Development of an Electrochemical Biosensor Based on Polypyrrole-3-carboxylic Acid/Polypyrrole/Au Nanoparticle Composites for Detection of Dopamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
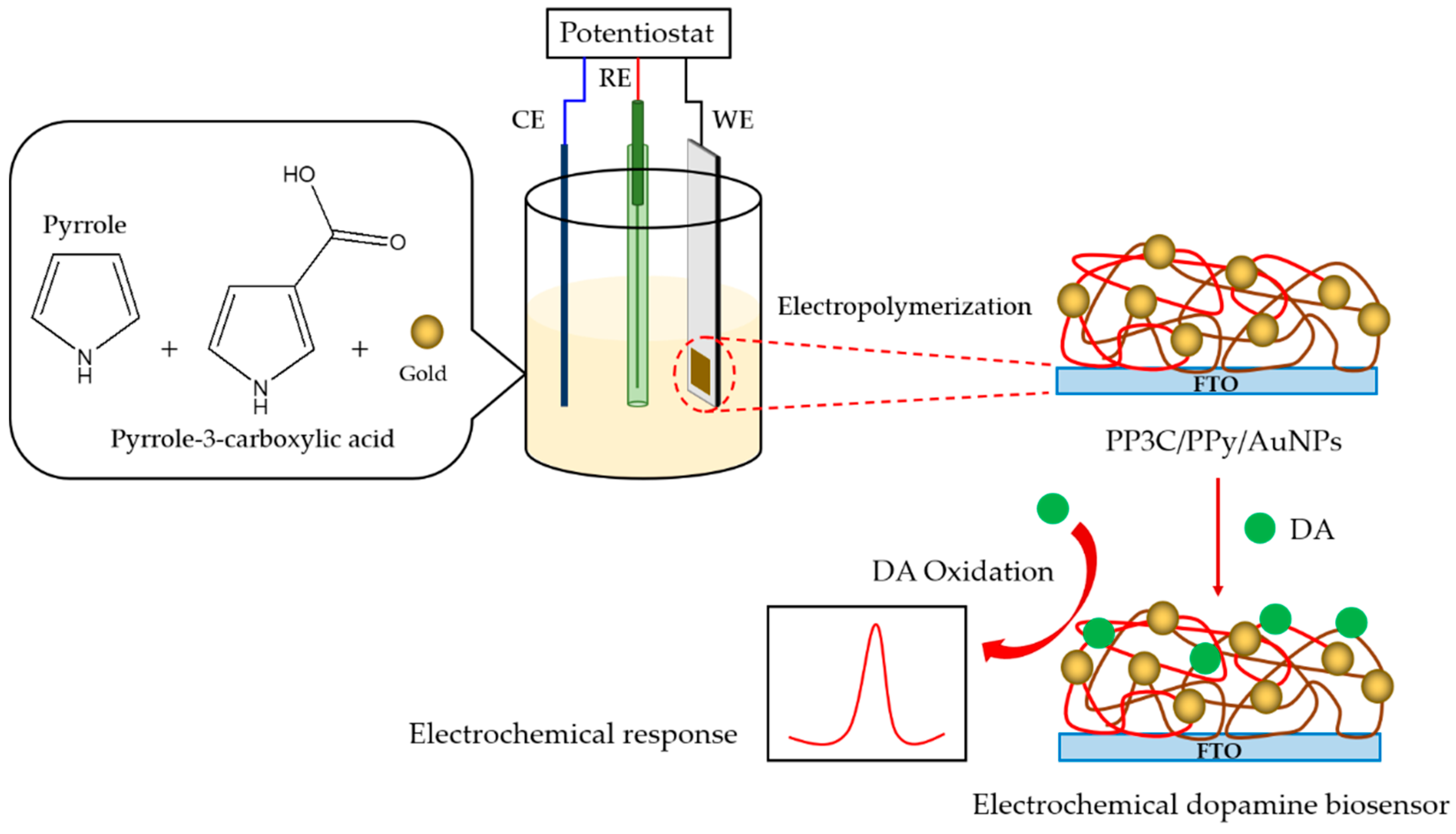
2.2. Electropolymerization of PP3C/PPy/AuNPs
2.3. Structural Characterizations of PP3C/PPy/AuNPs
2.4. Determination of DA
3. Results and Discussion
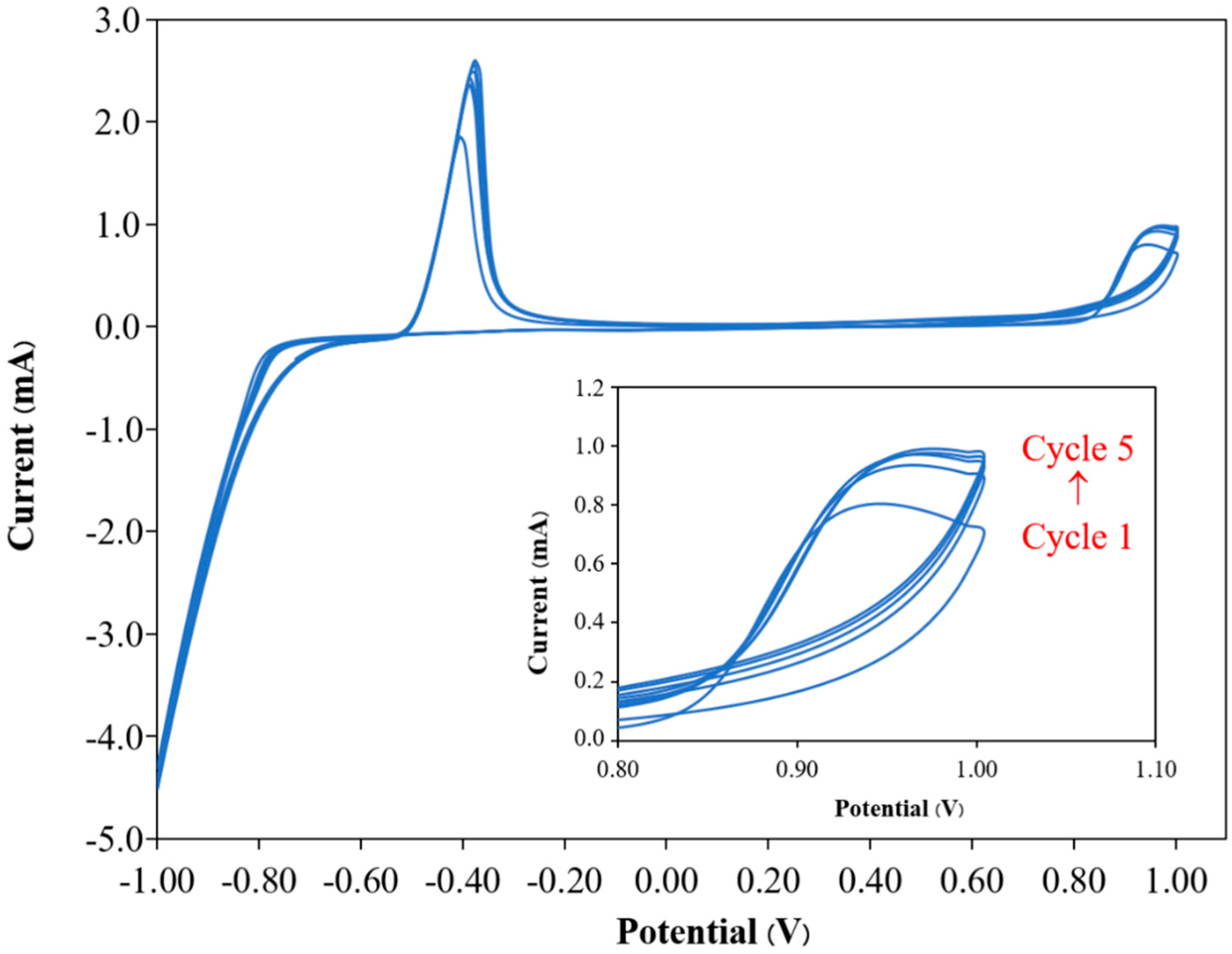
3.1. Fabrication of PP3C/PPy/AuNPs
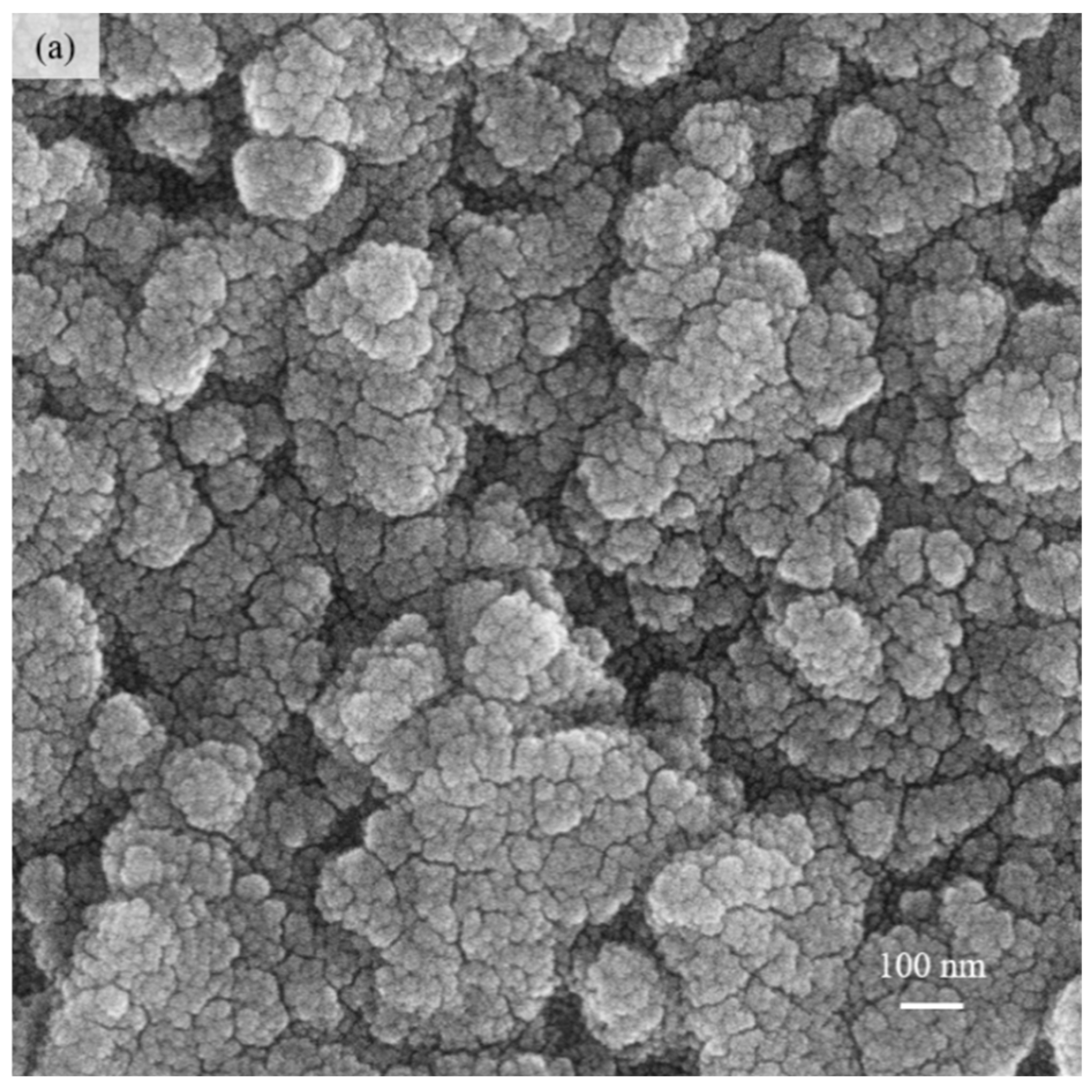
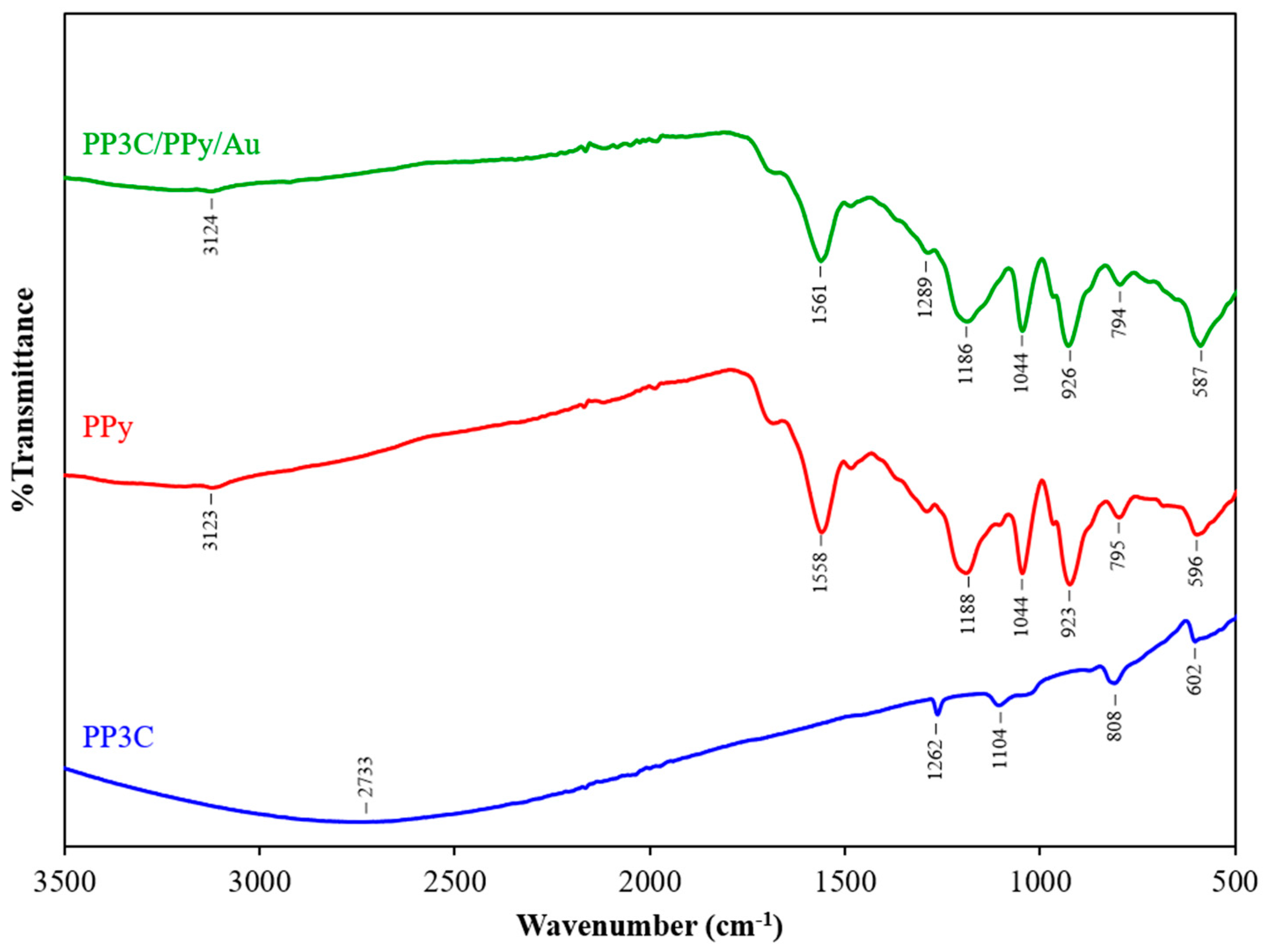
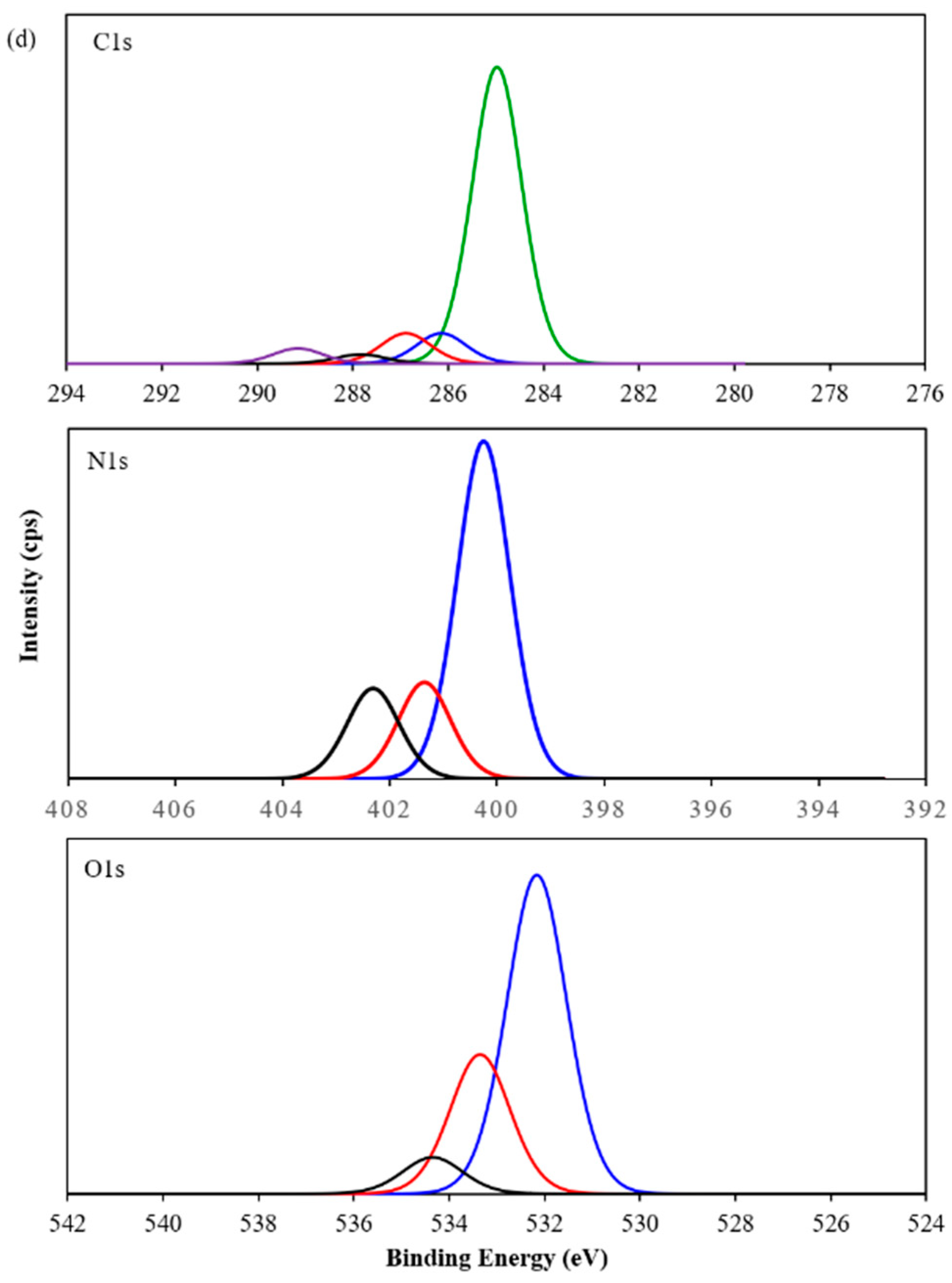
3.2. Characterization of PP3C/PPy/AuNPs Composite Thin Film
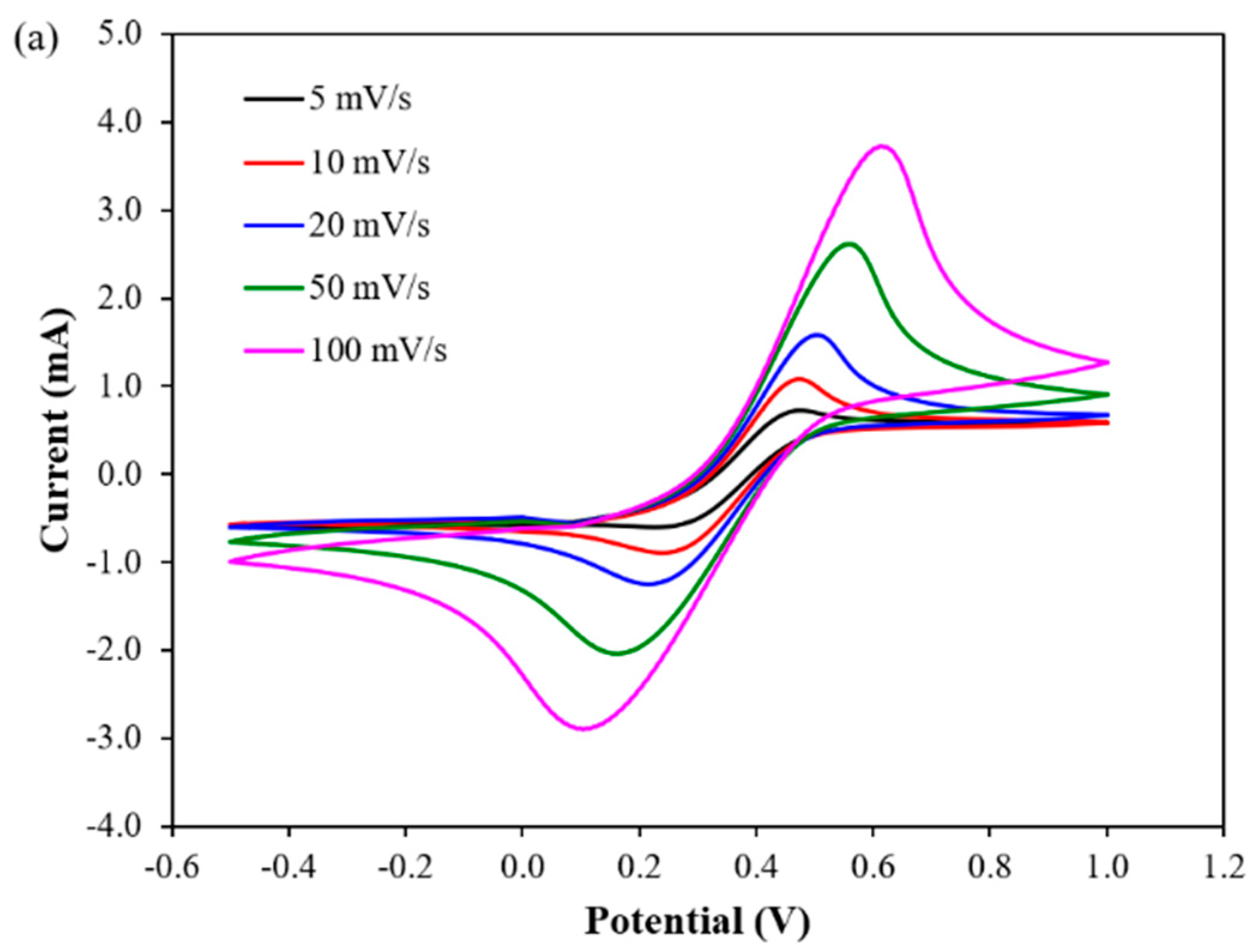
3.3. Electrochemical Detection of DA at PP3C/PPy/AuNPs
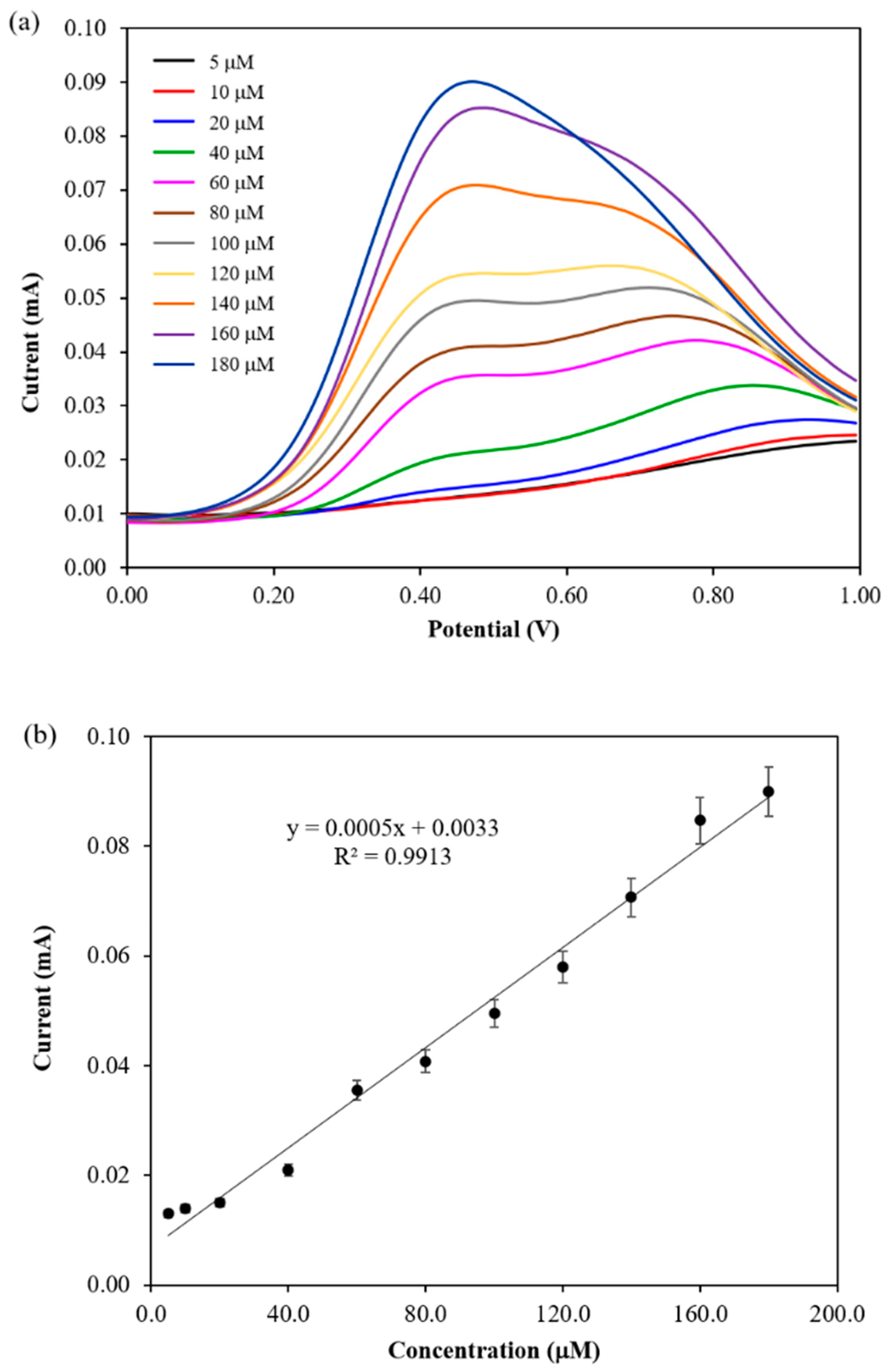
3.4. Performance of Electrochemical DA Biosensor
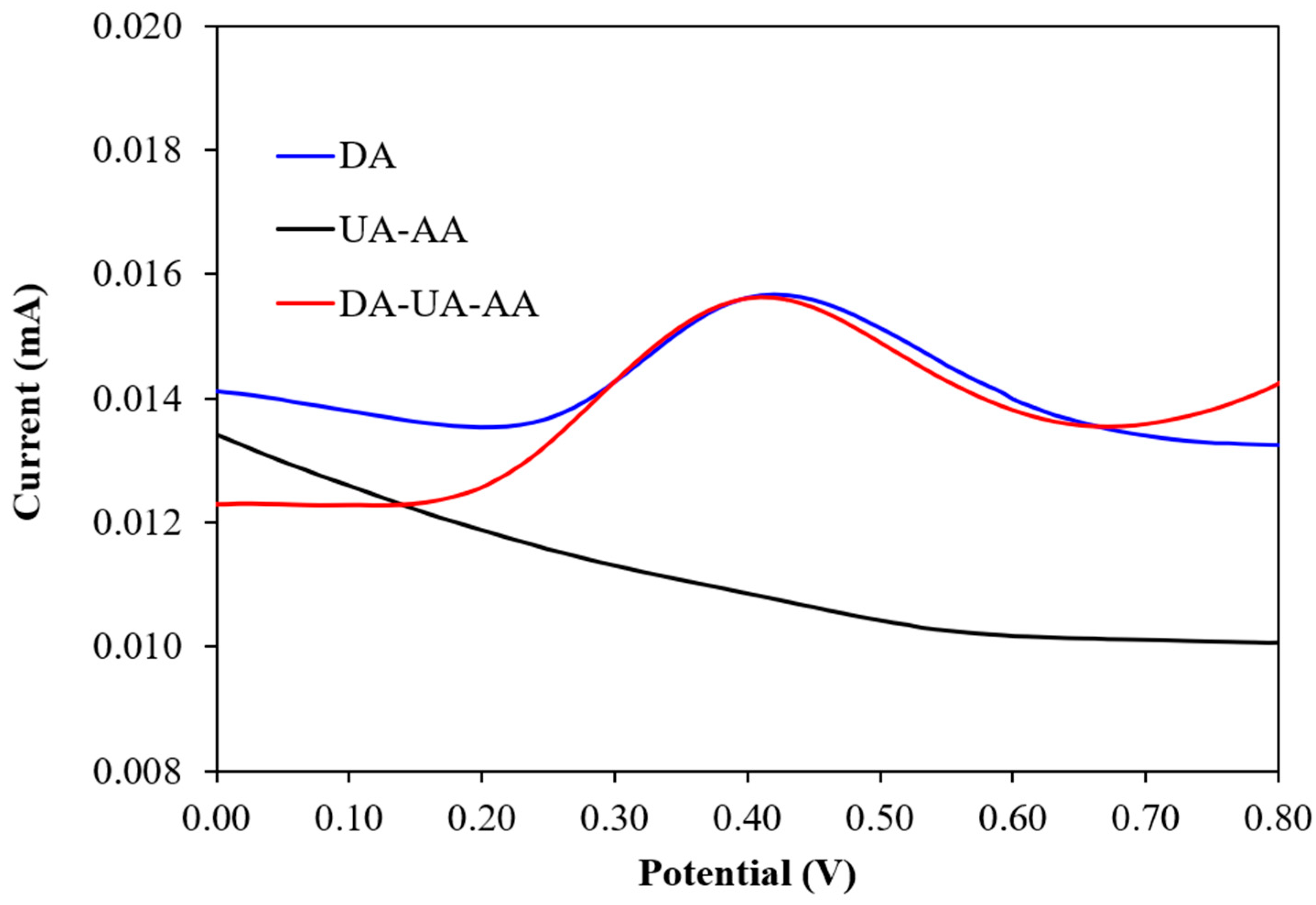
3.5. Determination of Selectivity, Stability, and Reproducibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, M. A dopamine electrochemical sensor based on gold nanoparticles/over-oxidized polypyrrole nanotube composite arrays. RSC Adv. 2015, 5, 9848–9851. [Google Scholar] [CrossRef]
- Qian, T.; Yu, C.; Zhou, X.; Ma, P.; Wu, S.; Xu, L.; Shen, J. Ultrasensitive dopamine sensor based on novel molecularly imprinted polypyrrole coated carbon nanotubes. Biosens. Bioelectron. 2014, 58, 237–241. [Google Scholar] [CrossRef]
- Panapimonlawat, T.; Phanichphant, S.; Sriwichai, S. Electrochemical Dopamine Biosensor Based on Poly(3-aminobenzylamine) Layer-by-Layer Self-Assembled Multilayer Thin Film. Polymers 2021, 13, 1488. [Google Scholar] [CrossRef] [PubMed]
- Anbumannan, V.; Kumar, R.T.R.; Suresh, K. Enhanced electrochemical detection of dopamine by graphene oxide/tungsten trioxide nanocomposite. Mater. Sci. Semicond. Process. 2021, 127, 105696. [Google Scholar] [CrossRef]
