Study on the Dispersion and Processing Performance of Activated Aluminum Hydroxide/Ammonium Polyphosphate Composite Flame Retardant System for Vinyl Ester Resin
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
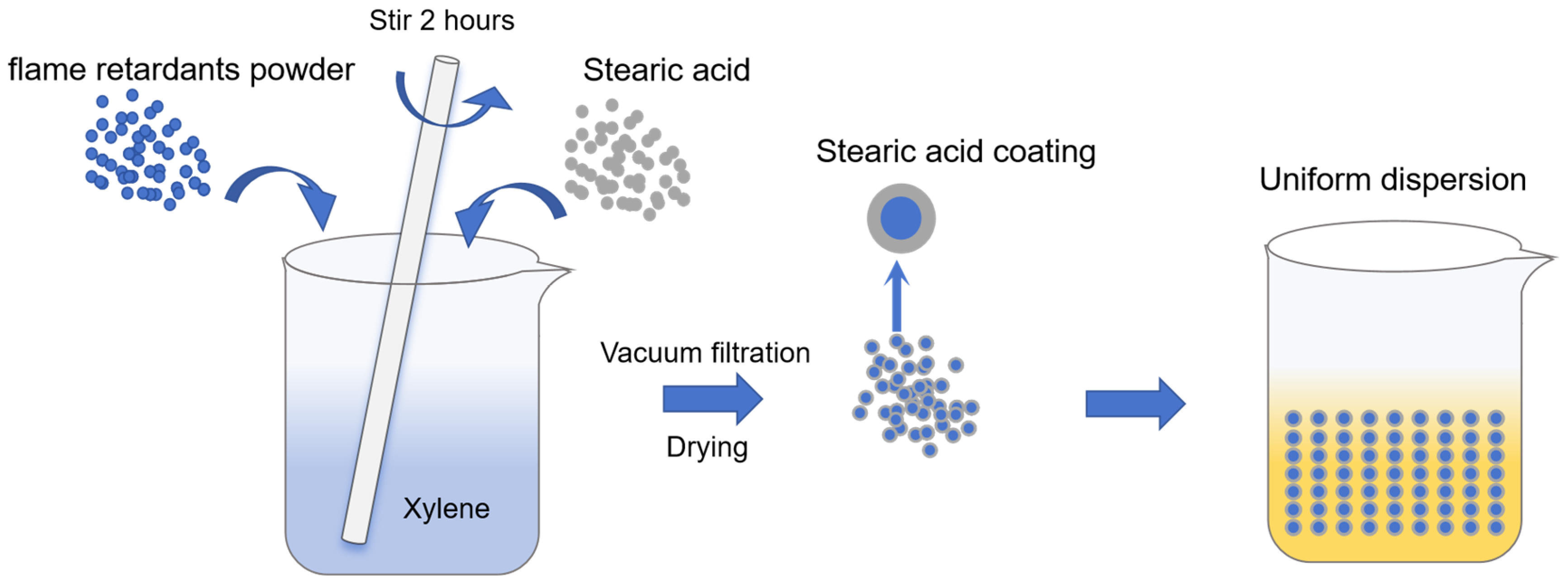
2.2. Surface Treatment of Flame-Retardant Powders
2.3. Preparation of Flame Retardant Vinyl Ester Resin
