Enhancing the Ductility and Properties of Non-Vulcanized Polylactic Acid-Based Thermoplastic Natural Rubber Using Acetyl Tributyl Citrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plasticized PLA (P-PLA)
2.3. NR Mastication
2.4. Preparation of TPNR
2.4.1. Effect of ATBC and NR Mastication Time
2.4.2. Effect of P-PLA/NR Weight Ratio
2.5. Mechanical Properties
2.6. Thermal Properties
2.7. Morphological Properties
2.8. Dynamic Mechanical Properties
2.9. Mw and Polydispersity Index
2.10. Rheological Measurements
2.11. Statistical Analysis
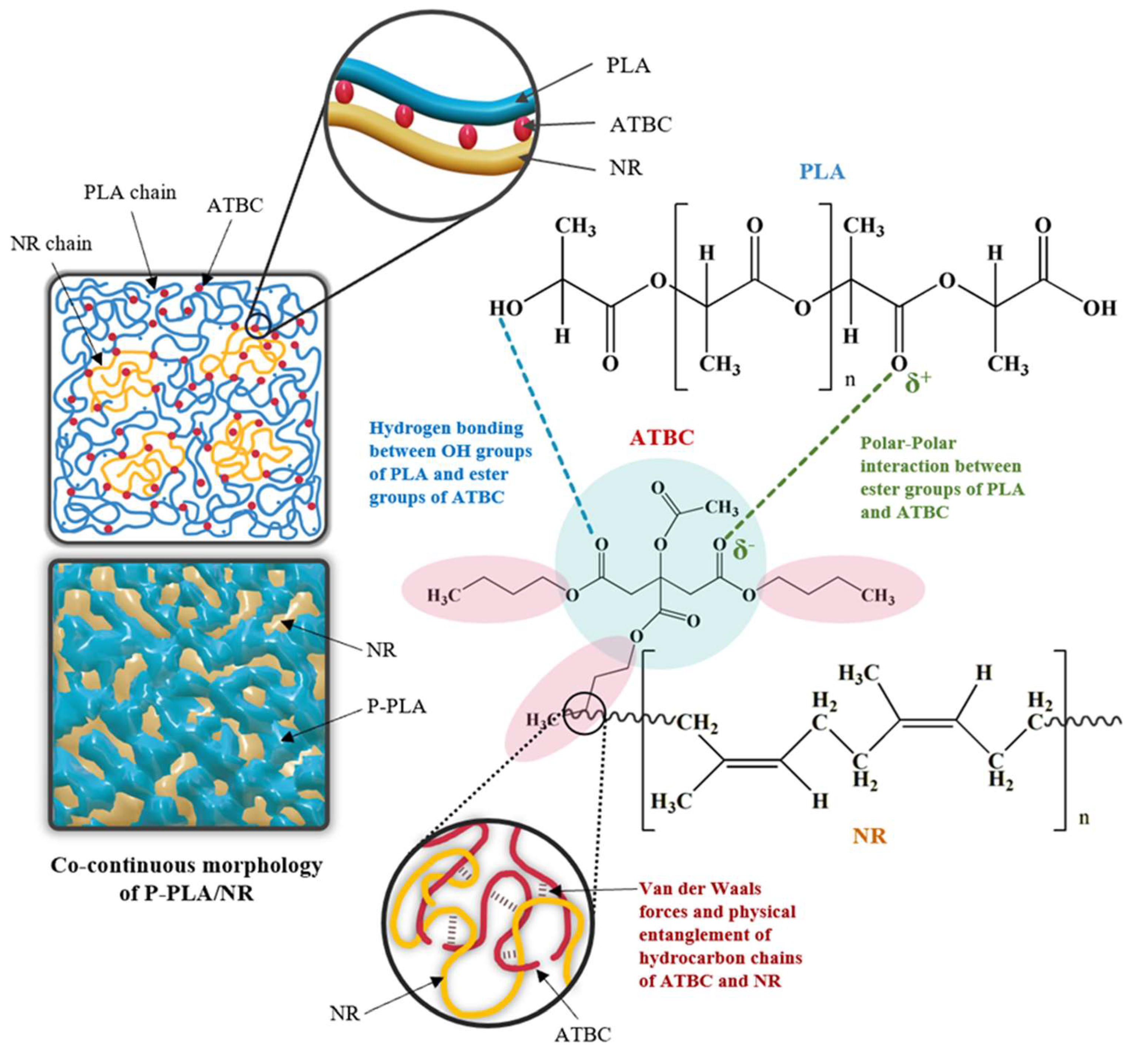
3. Results and Discussion
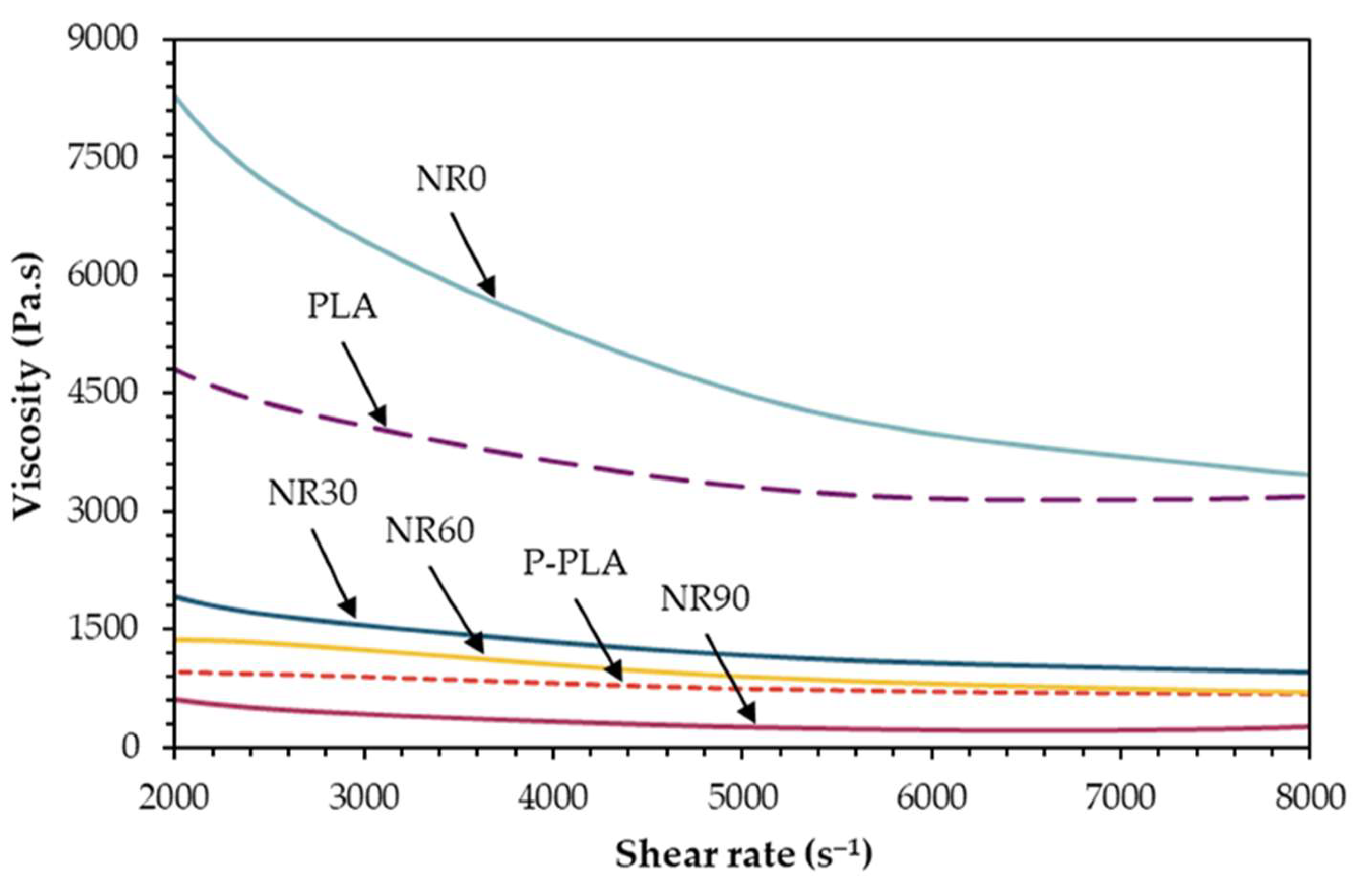
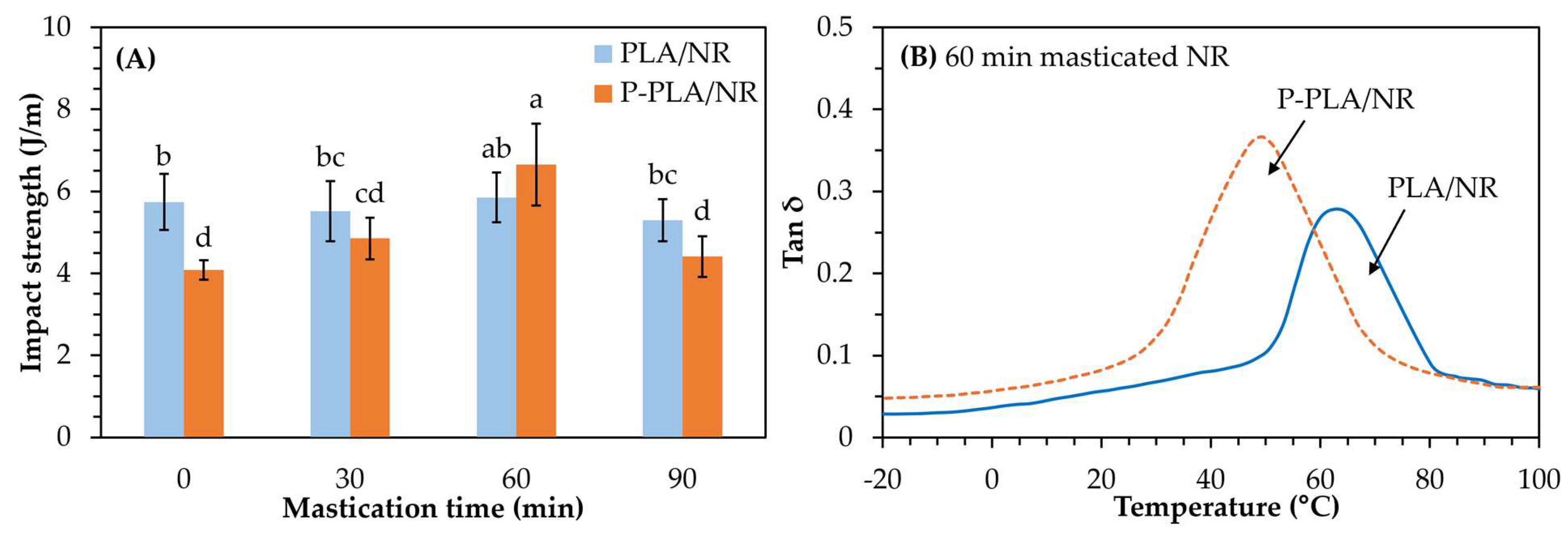
3.1. Effect of ATBC and NR Mastication Time
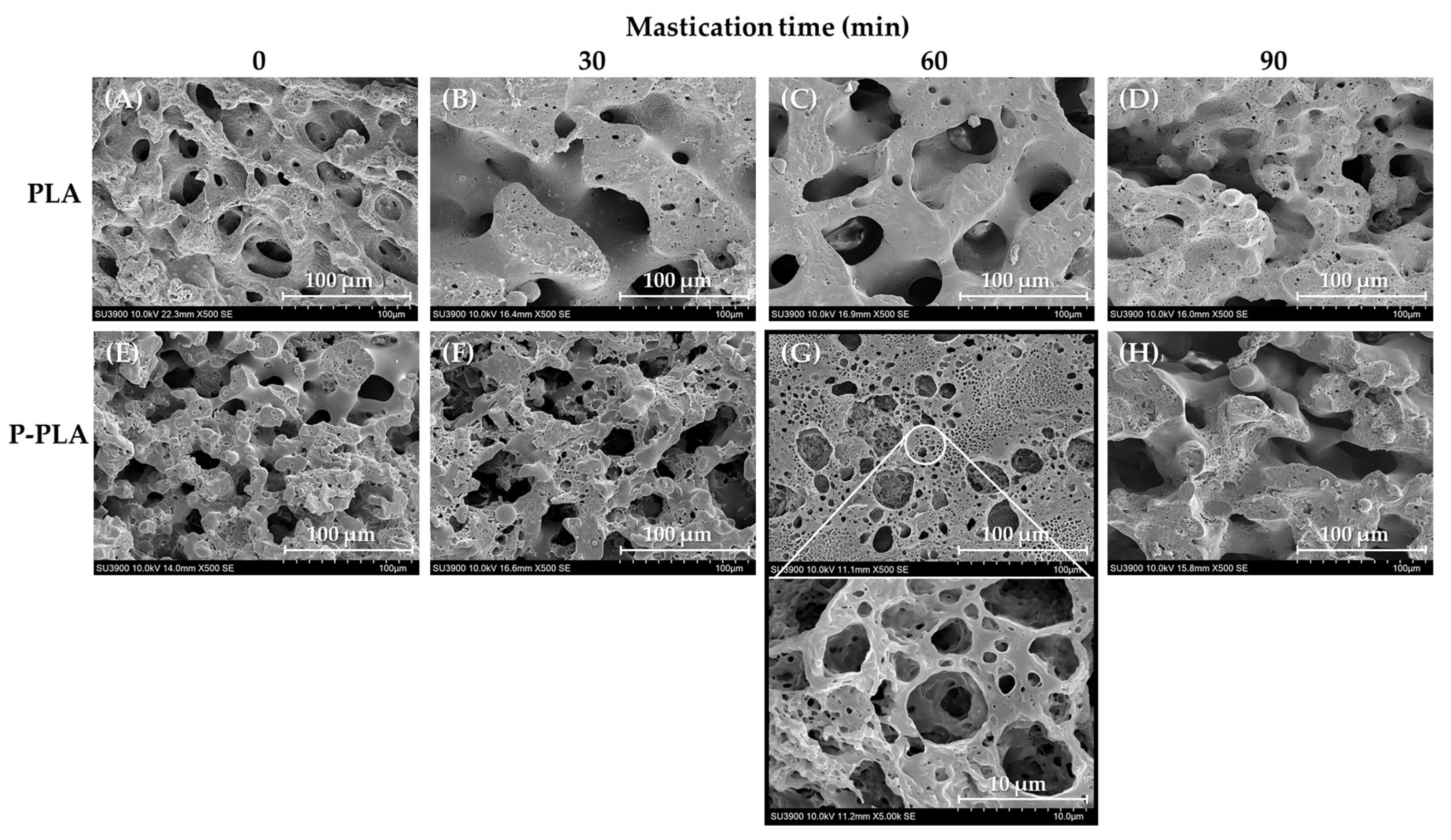
3.1.1. Morphological Properties
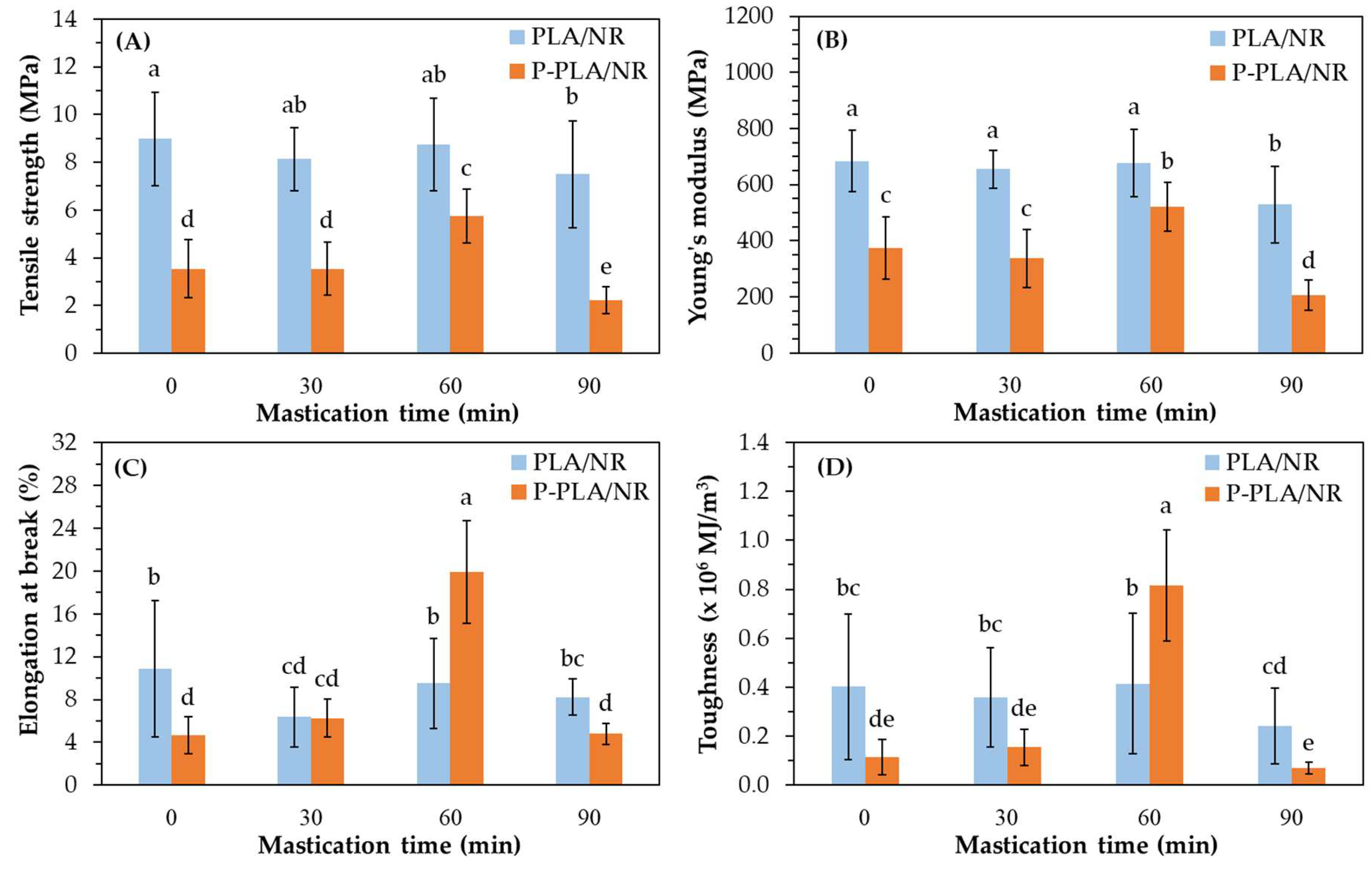
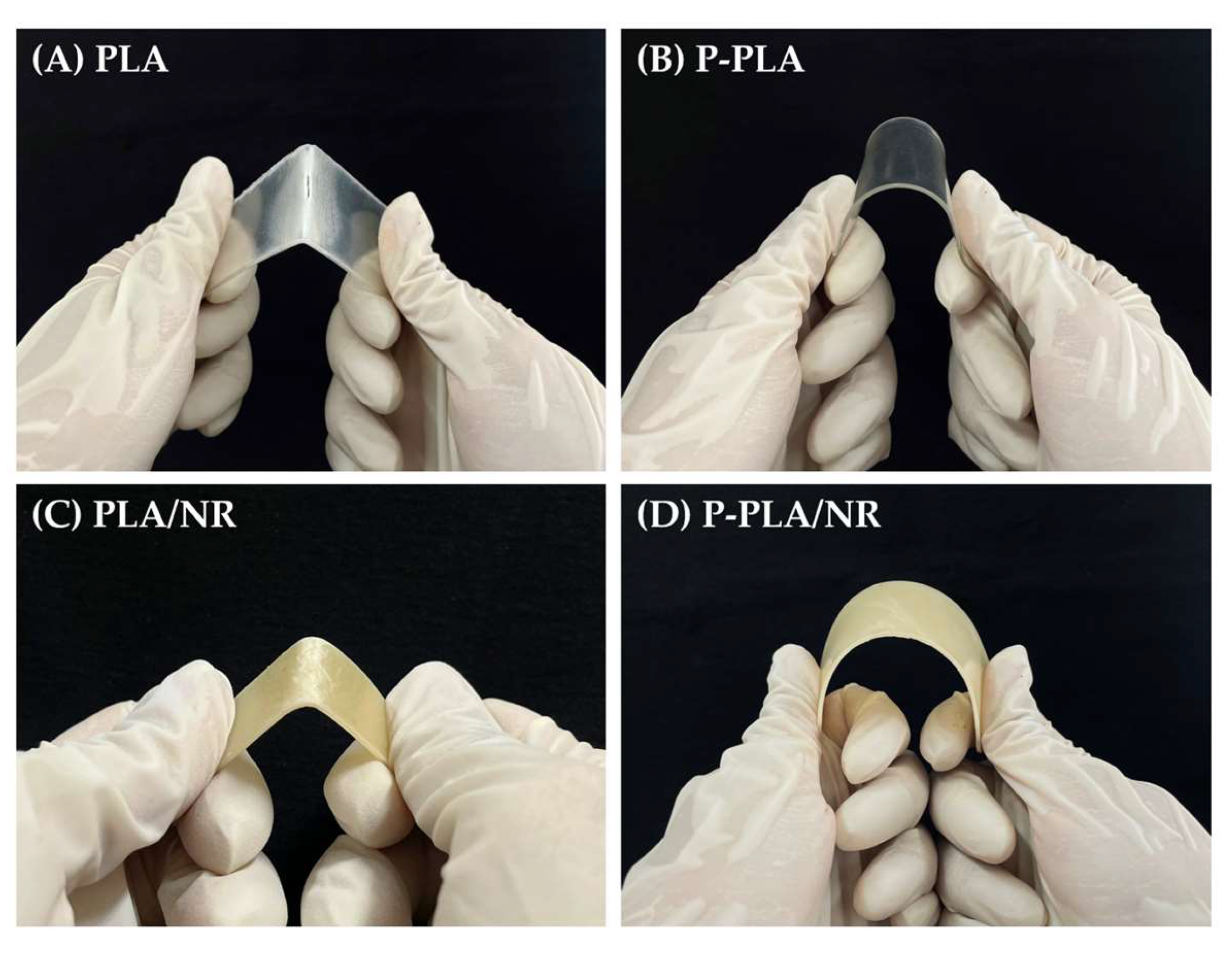
3.1.2. Mechanical Properties and Mw
3.2. Effect of P-PLA/NR Weight Ratio
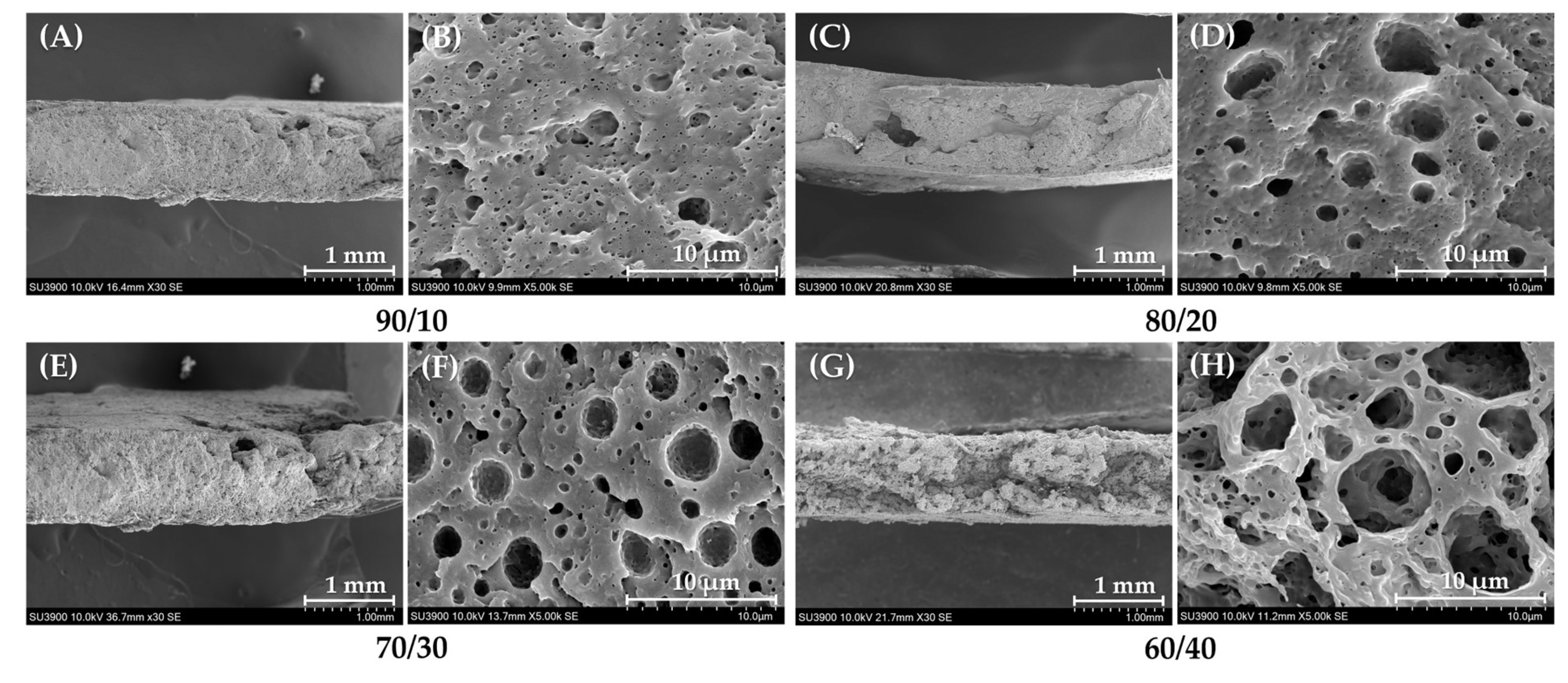
3.2.1. Morphological Properties
3.2.2. Mechanical Properties