- Özcan, A.; İlkbaş, S.; Atılır Özcan, A. Development of a disposable and low-cost electrochemical sensor for dopamine detection based on poly(pyrrole-3-carboxylic acid)-modified electrochemically over-oxidized pencil graphite electrode. Talanta 2017, 165, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Priyanto, S.A.N.; Yulianti, E.S.; Zakiyuddin, A.; Rahman, S.F. Amperometric Biosensor Detecting Dopamine Based on Polypyrrole/Reduced Graphene Oxide/Nickel Oxide/Glassy Carbon Electrode. J. Electr. Comput. Eng. 2024, 2024, 7453474. [Google Scholar] [CrossRef]
- Kaewda, C.; Sriwichai, S. Label-Free Electrochemical Dopamine Biosensor Based on Electrospun Nanofibers of Polyaniline/Carbon Nanotube Composites. Biosensors 2024, 14, 349. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Wei, X. A sensitive and selective sensor for dopamine determination based on a molecularly imprinted electropolymer of o-aminophenol. Sens. Actuators B Chem. 2009, 140, 663–669. [Google Scholar] [CrossRef]
- Matt, S.M.; Gaskill, P.J. Where Is Dopamine and how do Immune Cells See it?: Dopamine-Mediated Immune Cell Function in Health and Disease. J. Neuroimmune Pharmacol. 2020, 15, 114–164. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and sensors for dopamine detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Mahmoudian, M.R.; Basirun, W.J.; Binti Alias, Y. Sensitive Dopamine Biosensor Based on Polypyrrole-Coated Palladium Silver Nanospherical Composites. Ind. Eng. Chem. Res. 2016, 55, 6943–6951. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, Z.; Ye, F.; Zhao, S. Rapid and sensitive colorimetric detection of dopamine based on the enhanced-oxidase mimicking activity of cerium(iv). N. J. Chem. 2021, 45, 6780–6786. [Google Scholar] [CrossRef]
- Gu, H.; Varner, E.L.; Groskreutz, S.R.; Michael, A.C.; Weber, S.G. In Vivo Monitoring of Dopamine by Microdialysis with 1 min Temporal Resolution Using Online Capillary Liquid Chromatography with Electrochemical Detection. Anal. Chem. 2015, 87, 6088–6094. [Google Scholar] [CrossRef]
- Ramadan, M.A.A.; Almasri, I.; Khayal, G. Spectrophotometric Determination of Dopamine in Bulk and Dosage Forms Using 2,4-Dinitrophenylhydrazine. Turk. J. Pharm. Sci. 2020, 17, 679–685. [Google Scholar] [CrossRef]