2.4. Measurement of Activation Degree
2.5. Flame-Retardancy Testing
2.6. Other Tests and Characterization
3. Results and Discussion
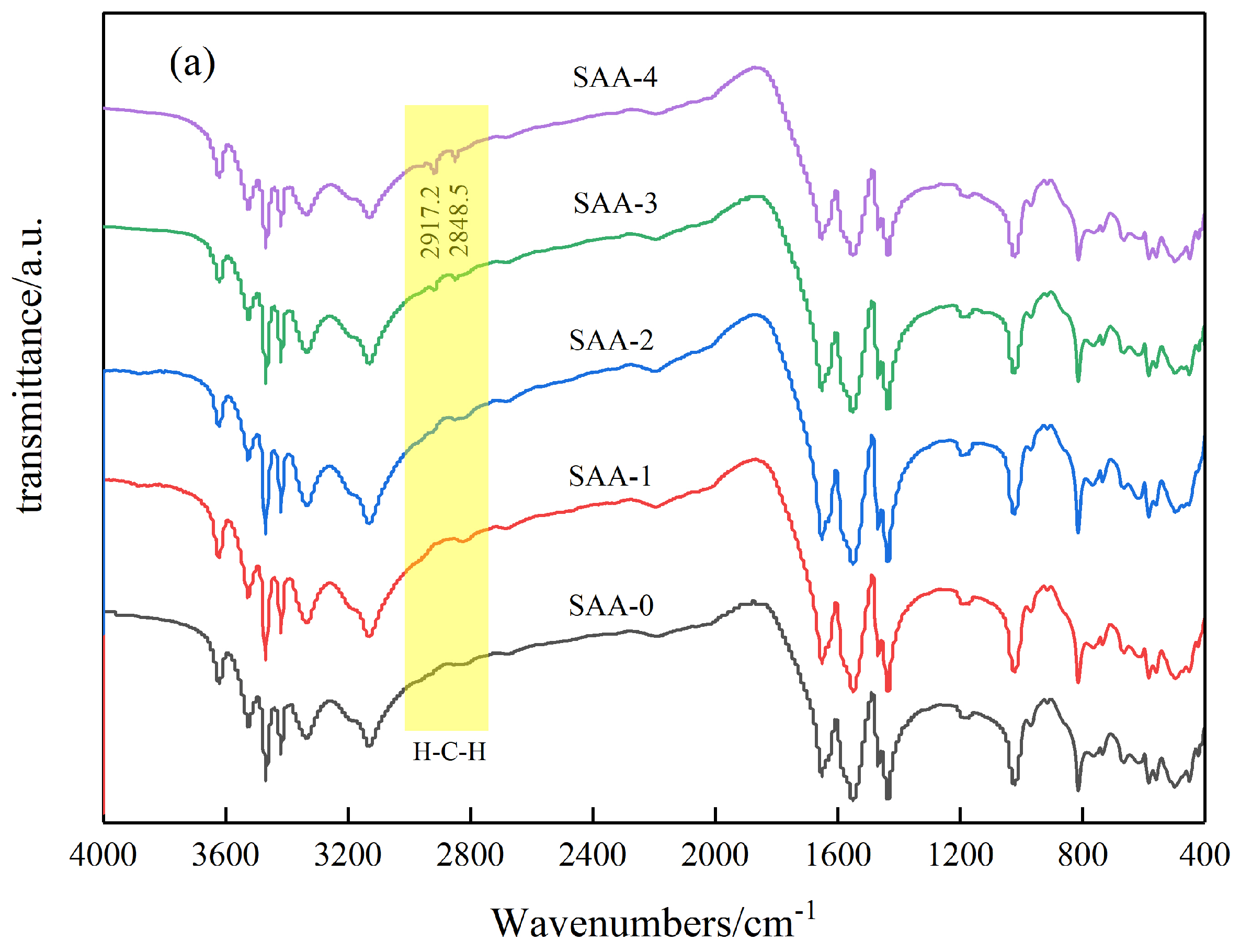
3.1. Surface Modification of Inorganic Powders by Stearic Acid
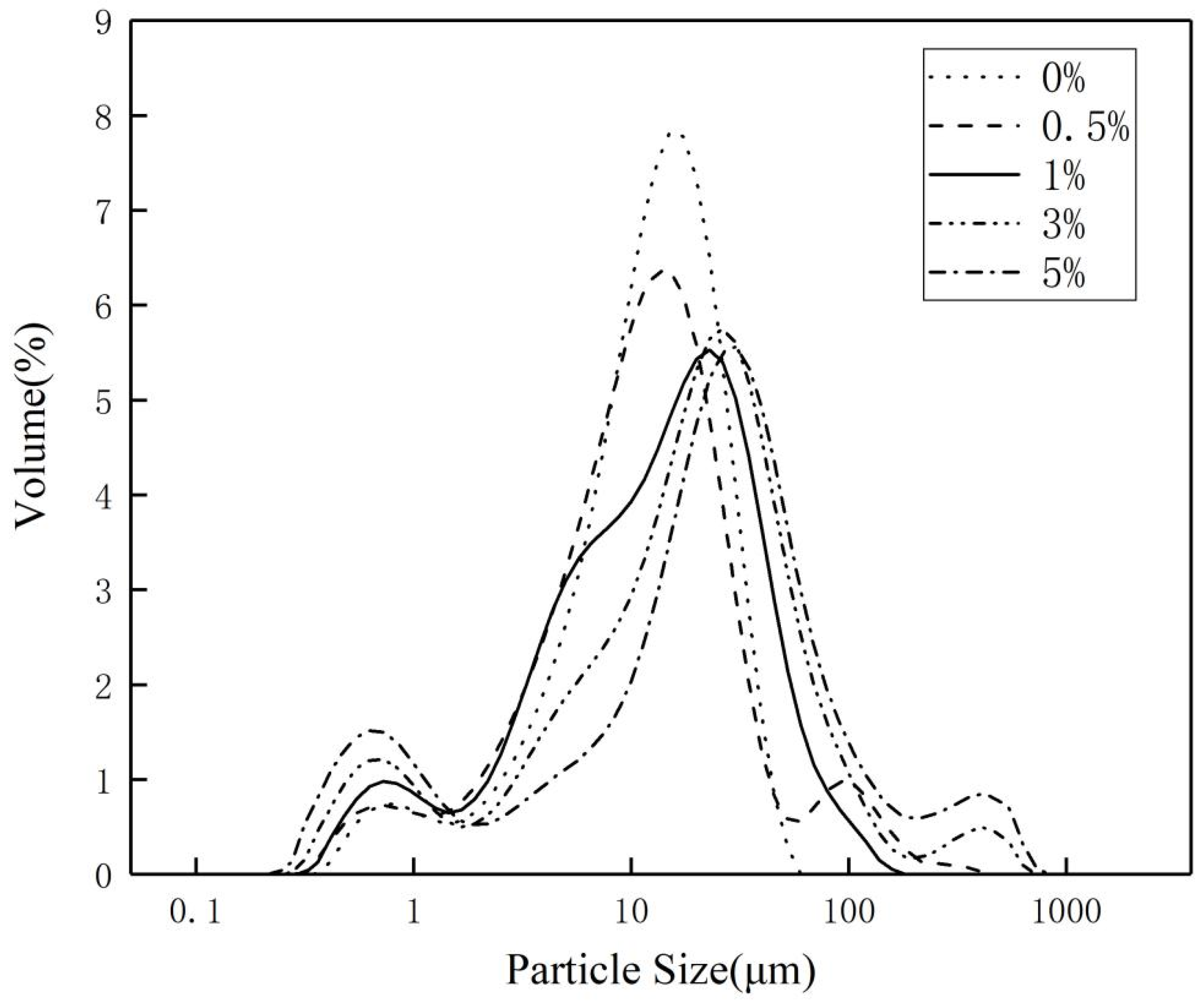
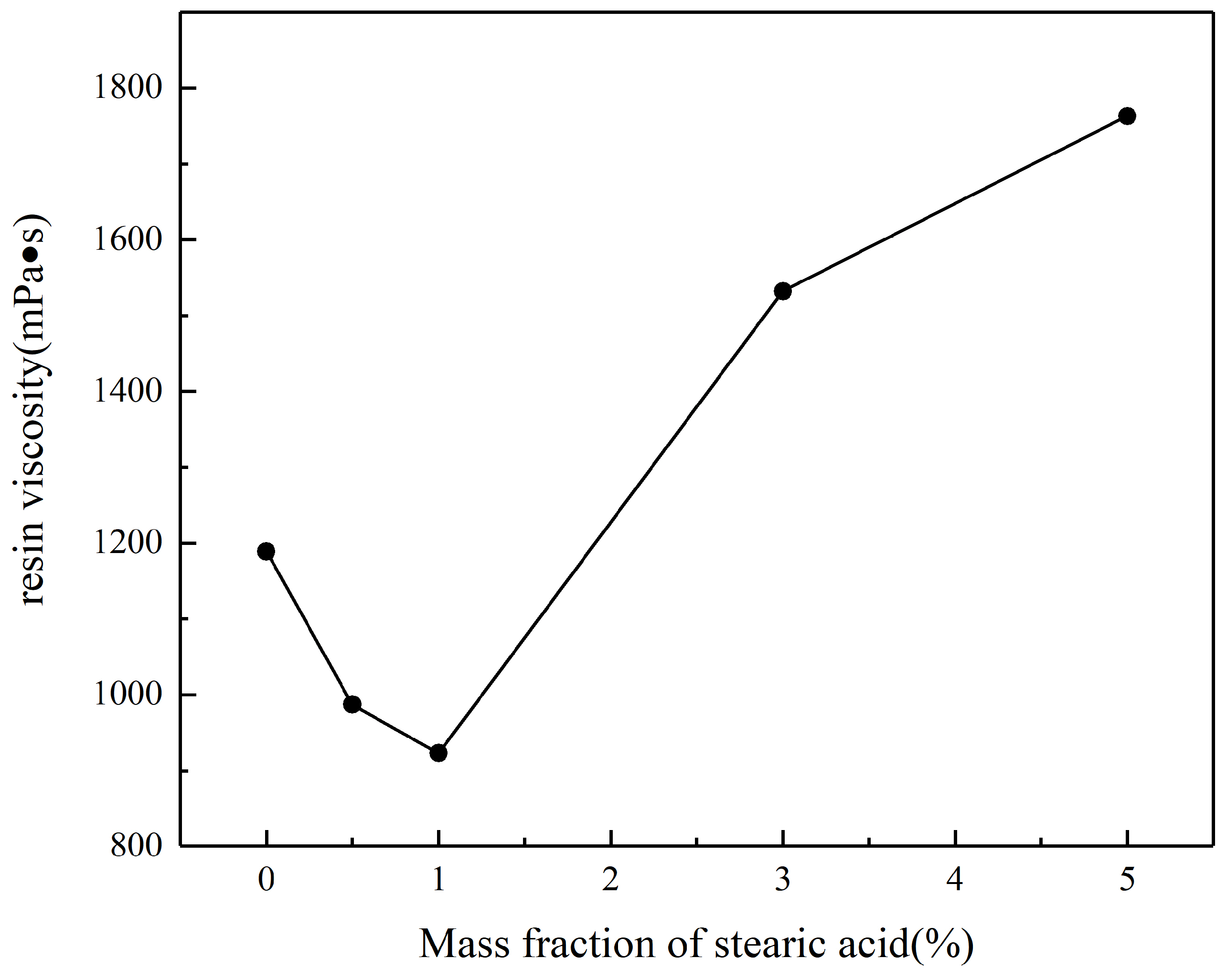