3.2.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farah, S.; Anderson, D.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Oz, A.; Süfer, Ö.; Çelebi Sezer, Y. Poly (lactic acid) films in food packaging systems. Food Sci. Nutr. Technol. 2017, 2, 000131. [Google Scholar]
- Taib, N.-A.; Rahman, M.; Huda, D.; Kuok, K.; Hamdan, S.; Bakri, M.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Li, Y. Synthesis and biological application of polylactic acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, Y.; Luo, C.; Jiang, L.; Dan, Y. Enhanced ductility of polylactide materials: Reactive blending with pre-hot sheared natural rubber. J. Polym. Res. 2013, 20, 121. [Google Scholar] [CrossRef]
- Si, W.-J.; Yuan, W.-Q.; Li, Y.-D.; Chen, Y.-K.; Zeng, J.-B. Tailoring toughness of fully biobased poly(lactic acid)/natural rubber blends through dynamic vulcanization. Polym. Test. 2018, 65, 249–255. [Google Scholar] [CrossRef]
- Chanthot, P.; Kaeophimmueang, N.; Larpsuriyakul, P.; Pattamaprom, C. The effect of dynamic vulcanization systems on the mechanical properties and phase morphology of PLA/NR reactive blends. J. Polym. Res. 2021, 28, 34. [Google Scholar] [CrossRef]
- Ali Shah, A.; Hasan, F.; Shah, Z.; Kanwal, N.; Zeb, S. Biodegradation of natural and synthetic rubbers: A review. Int. Biodeterior. Biodegrad. 2013, 83, 145–157. [Google Scholar] [CrossRef]
