Enhancing Radiation Shielding Efficiency of Nigella sativa Eumelanin Polymer Through Heavy Metals Doping
Abstract
1. Introduction
2. Materials and Methods
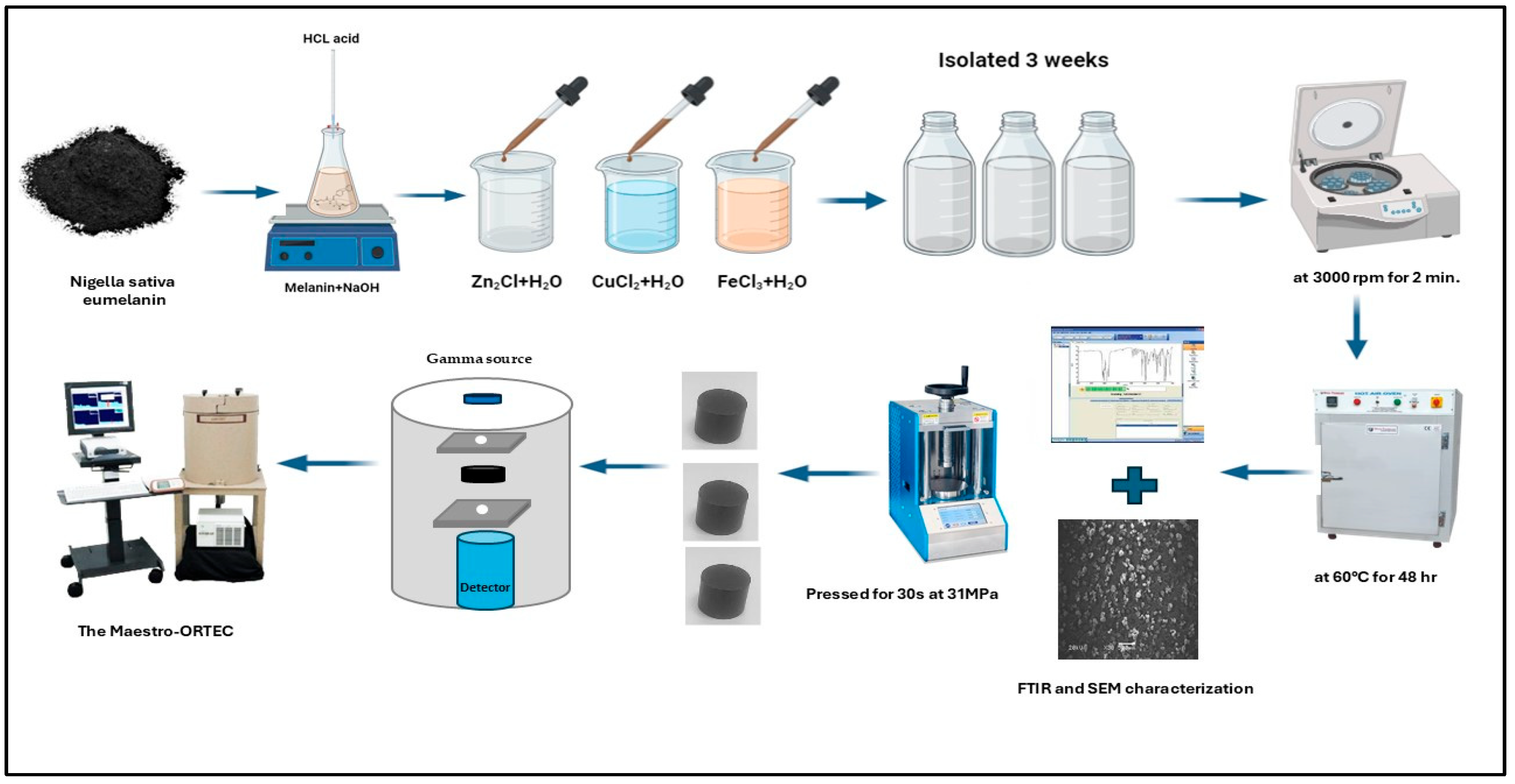
2.1. Sample Preparation
2.2. Evaluations of Gamma-Ray Shielding Parameters
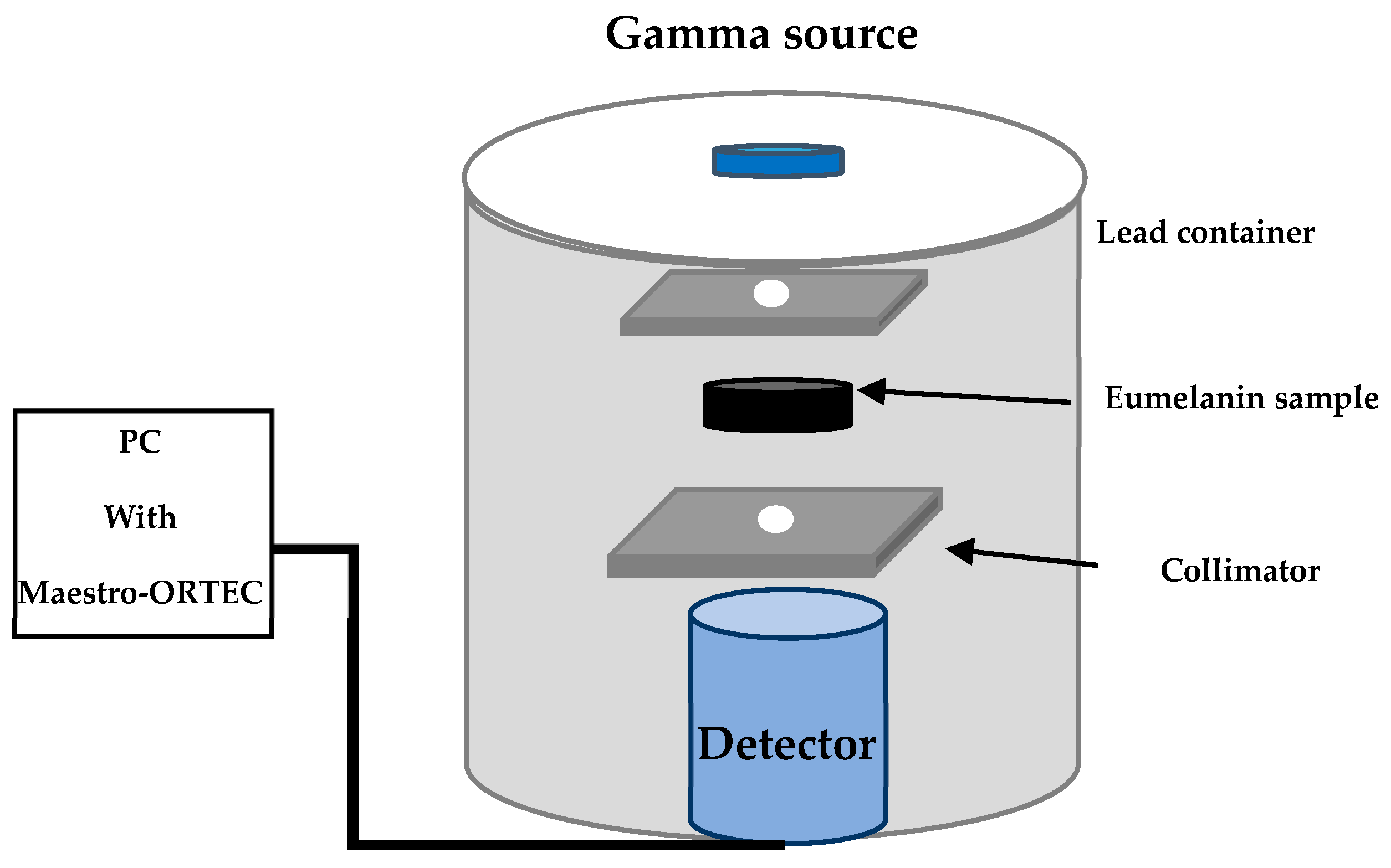
2.3. Experimental Setup
3. Results and Discussion
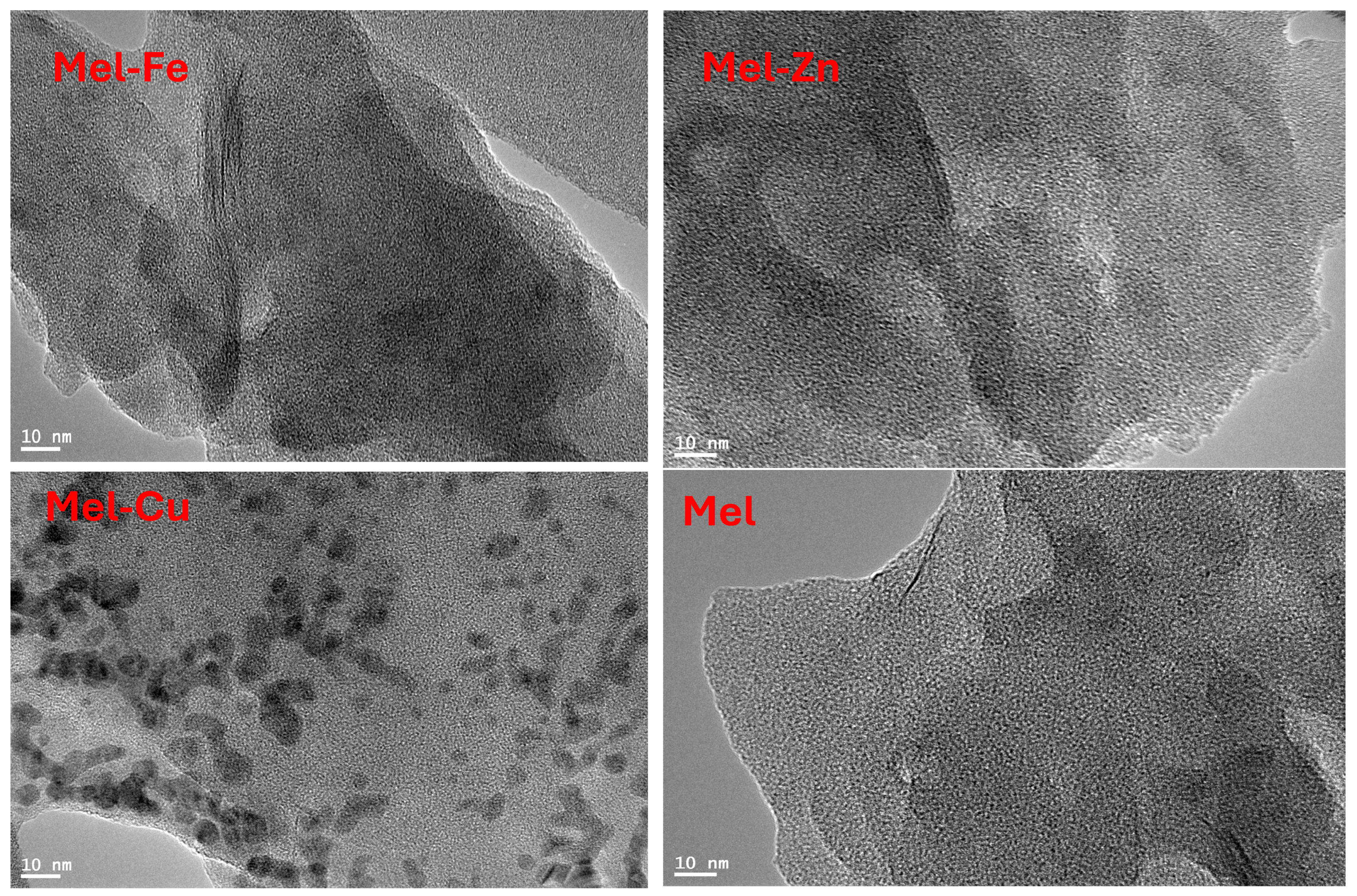
Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Samarth, R.; Kumar, M.; Matsumoto, Y.; Manda, K. The Effects of Ionizing and Non-Ionizing Radiation on Health. Recent Trends Adv. Environ. Health 2020, 179, 180–203. [Google Scholar]
- Thomas, G.A.; Symonds, P. Radiation exposure and health effects–is it time to reassess the real consequences? Clin. Oncol. 2016, 28, 231–236. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ionizing Radiation, Health Effects and Protective Measures; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Harrison, C.; Weaver, S.; Bertelsen, C.; Burgett, E.; Hertel, N.; Grulke, E. Polyethylene/boron nitride composites for space radiation shielding. J. Appl. Polym. Sci. 2008, 109, 2529–2538. [Google Scholar] [CrossRef]
- Hashim, A.; Agool, I.R.; Kadhim, K.J. Novel of (polymer blend-Fe3O4) magnetic nanocomposites: Preparation and characterization for thermal energy storage and release, gamma ray shielding, antibacterial activity and humidity sensors applications. J. Mater. Sci. Mater. Electron. 2018, 29, 10369–10394. [Google Scholar] [CrossRef]