- Moghzi, F.; Soleimannejad, J.; Sañudo, E.C.; Janczak, J. Dopamine Sensing Based on Ultrathin Fluorescent Metal–Organic Nanosheets. ACS Appl. Mater. Interfaces 2020, 12, 44499–44507. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wen, Y.; Qiao, Y.; Shen, K.Z.; Yuan, H. A Dopamine Detection Sensor Based on Au-Decorated NiS2 and Its Medical Application. Molecules 2024, 29, 2925. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.S.; Basirun, W.J.; Shalauddin, M.; Akhter, S. A dopamine electrochemical sensor based on a platinum–silver graphene nanocomposite modified electrode. RSC Adv. 2020, 10, 17336–17344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.; Yu, Y.; Chen, J.; Xu, W. Electrochemical Dopamine Biosensor Using Over-Oxidized Polypyrrole with Assistance of Graphene. In Proceedings of the 2016 13th IEEE International Conference on Solid-State and Integrated Circuit Technology (ICSICT), Hangzhou, China, 25–28 October 2016; pp. 671–673. [Google Scholar]
- Zablocka, I.; Wysocka-Zolopa, M.; Winkler, K. Electrochemical Detection of Dopamine at a Gold Electrode Modified with a Polypyrrole–Mesoporous Silica Molecular Sieves (MCM-48) Film. Int. J. Mol. Sci. 2019, 20, 111. [Google Scholar] [CrossRef]
- Mani, V.; Devasenathipathy, R.; Chen, S.-M.; Kohilarani, K.; Ramachandran, R. A Sensitive Amperometric Sensor for the Determination of Dopamine at Graphene and Bismuth Nanocomposite Film Modified Electrode. Int. J. Electrochem. Sci. 2015, 10, 1199–1207. [Google Scholar] [CrossRef]
- Demirkan, B.; Bozkurt, S.; Cellat, K.; Arıkan, K.; Yılmaz, M.; Şavk, A.; Çalımlı, M.H.; Nas, M.S.; Atalar, M.N.; Alma, M.H.; et al. Palladium supported on polypyrrole/reduced graphene oxidenanoparticles for simultaneous biosensing application of ascorbic acid, dopamine, anduric acid. Sci. Rep. 2020, 10, 2946. [Google Scholar] [CrossRef]
