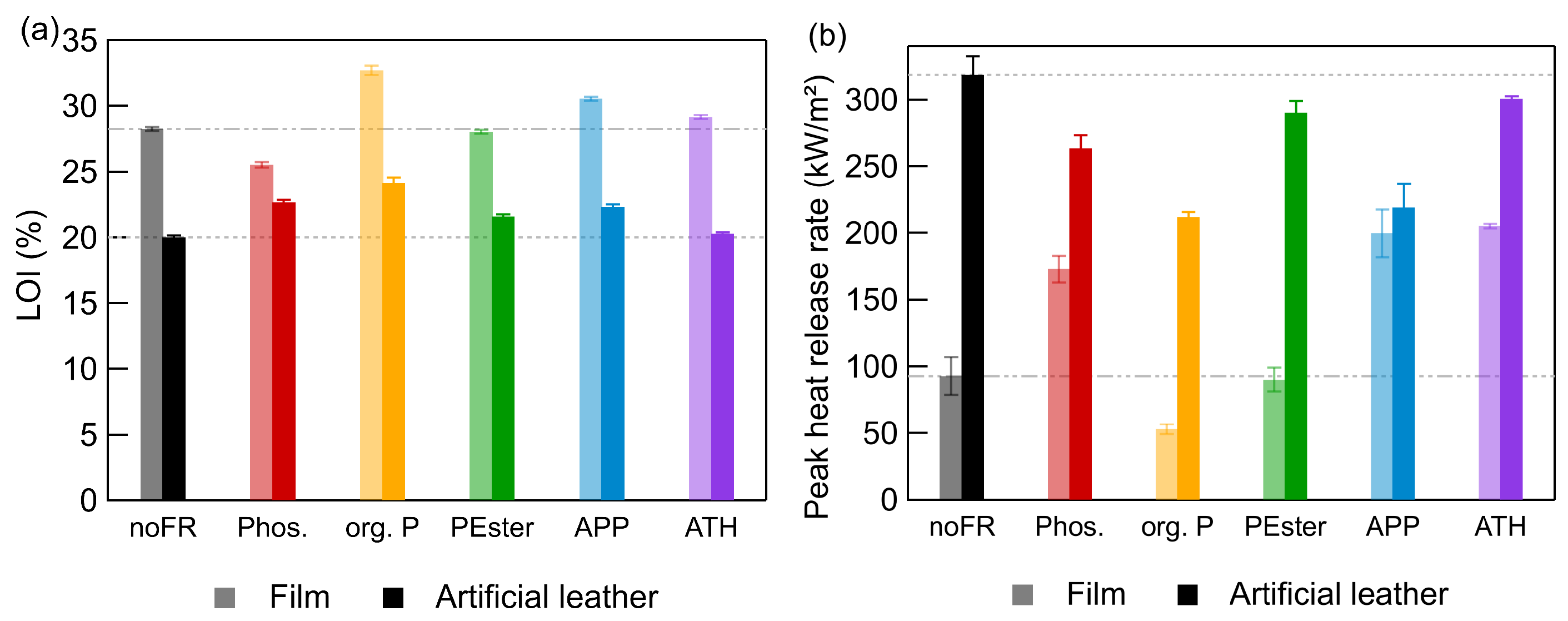
3.2. Comparing Results of Films and Artificial Leathers
3.3. Variation of Flame Retardant Concentration
3.4. Combination of Flame Retardants
3.5. Synergistic Effects
3.6. Mechanical Properties and Long-Term Durability
3.7. Transferability to Other High-Solid PURs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirschler, M.M. Polyurethane Foam and Fire Safety. Polym. Adv. Technol. 2008, 19, 521–529. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Commercial Flame Retardancy of Polyurethanes. J. Fire Sci. 2004, 22, 183–210. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Applications—A Review. Rsc Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- EN 15987; Leather-Terminology-Key Definitions for the Leather Trade. CEN: Brussels, Belgium, 2022.