- Harish, V.; Nagaiah, N.; Prabhu, T.N.; Varughese, K.T. Preparation and characterization of lead monoxide filled unsaturated polyester based polymer composites for gamma radiation shielding applications. J. Appl. Polym. Sci. 2009, 112, 1503–1508. [Google Scholar] [CrossRef]
- Marashdeh, M.; Abdulkarim, M. Determination of the attenuation coefficients of epoxy resin with carbopol polymer as a breast phantom material at low photon energy range. Polymers 2023, 15, 2645. [Google Scholar] [CrossRef] [PubMed]
- Marashdeh, M.W.; Mahmoud, K.A.; Akhdar, H.; Tharwat, M. Structural, physical, optical, and gamma ray shielding properties of SnO2-based boro-silicate glasses: The influence of substituting Na2O by SnO2. Nucl. Eng. Technol. 2024, 56, 3804–3811. [Google Scholar] [CrossRef]
- Zohuri, B. Heat Pipe Applications in Fission Driven Nuclear Power Plants; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Part, N.G. Radiation Protection and Safety of Radiation Sources International Basic Safety Standards, Interim ed.; International Atomic Energy Agency: Vienna, Austria, 2011. [Google Scholar]
- AbuAlRoos, N.J.; Amin, N.A.B.; Zainon, R. Conventional and new lead-free radiation shielding materials for radiation protection in nuclear medicine: A review. Radiat. Phys. Chem. 2019, 165, 108439. [Google Scholar] [CrossRef]