3.2. Effect of Surface-Modified Inorganic Flame-Retardant Powders on the Properties of Vinyl Ester Resin
4. Conclusions
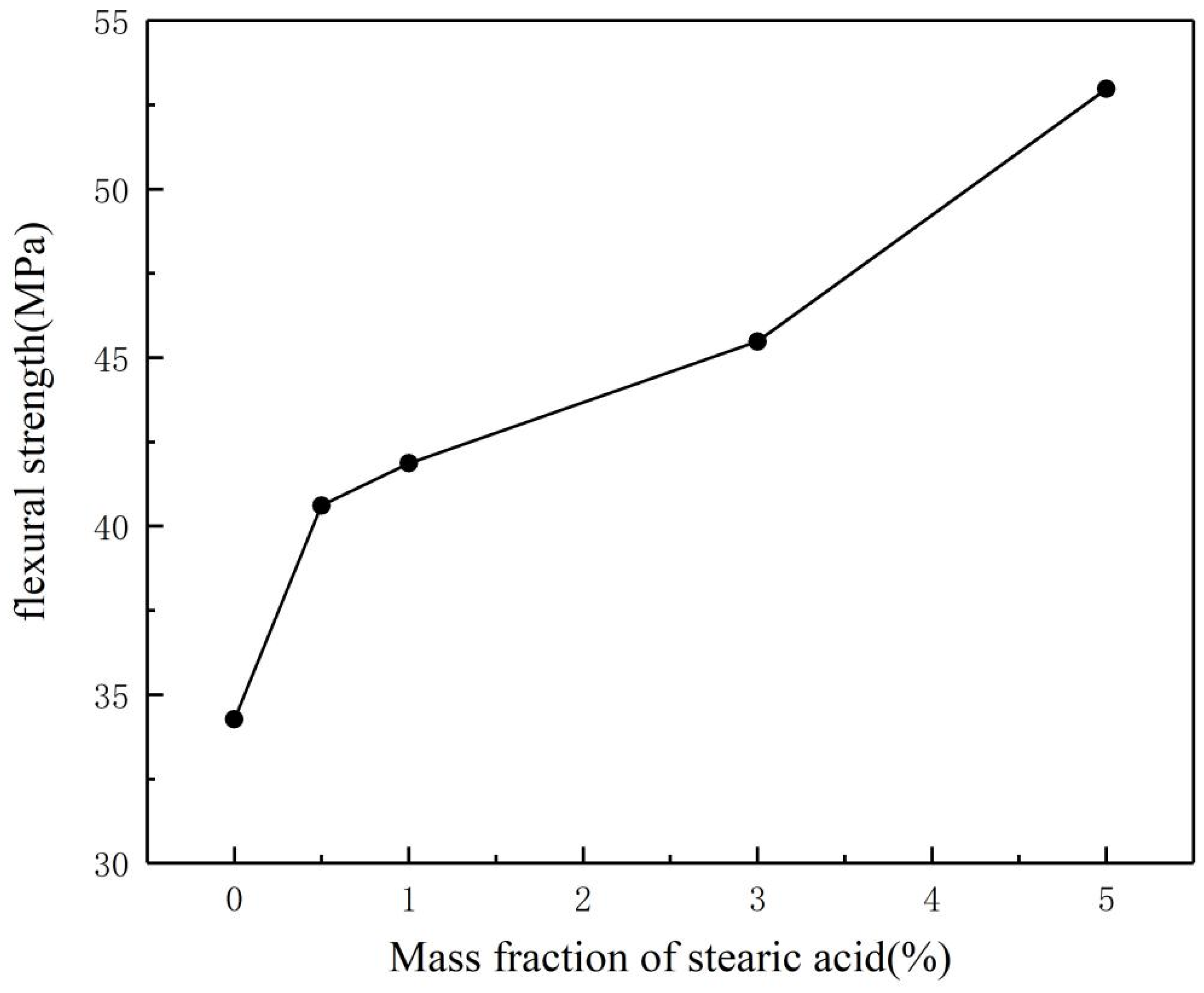
- Optimal stearic acid dosage: A stearic acid content of 1 wt % achieves the best balance between particle dispersion and processing performance. The modified powder (SAA-1) exhibits uniform particle size distribution (d(0.5) = 13.09 µm), high activation degree (73.6%), and minimal resin viscosity (923 mPa·s), while maintaining a bending strength of 41.86 MPa.
- Trade-off mechanism: Excessive SA (>1 wt %) induces particle agglomeration (d(0.5) up to 724.44 µm for SAA-4), leading to deteriorated flame retardancy (LOI decreased from 24.6% to 23.6%), despite improved mechanical strength (52.97 MPa for VER-4).
- Industrial relevance: The proposed surface modification method demonstrates scalability potential. This study provides a cost-effective strategy for optimizing halogen-free flame-retardant composites, suitable for applications requiring both mechanical robustness and processing efficiency.