- Alahmadi, N.; El-Said, W.A. Electrochemical Sensing of Dopamine Using Polypyrrole/Molybdenum Oxide Bilayer-Modified ITO Electrode. Biosensors 2023, 13, 578. [Google Scholar] [CrossRef]
- Ghadimi, H.; Mahmoudian, M.R.; Basirun, W.J. A sensitive dopamine biosensor based on ultra-thin polypyrrole nanosheets decorated with Pt nanoparticles. RSC Adv. 2015, 5, 39366–39374. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Wang, P.; Qin, J. Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer. Nanomaterials 2023, 13, 674. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Chang, Y.H.; Woi, P.M.; Alias, Y. The selective electrochemical detection of dopamine in the presence of ascorbic acid and uric acid using electro-polymerised-β-cyclodextrin incorporated f-MWCNTs/polyaniline modified glassy carbon electrode. Microchem. J. 2019, 148, 322–330. [Google Scholar] [CrossRef]
- Granero, A.M.; Pierini, G.D.; Robledo, S.N.; Di Nezio, M.S.; Fernández, H.; Zon, M.A. Simultaneous determination of ascorbic and uric acids and dopamine in human serum samples using three-way calibration with data from square wave voltammetry. Microchem. J. 2016, 129, 205–212. [Google Scholar] [CrossRef]
- Khan, R.; Anjum, S.; Fatima, N.; Farooq, N.; Shaheen, A.; Fernandez Garcia, J.; Khan, M.I.; Shanableh, A. Development of Electrochemical and Colorimetric Biosensors for Detection of Dopamine. Chemosensors 2024, 12, 126. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef]
- Iordănescu, A.; Tertis, M.; Cernat, A.; Suciu, M.; Săndulescu, R.; Cristea, C. Poly-(pyrrole-3-carboxylic acid) Based Nanostructured Platform for the Detection of Carcinoembryonic Antigen. Electroanalysis 2018, 30, 1100–1106. [Google Scholar] [CrossRef]
- Thunyakontirakun, W.; Sriwichai, S.; Phanichphant, S.; Janmanee, R. Fabrication of poly(pyrrole-3-carboxylic acid)/graphene oxide composite thin film for glucose biosensor. Mater. Today Proc. 2019, 17, 2070–2077. [Google Scholar] [CrossRef]