- Raj, K.; Das, A.P. Lead pollution: Impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Safari, A.; Rafie, P.; Taeb, S.; Najafi, M.; Mortazavi, S.M.J. Development of Lead-Free Materials for Radiation Shielding in Medical Settings: A Review. J. Biomed. Phys. Eng. 2024, 14, 229–244. [Google Scholar] [PubMed]
- Wozniak, A.I.; Ivanov, V.S.; Zhdanovich, O.A.; Nazarov, V.I.; Yegorov, A.S. Modern approaches to polymer materials protecting from ionizing radiation. J. Port Sci. Res. 2017, 33, 2148–2163. [Google Scholar] [CrossRef]
- Karkoszka, M.; Rok, J.; Wrześniok, D. Eumelanin Biopolymers in Pharmacology and Medicine—Skin Pigmentation Disorders, Implications for Drug Action, Adverse Effects and Therapy. Pharmaceuticals 2024, 17, 521. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Wang, S.; Zhao, J.; Cui, L.; Wang, X.; Cai, N.; Li, H.; Ren, S.; Li, T.; Shu, L. Synthesis and structural characteristics analysis of eumelanin pigments induced by blue light in Morchella sextelata. Front. Microbiol. 2023, 14, 1276457. [Google Scholar] [CrossRef]
- Madkhali, N.; Alqahtani, H.R.; Al-Terary, S.; Laref, A.; Hassib, A. Control of optical absorption and fluorescence spectroscopies of natural eumelanin at different solution concentrations. Opt. Quantum Electron. 2019, 51, 227. [Google Scholar] [CrossRef]
- Madkhali, N.; Algessair, S. Exploring the optical properties of metal-modified eumelanin following ultraviolet irradiation: An experimental and theoretical study using density functional theory. Heliyon 2024, 10, e29287. [Google Scholar] [CrossRef]