- Bitinis, N.; Verdejo, R.; Cassagnau, P.; Lopez-Manchado, M. Structure and properties of polylactide/natural rubber blends. Mater. Chem. Phys. 2011, 129, 823–831. [Google Scholar] [CrossRef]
- Pongtanayut, K.; Thongpin, C.; Santawitee, O. The effect of rubber on morphology, thermal properties and mechanical properties of PLA/NR and PLA/ENR blends. Energy Procedia 2013, 34, 888–897. [Google Scholar] [CrossRef]
- Pattamaprom, C.; Chareonsalung, W.; Teerawattananon, C.; Ausopron, S.; Prachayawasin, P.; Van Puyvelde, P. Improvement in impact resistance of polylactic acid by masticated and compatibilized natural rubber. Iran. Polym. J. 2016, 25, 169–178. [Google Scholar] [CrossRef]
- Chanthot, P.; Kerddonfag, N.; Pattamaprom, C. The Influence of peroxide on bubble stability and rheological properties of biobased poly(lactic acid)/natural rubber blown films. Chin. J. Polym. Sci. 2022, 40, 197–207. [Google Scholar] [CrossRef]
- Burkov, A.; Kraev, A.; Grishin, M.; Vesnin, R.; Fomin, S.; Iordanskii, A. Structural features and properties’ characterization of polylactic acid/natural rubber blends with epoxidized soybean oil. Polymers 2021, 13, 1101. