Silicone Rubber Catheters Modified by Poly(N-vinylpyrrolidone) Graft Promoted by Gamma Rays
Abstract
1. Introduction
2. Materials and Methods
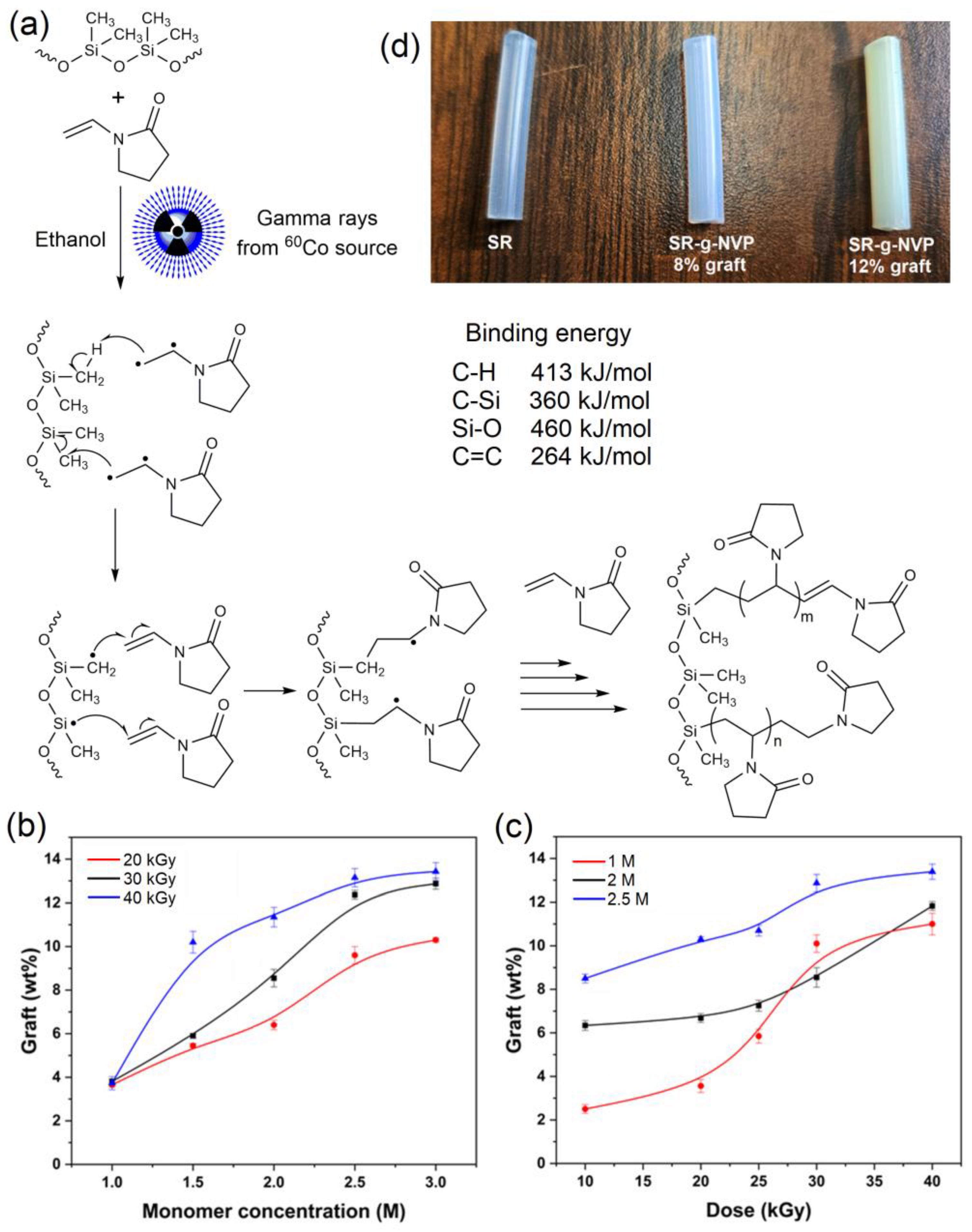
2.1. Modification of Silicone Catheters by Poly(N-vinylpyrrolidone) (SR-g-NVP)
2.2. Characterization
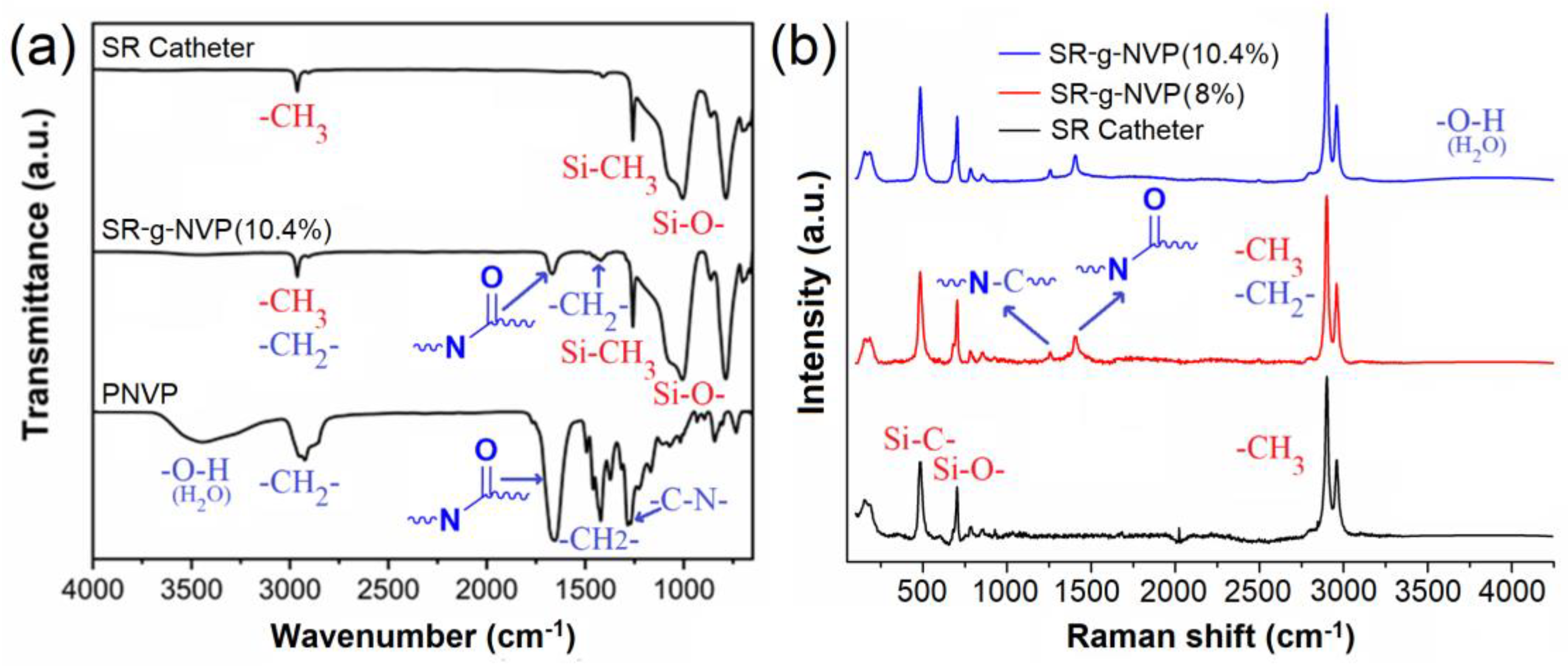
2.2.1. Structural Characterization Using ATR-IR and Raman Spectroscopy
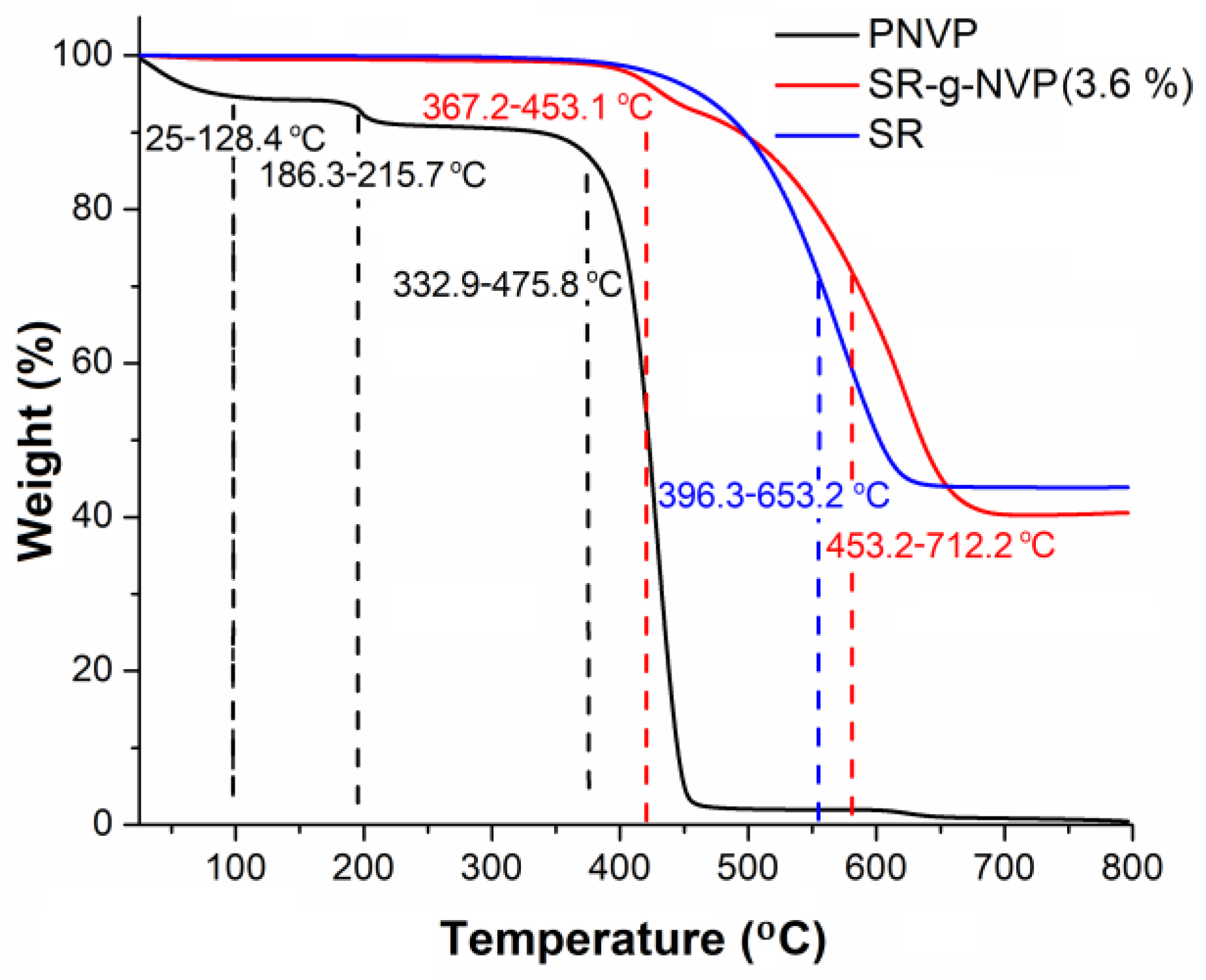
2.2.2. Thermal Characterization
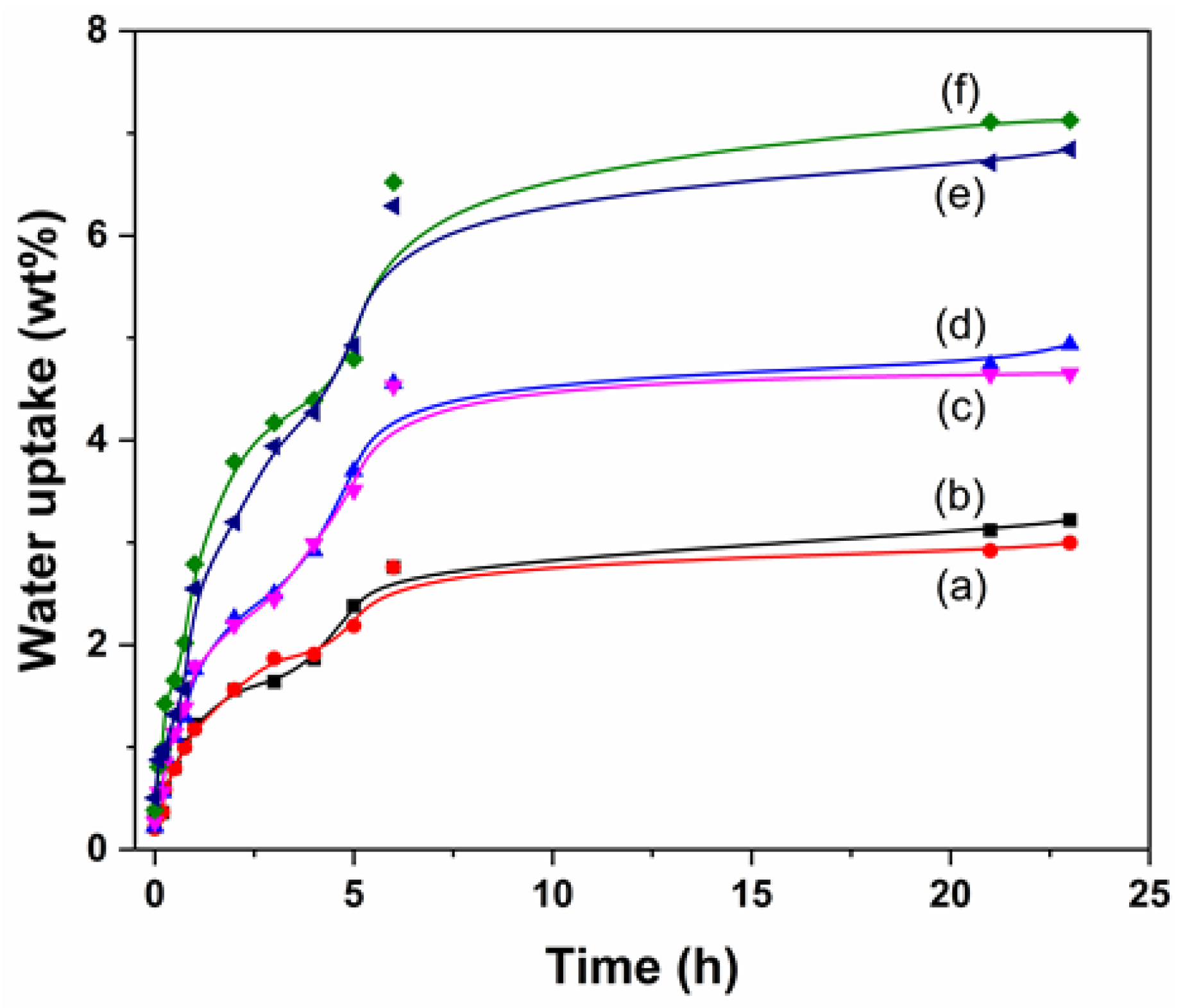
2.2.3. Wettability Capacity
2.2.4. Evaluation of Drug Loading
3. Results and Discussion
3.1. Synthesis of SR-g-NVP
3.2. Characterization
3.2.1. Infrared Spectroscopy (ATR-FTIR) and Raman Spectroscopy
3.2.2. Thermal Characterization
3.2.3. Wettability Degree
3.3. Drug Loading Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Liu, L.; Shou, L.; Yu, H.; Yao, J. Mechanical Properties and Corrosion Resistance of Vulcanized Silicone Rubber after Exposure to Artificial Urine. J. Macromol. Sci. Part B 2015, 54, 962–974. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; López-Saucedo, F.; Bucio, E.; Isoshima, T. Covalent Immobilization of Lysozyme in Silicone Rubber Modified by Easy Chemical Grafting. MRS Commun. 2017, 7, 904–912. [Google Scholar] [CrossRef]