- Laref, A.; Madkhali, N.; Alqahtani, H.R.; Wu, X.; Laref, S. Electronic structures and optical spectroscopies of 3d-transition metals-doped eumelanin for spintronic devices application. J. Magn. Magn. Mater. 2019, 491, 165513. [Google Scholar] [CrossRef]
- Xie, W.; Dhinojwala, A.; Gianneschi, N.C.; Shawkey, M.D. Interactions of Eumelanin with Electromagnetic Radiation: From Fundamentals to Applications. Chem. Rev. 2024, 124, 7165–7213. [Google Scholar] [CrossRef]
- Madkhali, N.; Alqahtani, H.R.; Al-Terary, S.; Laref, A.; Haseeb, A. The doping effect of Fe, Cu and Zn ions on the structural and electrochemical properties and the thermostability of natural eumelanin extracted from Nigella sativa L. J. Mol. Liq. 2019, 285, 436–443. [Google Scholar] [CrossRef]
- Madkhali, N. The optical and electronic properties of DHI monomer of eueumelanin doped with transition metals (TMs) based on LSDA approximation in DFT theory. AIP Conf. Proc. 2023, 2872, 020012. [Google Scholar]
- Liu, H.; Yang, Y.; Liu, Y.; Pan, J.; Wang, J.; Man, F.; Zhang, W.; Liu, G. Eumelanin-like nanomaterials for advanced biomedical applications: A versatile platform with extraordinary promise. Adv. Sci. 2020, 7, 1903129. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Xavier, J.R.; Nunes, C.D.; Vaz, P.D.; Humanes, M. Marine sponge melanin: A new source of an old biopolymer. Struct. Chem. 2012, 23, 115–122. [Google Scholar] [CrossRef]