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Maggiore, I.; Bertoldo, M.; Signori, F.; Bronco, S.; Ciardelli, F. Poly(lactic acid) properties as a consequence of poly(butylene adipate-co-terephthalate) blending and acetyl tributyl citrate plasticization. J. Appl. Polym. Sci. 2008, 110, 1250–1262. [Google Scholar] [CrossRef]
- Courgneau, C.; Domenek, S.; Guinault, A.; Avérous, L.; Ducruet, V. Analysis of the structure-properties relationships of different multiphase systems based on plasticized poly(lactic acid). J. Polym. Environ. 2011, 19, 362–371. [Google Scholar] [CrossRef]
- Chieng, B.; Ibrahim, N.; Yunus, W.; Hussein, M. Plasticized poly(lactic acid) with low molecular weight poly(ethylene glycol): Mechanical, thermal, and morphology properties. J. Appl. Polym. Sci. 2013, 130, 4576–4580. [Google Scholar] [CrossRef]
- Arrieta, M.; Samper, M.; López, J.; Jiménez, A. Combined effect of poly(hydroxybutyrate) and plasticizers on polylactic acid properties for film intended for food packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Bijarimi, M.; Ahmad, S.; Rasid, R.; Khushairi, M.; Zakir, M. Poly(lactic acid)/poly(ethylene glycol) blends: Mechanical, thermal and morphological properties. AIP Conf. Proc. 2016, 1727, 020002. [Google Scholar]