- Gupta, S.A.; Singh, J. A Study of conducting electrochemical sensors based on molecularly imprinted polymer on carbon nanostructure using polypyrrole film: A review. J. Sci. Res. 2021, 65, 110–115. [Google Scholar] [CrossRef]
- Sriwichai, S.; Netsuwan, P.; Phanichphant, S.; Baba, A. Electropolymerization and properties of poly (3-anilinethiophene) thin film. Chiang Mai J. Sci. 2016, 43, 863–869. [Google Scholar]
- Luceño-Sánchez, J.A.; Díez-Pascual, A.M. Grafting of Polypyrrole-3-carboxylic Acid to the Surface of Hexamethylene Diisocyanate-Functionalized Graphene Oxide. Nanomater. 2019, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, A.; Sethuraman, V.; Sasikumar, R. Non-enzymatic electrochemical detection of creatinine based on a glassy carbon electrode modified with a Pd/Cu2O decorated polypyrrole (PPy) nanocomposite: An analytical approach. Anal. Methods 2023, 15, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Padilla, A.; García-Guzmán, J.J.; López-Iglesias, D.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. E-tongues/noses based on conducting polymers and composite materials: Expanding the possibilities in complex analytical sensing. Sensors 2021, 21, 4976. [Google Scholar] [CrossRef]
- Janmanee, R.; Pinwattana, K. Electrochemical Fabrication of Polypyrrole/Poly(pyrrole-3-carboxylic acid)/Graphene Oxide Composite Thin Film for Biosensor Application. NU. Int. J. Sci. 2015, 1, 52–61. [Google Scholar]
- Kannan, A.; Radhakrishnan, S. Fabrication of an electrochemical sensor based on gold nanoparticles functionalized polypyrrole nanotubes for the highly sensitive detection of l-dopa. Mater. Today Commun. 2020, 25, 101330. [Google Scholar] [CrossRef]
- Kawakami, H.; Ito, Y.; Chien, Y.-A.; Chen, C.-Y.; Chiu, W.-T.; Chakraborty, P.; Nakamoto, T.; Sone, M.; Chang, T.-F.M. Development of polypyrrole/nano-gold composite for non-enzymatic glucose sensors. Micro Nano Eng. 2022, 14, 100109. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef]