- Chen, L.-M.; Liu, Y.-N. Surface-enhanced Raman detection of eueumelaninon silver-nanoparticle-decorated silver/carbon nanospheres: Effect of metal ions. ACS Appl. Mater. Interfaces 2011, 3, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, J.; Li, T.; Ma, P.; Bai, H.; Xie, Y.; Chen, M.; Dong, W. A novel UV-shielding and transparent polymer film: When bioinspired dopamine–eumelanin hollow nanoparticles join polymers. ACS Appl. Mater. Interfaces 2017, 9, 36281–36289. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, T.; Summerer, L. A biomimetic approach to shielding from ionizing radiation: The case of melanized fungi. PLoS ONE 2020, 15, e0229921. [Google Scholar] [CrossRef] [PubMed]
- Dadachova, E.; Casadevall, A. Melanin Nanoshells for Protection Against Radiation and Electronic Pulses. U.S. Patent No. 8,586,090, 19 November 2013. [Google Scholar]
- NBSIR-87-3597; XCOM: Photon Cross Sections on a Personal Computer. National Bureau of Standards: Washington, DC, USA, 1987.
- Sharma, A.; Sayyed, M.; Agar, O.; Kaçal, M.; Polat, H.; Akman, F. Photon-shielding performance of bismuth oxychloride-filled polyester concretes. Mater. Chem. Phys. 2020, 241, 122330. [Google Scholar] [CrossRef]
- Naseer, K.A.; Marimuthu, K.; Mahmoud, K.A.; Sayyed, M.I. Impact of Bi2O3 modifier concentration on barium–zincborate glasses: Physical, structural, elastic, and radiation-shielding properties. Eur. Phys. J. Plus 2021, 136, 116. [Google Scholar] [CrossRef]
- Marashdeh, M.; Al-Hamarneh, I.F. Evaluation of Gamma Radiation Properties of Four Types of Surgical Stainless Steel in the Energy Range of 17.50–25.29 keV. Materials 2021, 14, 6873. [Google Scholar] [CrossRef] [PubMed]