- Li, D.; Jiang, Y.; Lv, S.; Liu, X.; Gu, J.; Chen, Q.; Zhang, Y. Preparation of plasticized poly (lactic acid) and its influence on the properties of composite materials. PLoS ONE 2018, 13, e0193520. [Google Scholar] [CrossRef]
- Petchwattana, N.; Sanetuntikul, J.; Narupai, B. Plasticization of biodegradable poly(lactic acid) by different triglyceride molecular sizes: A comparative study with glycerol. J. Polym. Environ. 2018, 26, 1160–1168. [Google Scholar] [CrossRef]
- Li, X.-G.; Xie, Y.-B.; Huang, M.-R.; Umeyama, T.; Ohara, T.; Imahori, H. Effective role of eco-friendly acetyl tributyl citrate in large-scale catalyst-free synthesis of waterborne polyurethanes without volatile organic compounds. J. Clean. Prod. 2019, 237, 117543. [Google Scholar] [CrossRef]
- Rasmussen, L.; Sen, N.; Liu, X.; Craig, Z. Effects of oral exposure to the phthalate substitute acetyl tributyl citrate on female reproduction in mice. J. Appl. Toxicol. 2017, 37, 668–675. [Google Scholar] [CrossRef]
- Sookprasert, P.; Hinchiranan, N. Morphology, mechanical and thermal properties of poly(lactic acid) (PLA)/natural rubber (NR) blends compatibilized by NR-graft-PLA. J. Mater. Res. 2017, 32, 788–800. [Google Scholar] [CrossRef]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef]
- Shekhar, N.; Mondal, A. Synthesis, properties, environmental degradation, processing, and applications of polylactic acid (PLA): An overview. Polym. Bull. 2024, 81, 11421–11457. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tarachiwin, L. Recent advances in structural characterization of natural rubber. Rubber Chem. Technol. 2009, 82, 283–314. [Google Scholar] [CrossRef]
- Utara, S.; Boochathum, P. Effect of molecular weight of natural rubber on the compatibility and crystallization behavior of LLDPE/NR blends. Polym.-Plast. Technol. Eng. 2011, 50, 1019–1026. [Google Scholar] [CrossRef]
- Mohsen, A.; Ali, N. Mechanical, color and barrier, properties of biodegradable nanocomposites polylactic acid/nanoclay. J. Bioremediat. Biodegrad. 2018, 9, 455. [Google Scholar] [CrossRef]
- ASTM Standard D2240-15; Standard Test Method for Rubber Property–Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM Standard D256-23; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA, 2016.