- Levchik, S.V.; Weil, E.D. Thermal Decomposition, Combustion and Fire-Retardancy of Polyurethanes—A Review of the Recent Literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Lu, S.; Feng, Y.; Zhang, P.; Hong, W.; Chen, Y.; Fan, H.; Yu, D.; Chen, X. Preparation of Flame-Retardant Polyurethane and Its Applications in the Leather Industry. Polymers 2021, 13, 1730. [Google Scholar] [CrossRef]
- Tabatabaee, F.; Khorasani, M.; Ebrahimi, M.; González, A.; Irusta, L.; Sardon, H. Synthesis and Comprehensive Study on Industrially Relevant Flame Retardant Waterborne Polyurethanes Based on Phosphorus Chemistry. Prog. Org. Coat. 2019, 131, 397–406. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent Developments in the Fire Retardancy of Polymeric Materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K. Structural Engineering of Polyurethane Coatings for High Performance Applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Shaw, S. Halogenated Flame Retardants: Do the Fire Safety Benefits Justify the Risks? Rev. Environ. Health 2010, 25, 261–306. [Google Scholar] [CrossRef]
- Kim, Y.R.; Harden, F.A.; Toms, L.-M.L.; Norman, R.E. Health Consequences of Exposure to Brominated Flame Retardants: A Systematic Review. Chemosphere 2014, 106, 1–19. [Google Scholar] [CrossRef]
- Eriksson, P.; Jakobsson, E.; Fredriksson, A. Brominated Flame Retardants: A Novel Class of Developmental Neurotoxicants in Our Environment? Environ. Health Perspect. 2001, 109, 6. [Google Scholar]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molekulare Brandbekämpfung—Wie moderne Phosphorchemie zur Lösung der Flammschutzaufgabe beitragen kann. Angew. Chem. 2018, 130, 10608–10626. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-Based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Schartel, B. Flame Retardancy Mechanisms of Aluminium Phosphinate in Combination with Melamine Cyanurate in Glass-Fibre-Reinforced Poly(1,4-Butylene Terephthalate). Macromol. Mater. Eng. 2008, 293, 206–217. [Google Scholar] [CrossRef]
- Green, J. Mechanisms for Flame Retardancy and Smoke Suppression—A Review. J. Fire Sci. 1996, 14, 426–442. [Google Scholar]
- Hörold, S. Phosphorus-Based and Intumescent Flame Retardants. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 221–254. [Google Scholar]
- Joseph, P.; Ebdon, J.R. Phosphorus-Based Flame Retardants. In Fire Retardancy of Polymeric Materials, 2nd ed.; Wilkie, C.A., Morgan, A.B., Eds.; Taylor & Francis: Abingdon-on-Thames, UK, 2009; pp. 107–128. [Google Scholar]
- Lewin, M.; Weil, E.D. Mechanisms and Modes of Action in Flame Retardancy of Polymers. In Fire Retardant Materials; Elsevier: Amsterdam, The Netherlands, 2001; pp. 31–68. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal Stability and Flame Retardancy of Polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, X.; Wu, Z.; Wang, H.; Gu, Z.; Bao, C.; Tian, X. A Commercial Phosphorous-Nitrogen Containing Intumescent Flame Retardant for Thermoplastic Polyurethane. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Ma, Y.; Dang, X.; Shan, Z. Thermal Analysis and Identification of Potential Fire-Proof Energy Building Material Based on Artificial Leather. J. Therm. Sci. 2019, 28, 88–96. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, H.; Yang, H.; Huang, Z.; Qi, D. Solvent-Free Preparation of Reactive Flame-Retardant Polyurethane Resin for Synthetic Leather Applications. J. Appl. Polym. Sci. 2025, 14, e56684. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, B.; Zhao, Q.; Qi, Y.; Wang, Y.; Sun, Z.; Liu, B.; Zhang, N.; Hu, W.; et al. A Novel Phosphorus-Containing Lignin-Based Flame Retardant and Its Application in Polyurethane. Compos. Commun. 2020, 21, 100382. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhao, Q.; Li, L.; Yan, R.; Zhang, J.; Duan, J.C.; Liu, B.J.; Sun, Z.Y.; Zhang, M.Y.; Hu, W.; et al. Synthesis of a Lignin-Based Phosphorus-Containing Flame Retardant and Its Application in Polyurethane. RSC Adv. 2018, 8, 32252–32261. [Google Scholar] [CrossRef] [PubMed]
- Toldy, A.; Harakály, G.; Szolnoki, B.; Zimonyi, E.; Marosi, G. Flame Retardancy of Thermoplastics Polyurethanes. Polym. Degrad. Stab. 2012, 97, 2524–2530. [Google Scholar] [CrossRef]
- Ali, M.H.M.; Rahman, H.A.; Amirnordin, S.H.; Khan, N.A. Eco-Friendly Flame-Retardant Additives for Polyurethane Foams: A Short Review. Key Eng. Mater. 2018, 791, 19–28. [Google Scholar]
- Hejna, A. Clays as Inhibitors of Polyurethane Foams’ Flammability. Materials 2021, 14, 4826. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S.; Fontaine, G.; Bellayer, S.; Turf, T.; Samyn, F. Characterization and Reaction to Fire of Polymer Nanocomposites with and without Conventional Flame Retardants. Mol. Cryst. Liq. Cryst. 2008, 486, 325/[1367]–339/[1381]. [Google Scholar] [CrossRef]
- Xie, H.; Lai, X.; Zhou, R.; Li, H.; Zhang, Y.; Zeng, X.; Guo, J. Effect and Mechanism of N-Alkoxy Hindered Amine on the Flame Retardancy, UV Aging Resistance and Thermal Degradation of Intumescent Flame Retardant Polypropylene. Polym. Degrad. Stab. 2015, 118, 167–177. [Google Scholar] [CrossRef]
- Lopez-Cuesta, J.-M.; Jlassi, K.; Chehimi, M.M.; Thomas, S. Flame Retardancy Properties of Clay—Polymer Nanocomposites. In Clay-Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- ISO 5660-1; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- ISO 4589-2; Plastics—Determination of Burning Behaviour by Oxygen Index Part 2: Ambient-Temperature Test. ISO: Geneva, Switzerland, 2017.