- Akhdar, H.; Marashdeh, M.W.; AlAqeel, M. Investigation of gamma radiation shielding properties of polyethylene glycol in the energy range from 8.67 to 23.19 keV. Nucl. Eng. Technol. 2022, 54, 701–708. [Google Scholar] [CrossRef]
- Alshareef, R.; Marashdeh, M.; Almurayshid, M.; Alsuhybani, M. Study of radiation attenuation properties of HDPE/ZnO at energies between 47.5 and 266 keV. Prog. Nucl. Energy 2023, 165, 104909. [Google Scholar] [CrossRef]
- Singh, V.P.; Shirmardi, S.P.; Medhat, M.E.; Badiger, N.M. Determination of mass attenuation coefficient for some polymers using Monte Carlo simulation. Vacuum 2015, 119, 284–288. [Google Scholar] [CrossRef]
- Kucuk, N.; Cakir, M.; Isitman, N.A. Mass attenuation coefficients, effective atomic numbers and effective electron densities for some polymers. Radiat. Prot. Dosim. 2013, 153, 127–134. [Google Scholar] [CrossRef]
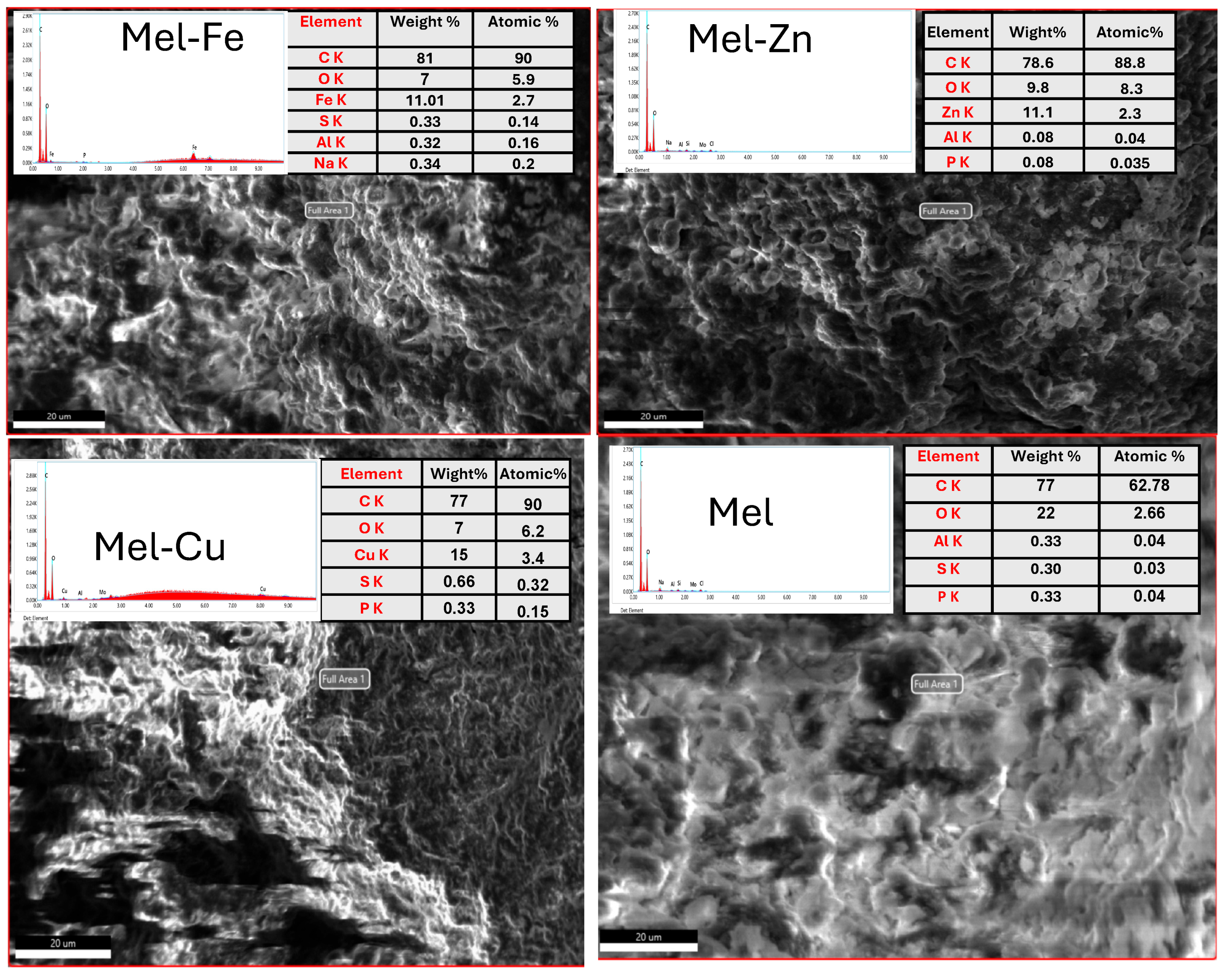




| Samples | Element Composition (%) | Density (g/cm3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Al | S | P | Fe | Cu | Na | Zn | ||
| Mel | 77.00 | 22.00 | 0.33 | 0.30 | 0.33 | - | - | - | - | 1.264 |
| Mel-Fe | 81.00 | 7.00 | 0.32 | 0.33 | - | 11.01 | 0.34 | - | 1.340 | |
| Mel-Cu | 77.00 | 7.00 | - | 0.66 | 0.33 | - | 15.00 | - | - | 1.327 |
| Mel-Zn | 78.60 | 9.80 | 0.08 | 0.08 | 0.08 | - | - | - | 11.10 | 1.304 |
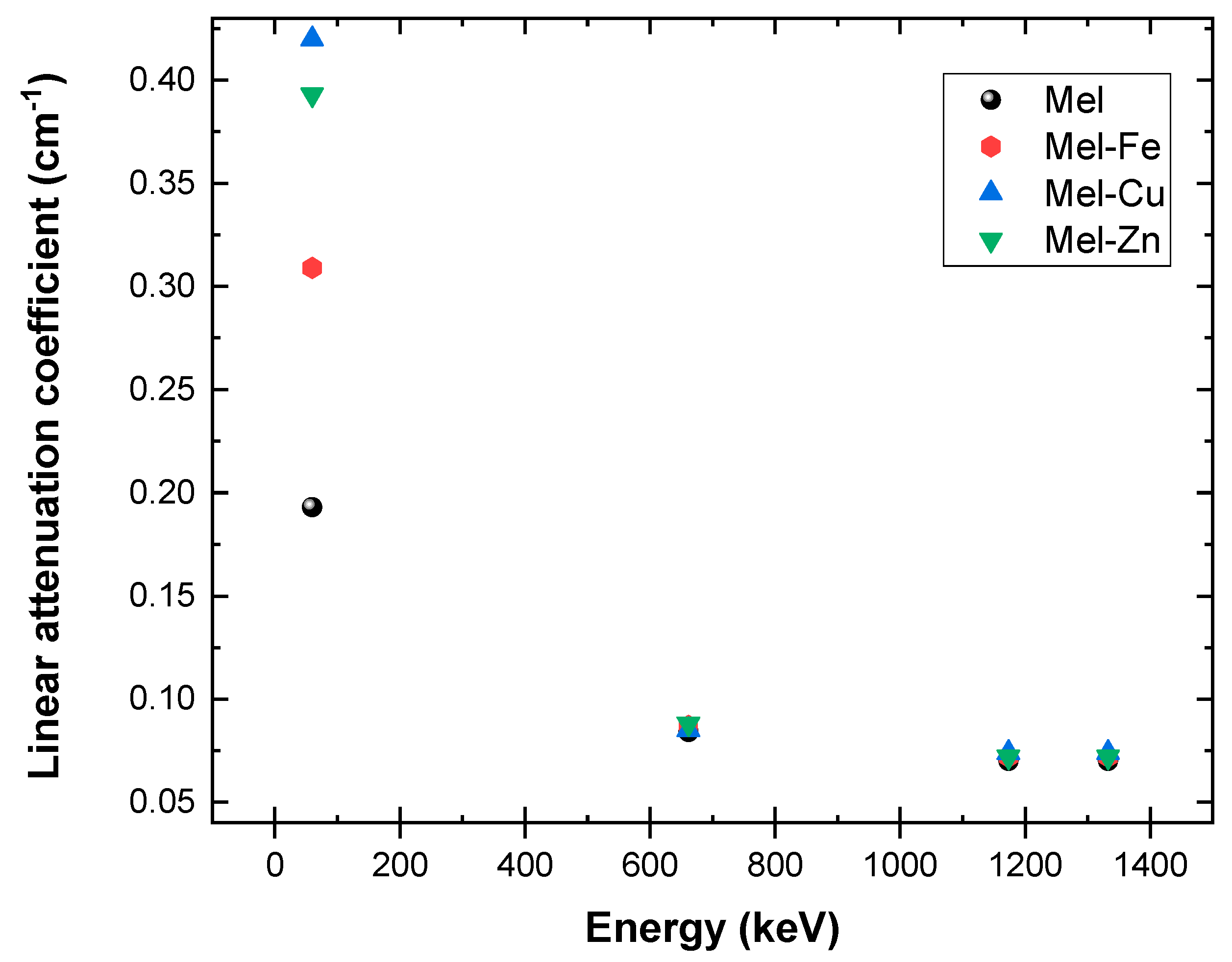