- Kalkornsurapranee, E.; Koedthip, D.; Songtipya, P.; Prodpran, T.; Johns, J.; Nakaramontri, Y.; Songtipya, L. Influence of modified natural rubbers as compatibilizers on the properties of flexible food contact materials based on NR/PBAT blends. Mater. Des. 2020, 196, 109134. [Google Scholar] [CrossRef]
- Phromma, W.; Magaraphan, R. Fabrication of Admicelled Natural Rubber by Polycaprolactone for Toughening Poly(lactic acid). J. Polym. Environ. 2018, 26, 2268–2280. [Google Scholar] [CrossRef]
- Thongseenuch, S.; Jindapaisan, S.; Taweepreda, W. Preparation and molecular weight controlled of liquid natural rubber using mastication. In Proceedings of the 2016 Management and Innovation Technology International Conference (MITicon), Bang-San, Thailand, 12–14 October 2016; pp. 4–7. [Google Scholar]
- Nakason, C.; Kaesaman, A.; Samoh, Z.; Homsin, S.; Kiatkamjornwong, S. Rheological properties of maleated natural rubber and natural rubber blends. Polym. Test. 2002, 21, 449–455. [Google Scholar] [CrossRef]
- Pattamaprom, C.; Saengklin, N. The rheological properties of masticated natural rubbers and their linear viscoelastic predictions. Rheol. Acta 2016, 55, 641–648. [Google Scholar] [CrossRef]
- Gonzalez-Garzon, M.; Shahbikian, S.; Huneault, M. Properties and phase structure of melt-processed PLA/PMMA blends. J. Polym. Res. 2018, 25, 58. [Google Scholar] [CrossRef]
- Celebi, H.; Gunes, E. Combined effect of a plasticizer and carvacrol and thymol on the mechanical, thermal, morphological properties of poly(lactic acid). J. Appl. Polym. Sci. 2018, 135, 45895. [Google Scholar] [CrossRef]
- Dimier, F.; Vergnes, B.; Vincent, M. Relationships between mastication conditions and rheological behavior of a natural rubber. Rheol. Acta 2004, 43, 196–202. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Chen, X.; Kong, Y.; Huang, Y.; Lv, Y.; Li, G. Effect of non-rubber components on the crosslinking structure and thermo-oxidative degradation of natural rubber. Polym. Degrad. Stab. 2022, 196, 109845. [Google Scholar] [CrossRef]
- Galiani, P.; Malmonge, J.; Soares, B.; Mattoso, L. Studies on thermal–oxidative degradation behaviours of raw natural rubber: PRI and thermogravimetry analysis. Plast. Rubber Compos. 2013, 42, 334–339. [Google Scholar] [CrossRef]
- von Kemenczky, O.; Buskirk, P.V. The use of capillary rheometry in predicting polymer processing performance. Polym. Polym. Compos. 1995, 3, 49–54. [Google Scholar]
- Fries, H.; Pandit, R. Mastication of Rubber. Rubber Chem. Technol. 1982, 55, 309–327. [Google Scholar] [CrossRef]
- Kraibut, A.; Saiwari, S.; Kaewsakul, W.; Noordermeer, J.; Sahakaro, K.; Dierkes, W. Dynamic response and molecular chain modifications associated with degradation during mixing of silica-reinforced natural rubber compounds. Polymers 2023, 15, 160. [Google Scholar] [CrossRef]
- Gecelter, R.; Ilyaguyeva, Y.; Thompson, N. The menisci are not shock absorbers: A biomechanical and comparative perspective. Anat. Rec. 2021, 305, 1051–1064. [Google Scholar] [CrossRef]
- Abdul Hadi, N.; Shuib, R. Effect of plasticizer on microstructure and dynamic mechanical performance of anisotropic magnetorheological elastomers. IOP Conf. Ser. Mater. Sci. Eng. 2019, 548, 012007. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Ferri, J.; Cardona, S.; Lora, J.; Fombuena, V. Dual plasticizer/thermal stabilizer effect of epoxidized chia seed oil (Salvia hispanica L.) to improve ductility and thermal properties of poly(lactic acid). Polymers 2021, 13, 1283. [Google Scholar] [CrossRef] [PubMed]
- Mirkhalaf, S.; Fagerström, M. The mechanical behavior of polylactic acid (PLA) films: Fabrication, experiments and modelling. Mech. Time-Depend. Mater. 2021, 25, 119–131. [Google Scholar] [CrossRef]
- Rapa, M.; Nita, R.; Vasile, C. Influence of plasticizers over some physico-chemical properties of PLA. Mater. Plast. 2017, 54, 73–78. [Google Scholar] [CrossRef]
- Thuy, N.; Ngoc Lan, P. Investigation of the impact of two types of epoxidized vietnam rubber seed oils on the properties of polylactic acid. Adv. Polym. Technol. 2021, 2021, 6698918. [Google Scholar] [CrossRef]
- Pivsa-Art, W.; Fujii, K.; Nomura, K.; Aso, Y.; Ohara, H.; Yamane, H. The effect of poly(ethylene glycol) as plasticizer in blends of poly(lactic acid) and poly(butylene succinate). J. Appl. Polym. Sci. 2016, 133, 43044. [Google Scholar] [CrossRef]
- Tessanan, W.; Chanthateyanonth, R.; Yamaguchi, M.; Phinyocheep, P. Improvement of mechanical and impact performance of poly(lactic acid) by renewable modified natural rubber. J. Clean. Prod. 2020, 276, 123800. [Google Scholar] [CrossRef]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.-J. Petro-based and bio-based plasticizers: Chemical structures to plasticizing properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Omar, A.; Mohd Hanafi, M.; Razak, N.; Ibrahim, A.; Ab Razak, N. A best-evidence review of bio-based plasticizer and the effects on the mechanical properties of PLA. Chem. Eng. Trans. 2021, 89, 241–246. [Google Scholar]