- Esmaeili, C.; Heng, L.Y.; Chiang, C.P.; Rashid, Z.A.; Safitri, E.; Malon Marugan, R.S.P. A DNA biosensor based on kappa-carrageenan-polypyrrole-gold nanoparticles composite for gender determination of Arowana fish (Scleropages formosus). Sens. Actuators B Chem. 2017, 242, 616–624. [Google Scholar] [CrossRef]
- Nowicka, A.M.; Fau, M.; Rapecki, T.; Donten, M. Polypyrrole-Au Nanoparticles Composite as Suitable Platform for DNA Biosensor with Electrochemical Impedance Spectroscopy Detection. Electrochim. Acta 2014, 140, 65–71. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P.; Paulmony, T. Evaluation of a New Biosensor Based on in Situ Synthesized PPy-Ag-PVP Nanohybrid for Selective Detection of Dopamine. J. Phys. Chem. B 2017, 121, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Malode, S.J.; Alodhayb, A.N.; Mondal, K.; Shetti, N.P. Conducting polymer-based electrochemical sensors: Progress, challenges, and future perspectives. Talanta Open 2025, 11, 100395. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Milikić, J.; Tapia, A.; Stamenović, U.; Vodnik, V.; Otoničar, M.; Škapin, S.; Santos, D.M.F.; Šljukić, B. High-performance metal (Au,Cu)–polypyrrole nanocomposites for electrochemical borohydride oxidation in fuel cell applications. Int. J. Hydrogen Energy 2022, 47, 36990–37001. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, T.; Ye, Z.; Feng, Y.; Chen, Z.; Wang, Y.; Sun, Y.; Wu, H.; Yang, Z.; Wang, Y.; et al. A One-Step Electropolymerized Biomimetic Polypyrrole Membrane-Based Electrochemical Sensor for Selective Detection of Valproate. Front. Bioeng. Biotechnol. 2022, 10, 851692. [Google Scholar] [CrossRef]
- Rozi, N.; Hanifah, S.A.; Zaid, M.H.M.; Abd Karim, N.H.; Ikeda, M. Feasible study on poly(Pyrrole-co-Pyrrole-3-Carboxylic Acid)-modified electrode for detection of 17β-Estradiol. Chem. Pap. 2021, 75, 3493–3503. [Google Scholar] [CrossRef]
- Sowmiya, G.; Velraj, G. Design of hollow nanosphere structured polypyrrole/Sn and SnO2 nanoparticles by COP approach for enhanced electron transport behavior. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5217–5223. [Google Scholar] [CrossRef]