| Samples | 214Am (59.5 keV) | 137Cs (661.7 keV) | 60Co (1173.2) | 60Co (1332.5 keV) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μ | μm | Error (±%) | μ | μm | Error (±%) | μ | μm | Error (±%) | μ | μm | Error (±%) | |
| Mel | 0.193 | 0.153 | 0.014 | 0.084 | 0.067 | 0.008 | 0.070 | 0.056 | 0.005 | 0.068 | 0.054 | 0.004 |
| Mel-Fe | 0.309 | 0.230 | 0.017 | 0.087 | 0.065 | 0.007 | 0.072 | 0.054 | 0.006 | 0.069 | 0.052 | 0.004 |
| Mel-Cu | 0.420 | 0.316 | 0.028 | 0.085 | 0.064 | 0.010 | 0.074 | 0.056 | 0.004 | 0.070 | 0.052 | 0.003 |
| Mel-Zn | 0.393 | 0.302 | 0.024 | 0.088 | 0.068 | 0.007 | 0.072 | 0.056 | 0.006 | 0.068 | 0.053 | 0.005 |
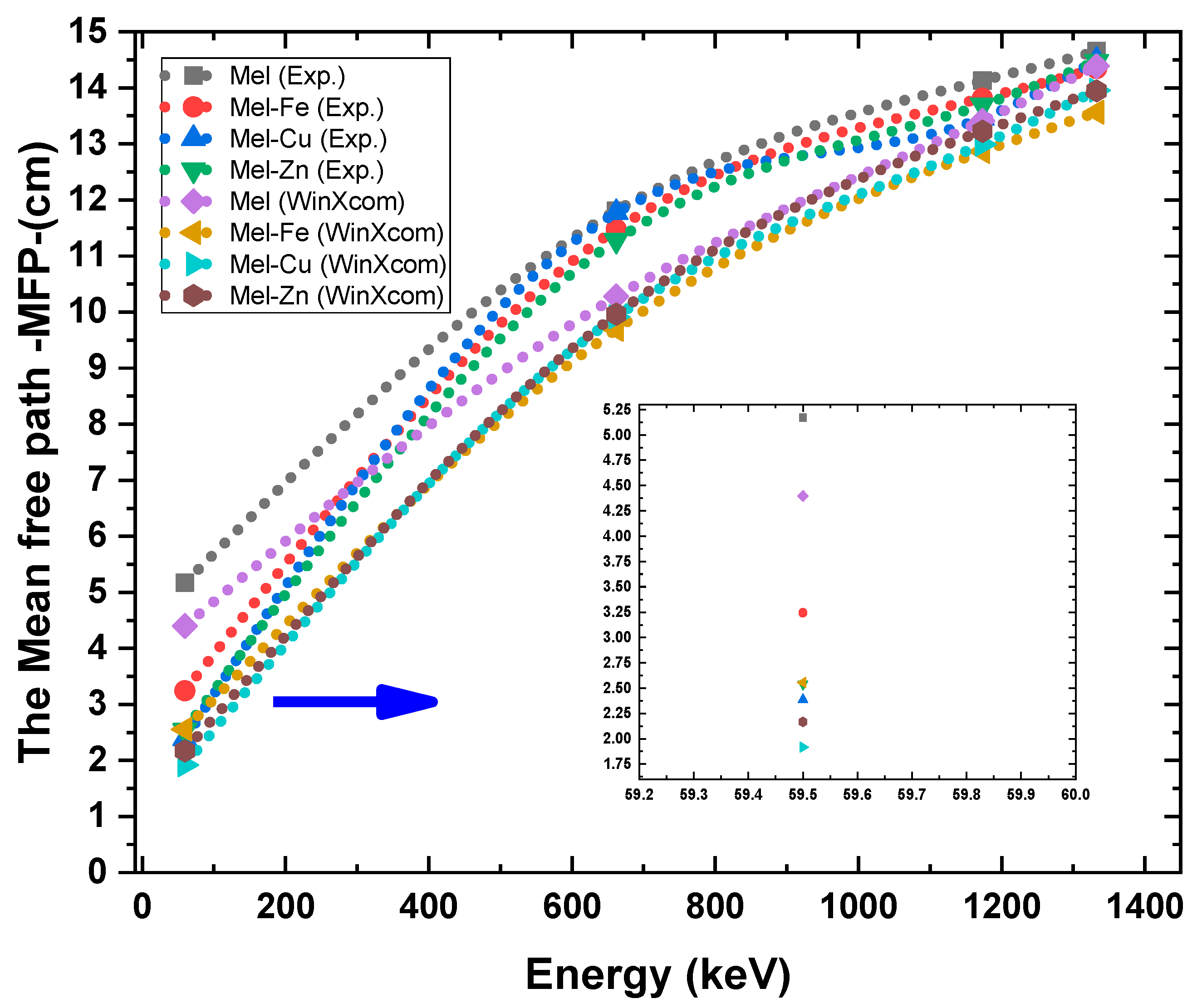
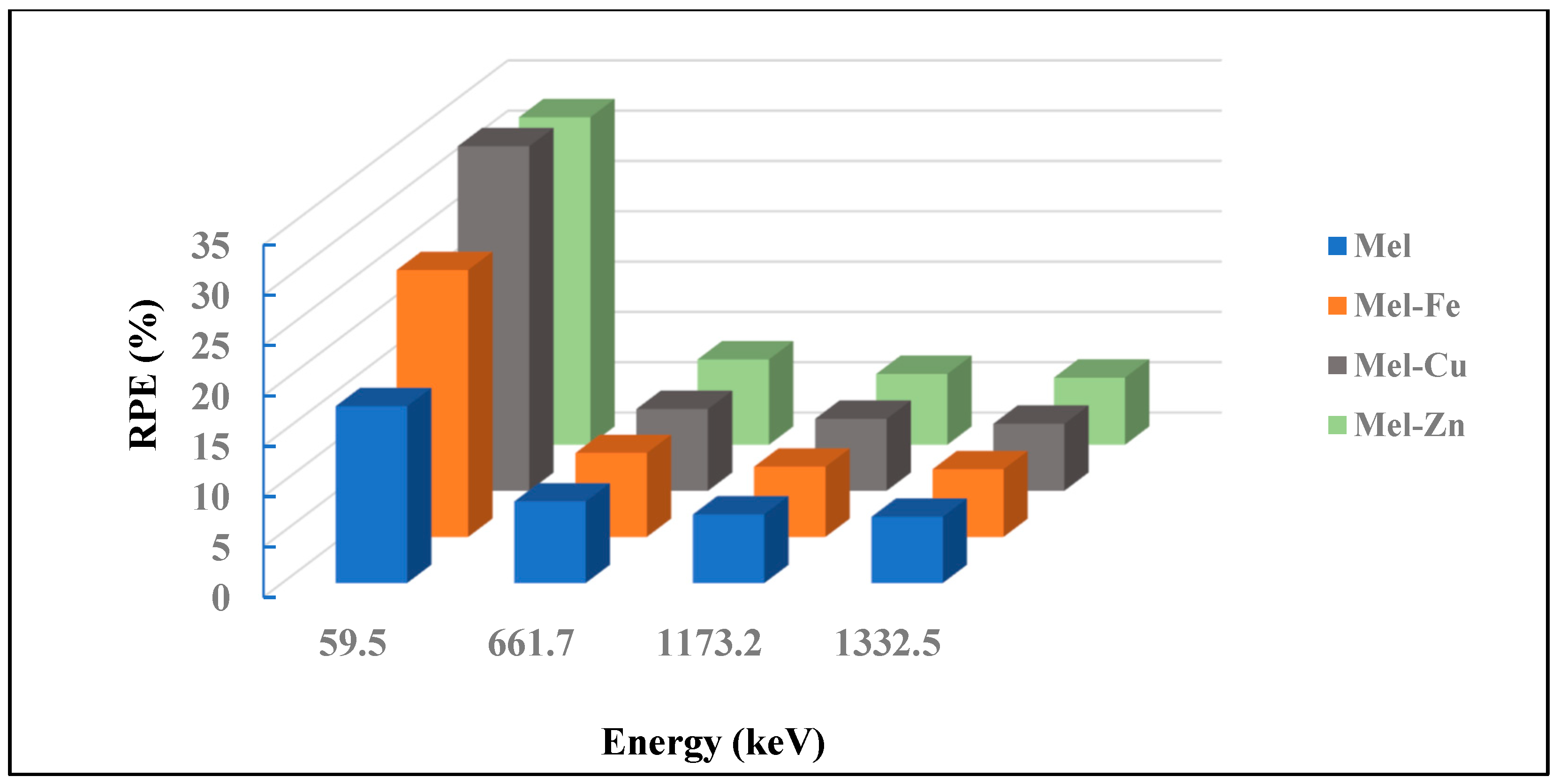
| Samples | Energy (keV) | Mass Attenuation Coefficients (cm2/g) | ||
|---|---|---|---|---|
| Experimental | Theoretical (WinXCOM) | Percentage Deviation% | ||
| Mel | 59.5 | 0.153 | 0.180 | 15.00 |
| 661.7 | 0.067 | 0.077 | 12.99 | |
| 1173.2 | 0.056 | 0.059 | 5.08 | |
| 1332.5 | 0.054 | 0.055 | 1.82 | |
| Mel-Fe | 59.5 | 0.230 | 0.292 | 21.23 |
| 661.7 | 0.065 | 0.077 | 15.58 | |
| 1173.2 | 0.054 | 0.058 | 6.90 | |
| 1332.5 | 0.052 | 0.055 | 5.45 | |
| Mel-Cu | 59.5 | 0.316 | 0.393 | 19.59 |
| 661.7 | 0.064 | 0.076 | 15.79 | |
| 1173.2 | 0.056 | 0.058 | 3.45 | |
| 1332.5 | 0.052 | 0.054 | 3.70 | |
| Mel-Zn | 59.5 | 0.302 | 0.354 | 14.69 |
| 661.7 | 0.068 | 0.077 | 11.69 | |
| 1173.2 | 0.056 | 0.058 | 3.45 | |
| 1332.5 | 0.053 | 0.055 | 3.64 | |
| Study | Study Theme | Material | |
|---|---|---|---|