- Silva-Santos, M.C.; Oliveira, M.S.; Giacomin, A.M.; Laktim, M.C.; Baruque-Ramos, J. Flammability on Textile of Business Uniforms: Use of Natural Fibers. Procedia Eng. 2017, 200, 148–154. [Google Scholar] [CrossRef]
- DIN 75200; Determination of Burning Behaviour of Interior Materials in Motor Vehicles. DIN: Berlin, German, 1980.
- Feng, J.; Ge, Z.; Chai, C.; Wang, S.; Yu, D.; Wu, G.; Luo, Y. Flame Retardant Modification of Waterborne Polyurethane Fabric Coating Agent with High Hydrostatic Pressure Resistance. Prog. Org. Coat. 2016, 97, 91–98. [Google Scholar]
- DIN EN ISO 527-3:2019-02; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. ISO: Geneva, Switzerland, 2018.
- ISO 32100:2010; Rubber- or Plastic-Coated Fabrics—Physical and Mechanical Tests—Determination of Flex Resistance by the Flexometer Method. ISO: Geneva, Switzerland, 2010.
- Mequanint, K.; Sanderson, R.; Pasch, H. Thermogravimetric Study of Phosphated Polyurethane Ionomers. Polym. Degrad. Stab. 2002, 77, 121–128. [Google Scholar] [CrossRef]
- Li, H.; Ning, N.; Zhang, L.; Wang, Y.; Liang, W.; Tian, M.; Chan, T.W. Effect of Content of Organophosphorus on Flame Retardancy Mode of Thermoplastic Polyurethane. Polymer 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Flame Retardants for Plastics and Textiles: Practical Applications; Hanser Publications: Cincinnati, OH, USA, 2009. [Google Scholar]
- Thirumal, M.; Singha, N.K.; Khastgir, D.; Manjunath, B.S.; Naik, Y.P. Halogen-Free Flame-Retardant Rigid Polyurethane Foams: Effect of Alumina Trihydrate and Triphenylphosphate on the Properties of Polyurethane Foams. J. Appl. Polym. Sci. 2010, 116, 2260–2268. [Google Scholar] [CrossRef]
- Duquesne, S.; Fontaine, G.; Cérin-Delaval, O.; Gardelle, B.; Tricot, G.; Bourbigot, S. Study of the Thermal Degradation of an Aluminium Phosphinate–Aluminium Trihydrate Combination. Thermochim. Acta 2013, 551, 175–183. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some Comments on the Use of Cone Calorimeter Data. Polym. Degrad. Stab. 2005, 88, 540–547. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of Fire-Retarded Materials—Interpretation of Cone Calorimeter Data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Li, H.; Ning, N.; Zhang, L.; Wang, Y.; Liang, W.; Tian, M. Different Flame Retardancy Effects and Mechanisms of Aluminium Phosphinate in PPO, TPU and PP. Polym. Degrad. Stab. 2014, 105, 86–95. [Google Scholar] [CrossRef]



| Flame Retardant | Abbr. | P-Content (%) | N-Content (%) | Mechanism |
|---|---|---|---|---|
| Phosphinate | Phos | 23.3–24 | - | Gas phase |
| Phosphinate | 23.5 | - | Gas phase | |
| Aluminum-Diethyl-Phosphinate | 23.3–24 | - | Gas phase | |
| Organic phosphorus compound | org. P | 24 | - | Charring |
| Oligomeric phosphate ester | PEster | 12.8 | - | Charring |
| Butylated triphenyl phosphate | 8.5 | - | Charring | |
| Ammonium polyphosphate | APP | 26 | 14 | Intumescence |
| Ammonium polyphosphate | 31–32 | 14–15 | Intumescence | |
| Ammonium polyphosphate/Melamine | 29–31 | 15–17 | Intumescence | |
| Ammonium polyphosphate/Melamine | 30 | 17 | Intumescence | |
| Ammonium polyphosphate/Silane | 31.4 | 14 | Intumescence | |