- Husain, A.; Mahajan, D.K. Effect of multi-walled carbon nanotubes on DC electrical conductivity and acetone vapour sensing properties of polypyrrole. Carbon Trends 2022, 9, 100193. [Google Scholar] [CrossRef]
- Bachhav, S.; Patil, D. Study of ethanol vapour sensing behaviour by polypyrrole-multiwall carbon nanotubes nanocomposites. J. Phys. Sci. 2018, 29, 137–152. [Google Scholar] [CrossRef]
- Tang, X.; Raskin, J.-P.; Kryvutsa, N.; Hermans, S.; Slobodian, O.; Nazarov, A.N.; Debliquy, M. An ammonia sensor composed of polypyrrole synthesized on reduced graphene oxide by electropolymerization. Sens. Actuators B Chem. 2020, 305, 127423. [Google Scholar] [CrossRef]
- Rashed, M.A.; Faisal, M.; Alsaiari, M.; Alsareii, S.A.; Harraz, F.A. MWCNT-doped polypyrrole-carbon black modified glassy carbon electrode for efficient electrochemical sensing of nitrite ions. Electrocatalysis 2021, 12, 650–666. [Google Scholar] [CrossRef]
- Sun, J.; Shu, X.; Tian, Y.; Tong, Z.; Bai, S.; Luo, R.; Li, D.; Liu, C.C. Facile preparation of polypyrrole-reduced graphene oxide hybrid for enhancing NH3 sensing at room temperature. Sens. Actuators B Chem. 2017, 241, 658–664. [Google Scholar] [CrossRef]
- Supakiet, C.; Chammari, P.; Nawee, K.; Kontad, O. A Poly(pyrrole-3-carboxylic acid) Thin Film Modified Screen Printed Carbon Electrode as Highly Sensitive and Selective Label-free Electrochemical Immunosensing Platform. Chiang Mai J. Sci. 2020, 47, 530–541. [Google Scholar]
- Choo, S.-S.; Kang, E.-S.; Song, I.; Lee, D.; Choi, J.-W.; Kim, T.-H. Electrochemical Detection of Dopamine Using 3D Porous Graphene Oxide/Gold Nanoparticle Composites. Sensors 2017, 17, 861. [Google Scholar] [CrossRef]
- Keene, S.T.; Lubrano, C.; Kazemzadeh, S.; Melianas, A.; Tuchman, Y.; Polino, G.; Scognamiglio, P.; Cinà, L.; Salleo, A.; van de Burgt, Y.; et al. A biohybrid synapse with neurotransmitter-mediated plasticity. Nat. Mater. 