- Braun, U.; Lorenz, E.; Weimann, C.; Sturm, H.; Karimov, I.; Ettl, J.; Meier, R.; Wohlgemuth, W.A.; Berger, H.; Wildgruber, M. Mechanic and Surface Properties of Central-Venous Port Catheters after Removal: A Comparison of Polyurethane and Silicon Rubber Materials. J. Mech. Behav. Biomed. Mater. 2016, 64, 281–291. [Google Scholar] [CrossRef]
- Aziz, T.; Waters, M.; Jagger, R. Analysis of the Properties of Silicone Rubber Maxillofacial Prosthetic Materials. J. Dent. 2003, 31, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.W.; Lebeau, J.E. The Effect of Implantation on the Physical Properties of Silicone Rubber. J. Biomed. Mater. Res. 1974, 8, 357–367. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; Pino-Ramos, V.H.; López-Saucedo, F.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Improved Covalent Immobilization of Lysozyme on Silicone Rubber-Films Grafted with Poly(Ethylene Glycol Dimethacrylate-Co-Glycidylmethacrylate). Eur. Polym. J. 2017, 95, 27–40. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, K.; Nie, J.; Du, B.; Tang, G. Poly(N-Vinylpyrrolidinone) Microgels: Preparation, Biocompatibility, and Potential Application as Drug Carriers. Biomacromolecules 2014, 15, 2285–2293. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. The Use of Poly(N-Vinyl Pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Mishra, R.K.; Datt, M.; Banthia, A.K. Synthesis and Characterization of Pectin/PVP Hydrogel Membranes for Drug Delivery System. AAPS PharmSciTech 2008, 9, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.; Reindel, W.; Steffen, R.; Mosehauer, G.; Chinn, J. Use of a Novel Extended Blink Test to Evaluate the Performance of Two Polyvinylpyrrolidone-Containing, Silicone Hydrogel Contact Lenses. Clin. Ophthalmol. 2018, 12, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Pino-Ramos, V.H.; Flores-Rojas, G.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Graft Copolymerization by Ionization Radiation, Characterization, and Enzymatic Activity of Temperature-Responsive SR-g-PNVCL Loaded with Lysozyme. React. Funct. Polym. 2018, 126, 74–82. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; Bucio, E. Radiation-Grafting of Ethylene Glycol Dimethacrylate (EGDMA) and Glycidyl Methacrylate (GMA) onto Silicone Rubber. Radiat. Phys. Chem. 2016, 127, 21–26. [Google Scholar] [CrossRef]
- Elzahaby, D.A.; Farrag, H.A.; Haikal, R.R.; Alkordi, M.H.; Abdeltawab, N.F.; Ramadan, M.A. Inhibition of Adherence and Biofilm Formation of Pseudomonas Aeruginosa by Immobilized ZnO Nanoparticles on Silicone Urinary Catheter Grafted by Gamma Irradiation. Microorganisms 2023, 11, 913. [Google Scholar] [CrossRef]
- Peng, J.; Wang, M.; Qiao, J.; Wei, G. Radiation-Induced Grafting Polymerization of MMA onto Polybutadiene Rubber Latex. Radiat. Phys. Chem. 2005, 72, 739–743. [Google Scholar] [CrossRef]
- Deng, B.; Li, J.; Hou, Z.; Yao, S.; Shi, L.; Liang, G.; Sheng, K. Microfiltration Membranes Prepared from Polyethersulfone Powder Grafted with Acrylic Acid by Simultaneous Irradiation and Their pH Dependence. Radiat. Phys. Chem. 2008, 77, 898–906. [Google Scholar] [CrossRef]
- Hoque, M.A.; Bradley, R.K.; Fan, J.; Fan, X. Effects of Humidity and Phosphor on Silicone/Phosphor Composite in White Light-Emitting Diode Package. J. Mater. Sci. Mater. Electron. 2019, 30, 20471–20478. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Peniche, C.; Zaldívar, D.; Pazos, M.; Páz, S.; Bulay, A.; Román, J.S. Study of the Thermal Degradation of Poly(N-vinyl-2-pyrrolidone) by Thermogravimetry–FTIR. J. Appl. Polym. Sci. 1993, 50, 485–493. [Google Scholar] [CrossRef]