- El-Hadi, A. The effect of additives interaction on the miscibility and crystal structure of two immiscible biodegradable polymers. Polímeros 2014, 24, 9–16. [Google Scholar] [CrossRef]
- Hindawi, I.; Higgins, J.; Weiss, R. Flow-induced mixing and demixing in polymer blends. Polymer 1992, 33, 2522–2529. [Google Scholar] [CrossRef]
- Pichaiyut, S.; Faibunchan, P.; Kummerlöwe, C.; Vennemann, N.; Nakason, C. Investigation of rheological, morphological and mechanical properties, thermal stability, biodegradability of the dynamically cured natural rubber/polyester blends. J. Polym. Environ. 2023, 31, 1051–1070. [Google Scholar] [CrossRef]
- Pawlak, A. Crystallization of polymers with a reduced density of entanglements. Crystals 2024, 14, 385. [Google Scholar] [CrossRef]

| Property | Plasticizer Contents (wt%) | |||
|---|---|---|---|---|
| 0 | 5 | 10 | 15 | |
| Tensile strength (MPa) | 57.50 ± 3.10 a | 53.02 ± 4.69 b | 49.08 ± 3.01 c | 25.96 ± 3.17 d |
| Elongation at break (%) | 6.95 ± 0.52 b | 7.11 ± 0.70 b | 6.81 ± 0.61 b | 234.44 ± 12.84 a |
| Young’s modulus (MPa) | 1441 ± 101 a | 1239 ± 134 b | 1235 ± 102 b | 954 ± 132 c |
| Toughness (106 MJ/m3) | 2.52 ± 0.33 b | 2.24 ± 0.27 b | 2.08 ± 0.27 b | 38.54 ± 6.08 a |
| Hardness (Shore D) | 81.00 ± 1.73 a | 78.60 ± 0.55 b | 75.60 ± 0.55 c | 70.60 ± 2.41 d |
| Impact strength (J/m) | 4.94 ± 0.82 a | 3.88 ± 0.84 b | 4.15 ± 0.47 b | 5.12 ± 0.75 a |
| Runs | Factor A (ATBC Contents, wt%) | Factor B (Mastication Time, min) |
|---|---|---|
| 1 | 0 (Neat PLA) | 0 |
| 2 | 30 | |
| 3 | 60 | |
| 4 | 90 | |
| 5 | 15 (P-PLA) | 0 |
| 6 | 30 | |
| 7 | 60 | |
| 8 | 90 |
| Sample | Mastication Time (min) | Mw (g/mol) | PDI |
|---|---|---|---|
| Unmasticated NR | 0 | 1,402,766 | 5.29 |
| Masticated NR | 30 | 324,491 | 2.25 |
| 60 | 296,152 | 2.02 | |
| 90 | 289,397 | 2.16 | |
| Neat PLA | 150,181 | 1.71 | |
| P-PLA | 156,344 | 1.90 |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | Toughness (×106 MJ/ m3) | Hardness (Shore D) |
|---|---|---|---|---|---|
| Neat PLA | 41.23 ± 3.50 a | 1914 ± 19 a | 6.85 ± 0.66 e | 1.61 ± 0.18 d | 81.00 ± 1.73 a |
| P-PLA/NR | |||||
| 100/0 * | 39.35 ± 2.94 b | 787 ± 94 b | 246.58 ± 10.90 a | 44.19 ± 4.16 a | 70.60 ± 2.41 b |
| 90/10 | 16.74 ± 0.80 c | 668 ± 72 c | 65.06 ± 32.18 b | 7.21 ± 3.58 b | 65.20 ± 0.45 c |
| 80/20 | 13.37 ± 0.96 d | 643 ± 47 c | 46.54 ± 23.54 c | 4.21 ± 1.83 c | 59.20 ± 0.84 d |
| 70/30 | 9.10 ± 1.13 e | 517 ± 73 d | 23.56 ± 12.09 d | 1.52 ± 0.83 d | 53.60 ± 0.89 e |
| 60/40 | 5.75 ± 1.13 f | 521 ± 86 d | 19.93 ± 4.80 de | 0.82 ± 0.23 d | 48.00 ± 0.71 f |
| Sample | Impact Strength (J/m) | % Fracture | Type of Failure | |
|---|---|---|---|---|
| Neat PLA | 4.19 ± 0.25 e | 100 ± 0 a | C |  |
| P-PLA/NR | ||||
| 100/0 * | 5.85 ± 0.57 c | 100 ± 0 a | C |  |
| 90/10 | 35.40 ± 2.56 a | 6.77 ± 0.98 c | NB |  |
| 80/20 | 15.37 ± 3.41 b | 46.92 ± 9.03 b | NB |  |
| 70/30 | 5.40 ± 0.56 cd | 100 ± 0 a | C |  |
| 60/40 | 5.18 ± 0.22 d | 100 ± 0 a | C |  |
| Sample | Tg (°C) | Tm (°C) | Tcc (°C) | (%) | |
|---|---|---|---|---|---|
| NR | PLA | ||||
| Neat PLA | - | 56.43 | 150.39 | 106.40 | 42.25 |
| P-PLA/NR | |||||
| 100/0 * | - | 33.86 | 134.14 | 84.59 | 31.82 |
| 90/10 | –64.03 | 23.52 | 130.10 | 82.44 | 31.10 |
| 80/20 | –67.17 | 24.95 | 131.14 | 83.38 | 29.97 |
| 70/30 | –67.78 | 28.11 | 132.71 | 85.65 | 30.14 |
| 60/40 | –66.93 | 32.83 | 135.06 | 86.45 | 25.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koedthip, D.; Kalkornsurapranee, E.; Sengloyluan, K.; Songtipya, P.; Songtipya, L. Enhancing the Ductility and Properties of Non-Vulcanized Polylactic Acid-Based Thermoplastic Natural Rubber Using Acetyl Tributyl Citrate. Polymers 2025, 17, 601. https://doi.org/10.3390/polym17050601
Koedthip D, Kalkornsurapranee E, Sengloyluan K, Songtipya P, Songtipya L. Enhancing the Ductility and Properties of Non-Vulcanized Polylactic Acid-Based Thermoplastic Natural Rubber Using Acetyl Tributyl Citrate. Polymers. 2025; 17(5):601. https://doi.org/10.3390/polym17050601
Chicago/Turabian StyleKoedthip, Donlaporn, Ekwipoo Kalkornsurapranee, Karnda Sengloyluan, Ponusa Songtipya, and Ladawan Songtipya. 2025. "Enhancing the Ductility and Properties of Non-Vulcanized Polylactic Acid-Based Thermoplastic Natural Rubber Using Acetyl Tributyl Citrate" Polymers 17, no. 5: 601. https://doi.org/10.3390/polym17050601
APA StyleKoedthip, D., Kalkornsurapranee, E., Sengloyluan, K., Songtipya, P., & Songtipya, L. (2025). Enhancing the Ductility and Properties of Non-Vulcanized Polylactic Acid-Based Thermoplastic Natural Rubber Using Acetyl Tributyl Citrate. Polymers, 17(5), 601. https://doi.org/10.3390/polym17050601