2020, 19, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Koyun, O.; Gursu, H.; Gorduk, S.; Sahin, Y. Highly Sensitive Electrochemical Determination of Dopamine with an Overoxidized Polypyrrole Nanofiber Pencil Graphite Electrode. Int. J. Electrochem. Sci. 2017, 12, 6428–6444. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajheidari, N. ZnO-CuxO/polypyrrole nanocomposite modified electrode for simultaneous determination of ascorbic acid, dopamine, and uric acid. Anal. Biochem. 2015, 473, 53–62. [Google Scholar] [CrossRef]






| Modified Electrode | Method | Linear Range (μM) | LOD (μM) | Sensitivity | Ref. |
|---|---|---|---|---|---|
| PPy–MCM-48 | SWV | 2–250 | 0.70 | 0.0058 (μA μM−1) | [19] |
| PPy/rGO/NiO/GCE | CV | 10–1000 | 0.195 | 25.89 (A mM−1 cm−2) | [6] |
| OONfPPy/PGE | DPV | 1–1000 | 0.00695 | - | [60] |
| ZnO-CuxO-PPy/GCE | DPV | 0.1–130 | 0.04 | 0.28 (μA μM−1) | [61] |
| PP3C/EOPGE | AdSDPV | 0.01–7.5 | 0.0025 | 17.23 (μA μM−1) | [5] |
| PP3C/PPy/AuNPs | DPV | 5–180 | 0.00972 | 2 (μA μM−1 cm−2) | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janmanee, R.; Sriwichai, S. Development of an Electrochemical Biosensor Based on Polypyrrole-3-carboxylic Acid/Polypyrrole/Au Nanoparticle Composites for Detection of Dopamine. Polymers 2025, 17, 754. https://doi.org/10.3390/polym17060754
Janmanee R, Sriwichai S. Development of an Electrochemical Biosensor Based on Polypyrrole-3-carboxylic Acid/Polypyrrole/Au Nanoparticle Composites for Detection of Dopamine. Polymers. 2025; 17(6):754. https://doi.org/10.3390/polym17060754
Chicago/Turabian StyleJanmanee, Rapiphun, and Saengrawee Sriwichai. 2025. "Development of an Electrochemical Biosensor Based on Polypyrrole-3-carboxylic Acid/Polypyrrole/Au Nanoparticle Composites for Detection of Dopamine" Polymers 17, no. 6: 754. https://doi.org/10.3390/polym17060754
APA StyleJanmanee, R., & Sriwichai, S. (2025). Development of an Electrochemical Biosensor Based on Polypyrrole-3-carboxylic Acid/Polypyrrole/Au Nanoparticle Composites for Detection of Dopamine. Polymers, 17(6), 754. https://doi.org/10.3390/polym17060754