- Industrial application potential: The proposed surface modification method demonstrates scalability potential. This modification method requires only one step, is easy to operate, and offers simplicity and effectiveness for large-scale production. This study provides an economical and efficient strategy for optimizing halogen-free flame-retardant composites.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J. Vinyl Ester Resins and Their Applications; Chemical Industry Press: Beijing, China, 2014. [Google Scholar]
- Zhang, W.; Qin, Z.; Lan, Y.; Zhang, X.; Zhang, W.; Pan, Y.; Yang, R. Flame retardant composites of ladder phenyl/vinyl polysilsesquioxane-reinforced vinyl ester. J. Mater. Sci. 2021, 56, 457–473. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Zhang, S.; Zhou, H. Study on the Flame Retardant and Thermal Properties of POSS modified vinyl resin containing phosphorus and nitrogen. Chemistry 2021, 84, 1066–1073. [Google Scholar] [CrossRef]
- Cai, Y. Handbook of Modern Flame Retardant Technology; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Cai, Y.; Liu, J. Flame Retardant Technical Manual for Polymer Materials; Chemical Industry Press: Beijing, China, 1993. [Google Scholar]
- Miao, J.; Fang, Y.; Yang, X.; Zhu, Y.; Hu, A.; Wang, G. Fabrication, flame retardancy and physical properties of phosphorus containing porous organic polymers/epoxy resin composites. Polym. Degrad. Stab. 2020, 176, 109159. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Zeng, G.; Du, J.; Zhang, W.; Yang, R. The Effect of Different Smoke Suppressants with APP for Enhancing the Flame Retardancy and Smoke Suppression on Vinyl Ester Resin. Polym. Eng. Sci. 2020, 60, 314–322. [Google Scholar] [CrossRef]
- Rishubh, G.; Manoj, K.; Sanjay, M.; Suchart, S.; Hom, N.; Sunny, Z. Recent progress in additive inorganic flame retardants polymer composites: Degradation mechanisms, modeling and applications. Heliyon 2024, 10, e39662. [Google Scholar] [CrossRef]
- Levchik, S.V. A Review of Recent Progress in Phosphorus-based Flame Retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Mochane, M.J.; Mokhothu, T.H.; Mokhena, T.C. Synthesis, mechanical, and flammability properties of metal hydroxide reinforced polymer composites: A review. Polym. Eng. Sci. 2022, 62, 44–65. [Google Scholar] [CrossRef]
- Qiu, T.; Lu, Z.; Li, J.; Niu, Y. Effect of Particle Size and Dosage of Aluminum Hydroxide on Performance of Unsaturated Polyester Resin. China Powder Sci. Technol. 2014, 20, 6–12. [Google Scholar] [CrossRef]
- Lin, Y.; Fu, T.; Guo, D.; Wang, Y. Research on the flame retardant performance and mechanism of vinyl ester resin modified by ammonium polyphosphate. J. Sichuan Univ. 2023, 60, 190–195. [Google Scholar] [CrossRef]
- Zhang, S. Ultrafine Powder Processing Technology and Application, 3rd ed.; Chemical Industry Press: Beijing, China, 2021. [Google Scholar]
- Su, S.; Yi, J.; Zhang, X. Experimental Study on Surface Modification of Aluminum Hydroxide Powder. Met. World 2024, 3, 20–24. [Google Scholar] [CrossRef]
- Wu, D.; Guo, J. Preparation and organic coating of ultra-fine ammonium polyphosphate. New Chem. Mater. 2008, 92, 84–85. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Wen, S.; Chen, J.; Gu, F.; Wu, J.; Meng, Y. Research Progress of Modifications and Applications of Ammonium Polyphosphate Flame Retardants. China Plast. Ind. 2024, 52, 26–35. [Google Scholar] [CrossRef]
- Fang, H.; Li, G.; Wang, K.; Wu, F. Significant Improvement of Thermal Conductivity of Polyamide 6/Boron Nitride Composites by Adding a Small Amount of Stearic Acid. Polymers 2023, 15, 1887. [Google Scholar] [CrossRef] [PubMed]
- Yassene, A.A.M.; Awad, E.H.; Hegazy, A.A. The effect of gamma rays and stearic acid on calcium carbonate and its impact on the properties of epoxy-based composites. Radiochim. Acta 2024, 112, 339–350. [Google Scholar] [CrossRef]
- Kow, K.W.; Abdullah, E.C.; Aziz, A.R. Effects of ultrasound in coating nano-precipitated CaCO3 with stearic acid. Asia-Pac. J. Chem. Eng. 2009, 4, 807–813. [Google Scholar] [CrossRef]
- Huang, H.; Tian, M.; Yang, J.; Li, H.; Liang, W.; Zhang, L.; Li, X. Stearic acid surface modifying Mg(OH)2: Mechanism and its effect on properties of ethylene vinyl acetate/Mg(OH)2 composites. J. Appl. Polym. Sci. 2008, 107, 3325–3331. [Google Scholar] [CrossRef]
- Ma, M.; Wu, Z.; Hu, Y.; Han, X. Study on Surface Modification of Nano-meter Calcium Carbonate With Stearic Acid. Contemp. Chem. Ind. 2024, 53, 582–586. [Google Scholar] [CrossRef]
- Liu, L.; Luan, Z.; Chen, M.; Zhang, J.; An, X. Surface Modification of Aluminum Hydroxide as Flame Retardant and Its Application in Soft PVC. J. Cap. Norm. Univ. 2019, 40, 37–41. [Google Scholar] [CrossRef]
- GB/T 2406-1993; Plastics Determination of Fammability by Oxygen Index. Beijing FRP Research Institute: Beijing, China, 1993.