| Aluminum trihydroxide | ATH | - | - | Dilution |
| High Solid | Abbr. | Crosslinker | Preheating | Gelation Parameter | Scraper Gap | |
|---|---|---|---|---|---|---|
| Topcoat | Polycarbonate ester PUR | TC | - 1 | 2 min 90 °C | 3 min 160 °C | 0.1 mm |
| Intermediate coat | Aromatic high solid | HS 1 | 9.2 phr | 2 min 80 °C | 3 min 165 °C | 0.4 mm |
| Intermediate coat | Aromatic high solid | HS 2 | 6.9 phr | 2 min 80 °C | 2 min 160 °C 2 min 170 °C | 0.4 mm |
| Intermediate coat | Aliphatic high solid | HS 3 | 14.9 phr | 2 min 80 °C | 4 min 175 °C | 0.4 mm |
| Adhesive Coat | Anionic, aromatic polyether PUR | AC | 6.0 ph 1 | 2 min 80 °C | 3 min 165 °C | 0.1 mm |
| Films | Artificial Leather | |||||
|---|---|---|---|---|---|---|
| LOI | PHRR | TGA 1 | LOI | PHRR | TGA 1 | |
| Phos |  |  |  |  |  |  |
| Org. P |  |  |  |  |  |  |
| PEster |  |  |  |  |  |  |
| APP |  |  |  |  |  |  |
| ATH |  |  |  |  |  |  |
| FR (phr) | LOI (%) | Burning Rate (mm/min) | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | TSP (m2) | |
|---|---|---|---|---|---|---|---|
| Without FR | - | 20.0 | 62.9 | 18.3 | 318.3 | 9.1 | 0.52 |
| Phos | 5 | 21.2 | 49.1 | 16.3 | 249.4 | 7.4 | n.d. |
| 10 | 22.3 | 41.8 | 16.8 | 216.1 | 6.8 | n.d. | |
| 20 | 22.7 | 54.2 | 14 | 260.0 | 7.3 | 1.0 | |
| 30 | 22.7 | 49.3 | 20.8 | 230.4 | 6.2 | n.d. | |
| Org. P | 5 | 22.0 | 37.8 | 19.7 | 322.4 | 10.2 | n.d. |
| 10 | 23.5 | 27.9 | 18.5 | 291.3 | 9.8 | n.d. | |
| 20 | 24.2 | 0 | 21 | 212.1 | 5.1 | 1.7 | |
| 30 | 25.1 | 0 | 24.3 | 197.1 | 8.0 | n.d. | |
| PEster | 5 | 20.7 | 53.1 | 15.5 | 315.6 | 9.1 | n.d. |
| 10 | 21.9 | 56.1 | 17.3 | 311.6 | 10.2 | n.d. | |
| 20 | 21.6 | 40.4 | 17.5 | 290.1 | 8.7 | 1.42 | |
| 30 | 22.0 | 32.5 | 19 | 247.6 | 7.1 | n.d. | |
| APP | 5 | 21.6 | 60.0 | 17 | 276.0 | 8.8 | n.d. |
| 10 | 21.7 | 57.5 | 23 | 244.9 | 5.7 | n.d. | |
| 20 | 22.3 | 79.5 | 16 | 219.0 | 6.3 | 0.78 | |
| 30 | 24.4 | 66.1 | 16 | 154.0 | 5.1 | n.d. | |
| ATH | 5 | 19.9 | 66.7 | 15 | 271.7 | 7.8 | n.d. |
| 10 | 20.1 | 57.4 | 19 | 270.0 | 7.8 | n.d. | |
| 20 | 20.3 | 68.3 | 19 | 296.8 | 9.9 | 3.8 |
| LOI | Horizontal Burning | Cone Calorimetry | |
|---|---|---|---|
| Phos |  |  |  |
| Org. P |  |  |  |
| PEster |  |  |  |
| APP |  |  |  |
| ATH |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bader, M.; Lehmann, M.; Meyer, M. Phosphorous-Based, Halogen-Free Flame Retardants for Thin, Flexible Polyurethane Artificial Leathers. Polymers 2025, 17, 841. https://doi.org/10.3390/polym17070841
Bader M, Lehmann M, Meyer M. Phosphorous-Based, Halogen-Free Flame Retardants for Thin, Flexible Polyurethane Artificial Leathers. Polymers. 2025; 17(7):841. https://doi.org/10.3390/polym17070841
Chicago/Turabian StyleBader, Miriam, Maren Lehmann, and Michael Meyer. 2025. "Phosphorous-Based, Halogen-Free Flame Retardants for Thin, Flexible Polyurethane Artificial Leathers" Polymers 17, no. 7: 841. https://doi.org/10.3390/polym17070841
APA StyleBader, M., Lehmann, M., & Meyer, M. (2025). Phosphorous-Based, Halogen-Free Flame Retardants for Thin, Flexible Polyurethane Artificial Leathers. Polymers, 17(7), 841. https://doi.org/10.3390/polym17070841