| Singh et al. [35] | Simulation (MCNP) | phenol-formaldehyde resin (ρ =1.36 g/cm3) | 0.173 |
| Simulation (MCNP) | Polycarbonate (ρ = 1.22 g/cm3) | 0.172 | |
| Kucuk et al. [36] | Experimental | Poly(methyl methacrylate) (ρ = 1.18 g/cm3) | 0.109 |
| Experimental | Polypropylene (ρ = 0.946 g/cm3) | 0.126 | |
| Experimental | Polyethylene (ρ = 0.920 g/cm3) | 0.112 | |
| This study | Experimental | eumelanin (Mel) | 0.153 |
| Theoretical (XCOM) | eumelanin (Mel) | 0.180 | |
| Experimental | Mel-Fe | 0.230 | |
| Experimental | Mel-Cu | 0.316 | |
| Experimental | Mel-Zn | 0.302 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marashdeh, M.; Madkhali, N. Enhancing Radiation Shielding Efficiency of Nigella sativa Eumelanin Polymer Through Heavy Metals Doping. Polymers 2025, 17, 609. https://doi.org/10.3390/polym17050609
Marashdeh M, Madkhali N. Enhancing Radiation Shielding Efficiency of Nigella sativa Eumelanin Polymer Through Heavy Metals Doping. Polymers. 2025; 17(5):609. https://doi.org/10.3390/polym17050609
Chicago/Turabian StyleMarashdeh, Mohammad, and Nawal Madkhali. 2025. "Enhancing Radiation Shielding Efficiency of Nigella sativa Eumelanin Polymer Through Heavy Metals Doping" Polymers 17, no. 5: 609. https://doi.org/10.3390/polym17050609
APA StyleMarashdeh, M., & Madkhali, N. (2025). Enhancing Radiation Shielding Efficiency of Nigella sativa Eumelanin Polymer Through Heavy Metals Doping. Polymers, 17(5), 609. https://doi.org/10.3390/polym17050609