- GB/T 2408-1996; Plastics—Determination of Burning Characteristics—Horizontal and Vertical Test. Beijing FRP Research Institute: Beijing, China, 1996.
- GB/T 1449-2005; Fibre-Reinforced Plastic Composites-Determination of Flexural Properties. Beijing FRP Research Institute: Beijing, China, 2005.
- Zhen, B.; Xie, X.; Tang, Q.; Li, C.; Zhu, W.; Wang, M.; Guo, H.; Wang, L. Effect of Kaolinite Particle Size on Stability of Pickering Emulsion. J. Synth. Cryst. 2019, 48, 705–711. [Google Scholar] [CrossRef]



| Samples | ATH/G | APP/G | Stearic Acid/G |
|---|---|---|---|
| SAA-0 | 40 | 60 | 0 |
| SAA-1 | 40 | 60 | 0.5 |
| SAA-2 | 40 | 60 | 1.0 |
| SAA-3 | 40 | 60 | 3.0 |
| SAA-4 | 40 | 60 | 5.0 |
| Samples | Resin/G | Flame-Retardant Powder Added |
|---|---|---|
| VER-0 | 120 | 50 g SAA-0 |
| VER-1 | 120 | 50 g SAA-1 |
| VER-2 | 120 | 50 g SAA-2 |
| VER-3 | 120 | 50 g SAA-3 |
| VER-4 | 120 | 50 g SAA-4 |
| Samples | d(0.5)/µm | d(0.9)/µm | Maximum Particle Size/µm |
|---|---|---|---|
| SAA-0 | 11.93 | 26.06 | 45.71 |
| SAA-1 | 12.49 | 38.14 | 416.87 |
| SAA-2 | 13.09 | 40.37 | 138.04 |
| SAA-3 | 18.33 | 61.42 | 549.54 |
| SAA-4 | 22.28 | 97.43 | 724.24 |
| Samples | d(0.9) of the Added Powder/µm | Maximum Particle Size/µm | Viscosity of the Resin/mPa·s |
|---|---|---|---|
| VER-0 | 26.06 | 45.71 | 1189 |
| VER-1 | 38.14 | 416.87 | 987 |
| VER-2 | 40.37 | 138.04 | 923 |
| VER-3 | 61.42 | 549.54 | 1532 |
| VER-4 | 97.43 | 724.24 | 1763 |
| Samples | LOI | Vertical Fire Test |
|---|---|---|
| VER-0 | 24.6 | FV-1 |
| VER-1 | 24.3 | FV-1 |
| VER-2 | 24.0 | FV-1 |
| VER-3 | 23.8 | FV-2 |
| VER-4 | 23.6 | FV-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, J.; Xie, Y.; Chen, R.; Qin, Y. Study on the Dispersion and Processing Performance of Activated Aluminum Hydroxide/Ammonium Polyphosphate Composite Flame Retardant System for Vinyl Ester Resin. Polymers 2025, 17, 667. https://doi.org/10.3390/polym17050667
Dou J, Xie Y, Chen R, Qin Y. Study on the Dispersion and Processing Performance of Activated Aluminum Hydroxide/Ammonium Polyphosphate Composite Flame Retardant System for Vinyl Ester Resin. Polymers. 2025; 17(5):667. https://doi.org/10.3390/polym17050667
Chicago/Turabian StyleDou, Jipeng, Yong Xie, Rui Chen, and Yan Qin. 2025. "Study on the Dispersion and Processing Performance of Activated Aluminum Hydroxide/Ammonium Polyphosphate Composite Flame Retardant System for Vinyl Ester Resin" Polymers 17, no. 5: 667. https://doi.org/10.3390/polym17050667
APA StyleDou, J., Xie, Y., Chen, R., & Qin, Y. (2025). Study on the Dispersion and Processing Performance of Activated Aluminum Hydroxide/Ammonium Polyphosphate Composite Flame Retardant System for Vinyl Ester Resin. Polymers, 17(5), 667. https://doi.org/10.3390/polym17050667