- Basha, A.-F.; Basha, M.A.F. Structural and Thermal Degradation Studies on Thin Films of the Nanocomposite System PVP–Ce(SO4)2·4H2O. Polym. Bull. 2012, 68, 151–165. [Google Scholar] [CrossRef]
- Cornejo-Bravo, J.M.; Palomino, K.; Palomino-Vizcaino, G.; Pérez-Landeros, O.M.; Curiel-Alvarez, M.; Valdez-Salas, B.; Bucio, E.; Magaña, H. Poly(N-Vinylcaprolactam) and Salicylic Acid Polymeric Prodrug Grafted onto Medical Silicone to Obtain a Novel Thermo- and pH-Responsive Drug Delivery System for Potential Medical Devices. Materials 2021, 14, 1065. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hou, X.; Zhao, Q.; Cai, X.; Bian, C.; Cheng, J.; Feng, X. Pyrolysis Mechanism of Silicone Rubber Thermal Protection System Materials in Service Environment. Polym. Degrad. Stab. 2024, 229, 110951. [Google Scholar] [CrossRef]
- Navarrete-Germán, L.; Gómez-Lázaro, B.; López-Saucedo, F.; Bucio, E. Antimicrobial Functionalization of Silicone-Graft-Poly(N-Vinylimidazole) Catheters. Molecules 2024, 29, 2225. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Stratidakis, A.K.; Goryachaya, A.V.; Tzatzarakis, M.N.; Stivaktakis, P.D.; Docea, A.O.; Berdiaki, A.; Nikitovic, D.; Velonia, K.; Shtilman, M.I.; et al. In Vitro Blood Compatibility and in Vitro Cytotoxicity of Amphiphilic Poly-N-Vinylpyrrolidone Nanoparticles. Food Chem. Toxicol. 2019, 127, 42–52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Meza, J.E.; Pino-Ramos, V.H.; Flores-Rojas, G.G.; Mendizabal, E.; Bucio, E. Silicone Rubber Catheters Modified by Poly(N-vinylpyrrolidone) Graft Promoted by Gamma Rays. Polymers 2025, 17, 600. https://doi.org/10.3390/polym17050600
López-Meza JE, Pino-Ramos VH, Flores-Rojas GG, Mendizabal E, Bucio E. Silicone Rubber Catheters Modified by Poly(N-vinylpyrrolidone) Graft Promoted by Gamma Rays. Polymers. 2025; 17(5):600. https://doi.org/10.3390/polym17050600
Chicago/Turabian StyleLópez-Meza, Jesús Enrique, Víctor Hugo Pino-Ramos, Guadalupe Gabriel Flores-Rojas, Eduardo Mendizabal, and Emilio Bucio. 2025. "Silicone Rubber Catheters Modified by Poly(N-vinylpyrrolidone) Graft Promoted by Gamma Rays" Polymers 17, no. 5: 600. https://doi.org/10.3390/polym17050600
APA StyleLópez-Meza, J. E., Pino-Ramos, V. H., Flores-Rojas, G. G., Mendizabal, E., & Bucio, E. (2025). Silicone Rubber Catheters Modified by Poly(N-vinylpyrrolidone) Graft Promoted by Gamma Rays. Polymers, 17(5), 600. https://doi.org/10.3390/polym17050600








