Adhesive Hemostatic Flake Particulates Composed of Calcium Alginate–Starch–Polyacrylamide/Poly(Acrylic Acid) Ionic Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Alpha-Starch Powder
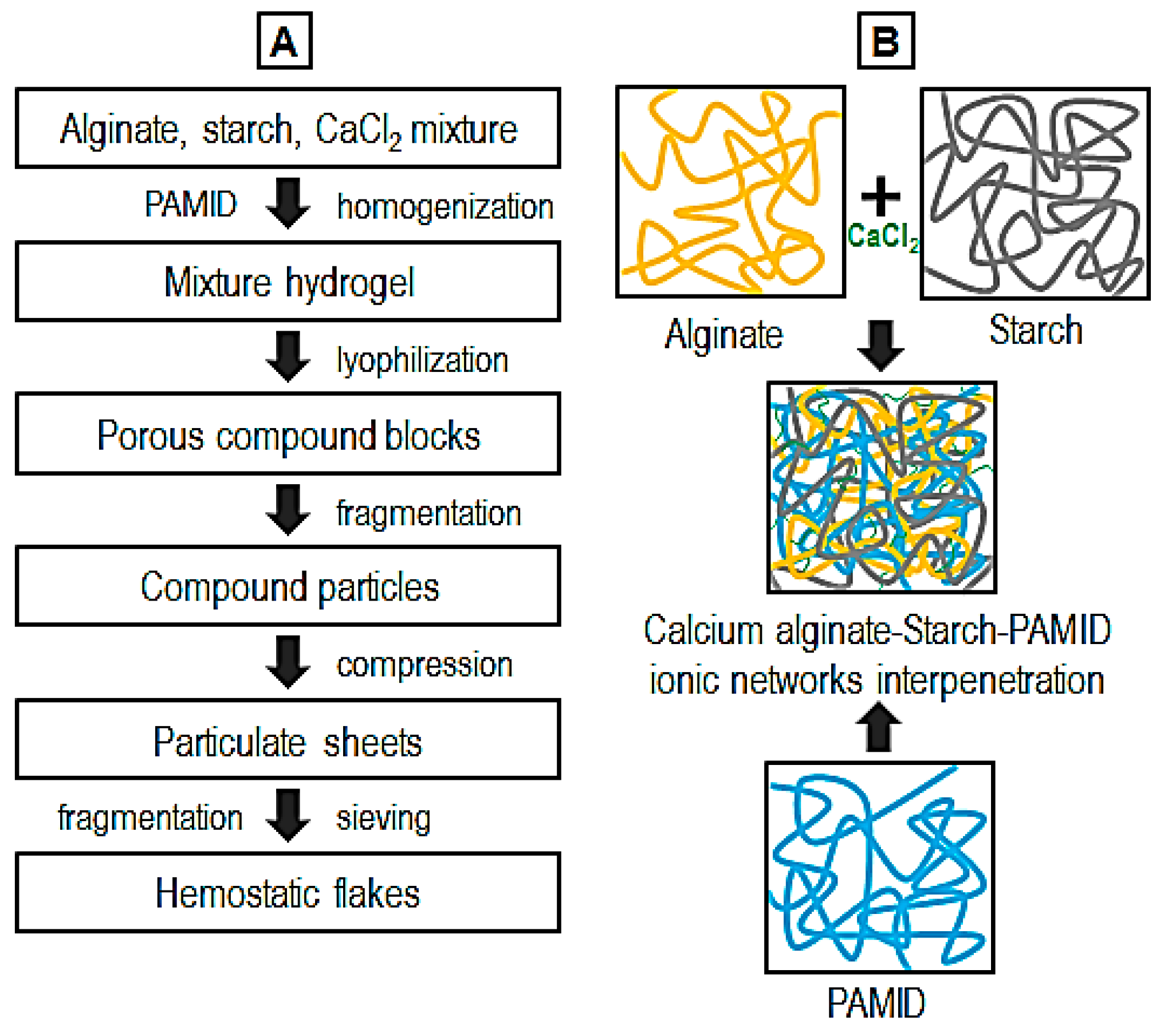
2.2. Fabrication of Calcium Alginate–Starch–Polyacrylamide/Poly(Acrylic Acid) Flakes
2.3. Chemical Analysis and Microstructure
2.4. Blood Infiltration Test
2.5. In Vitro Blood Absorption Amount and Rate
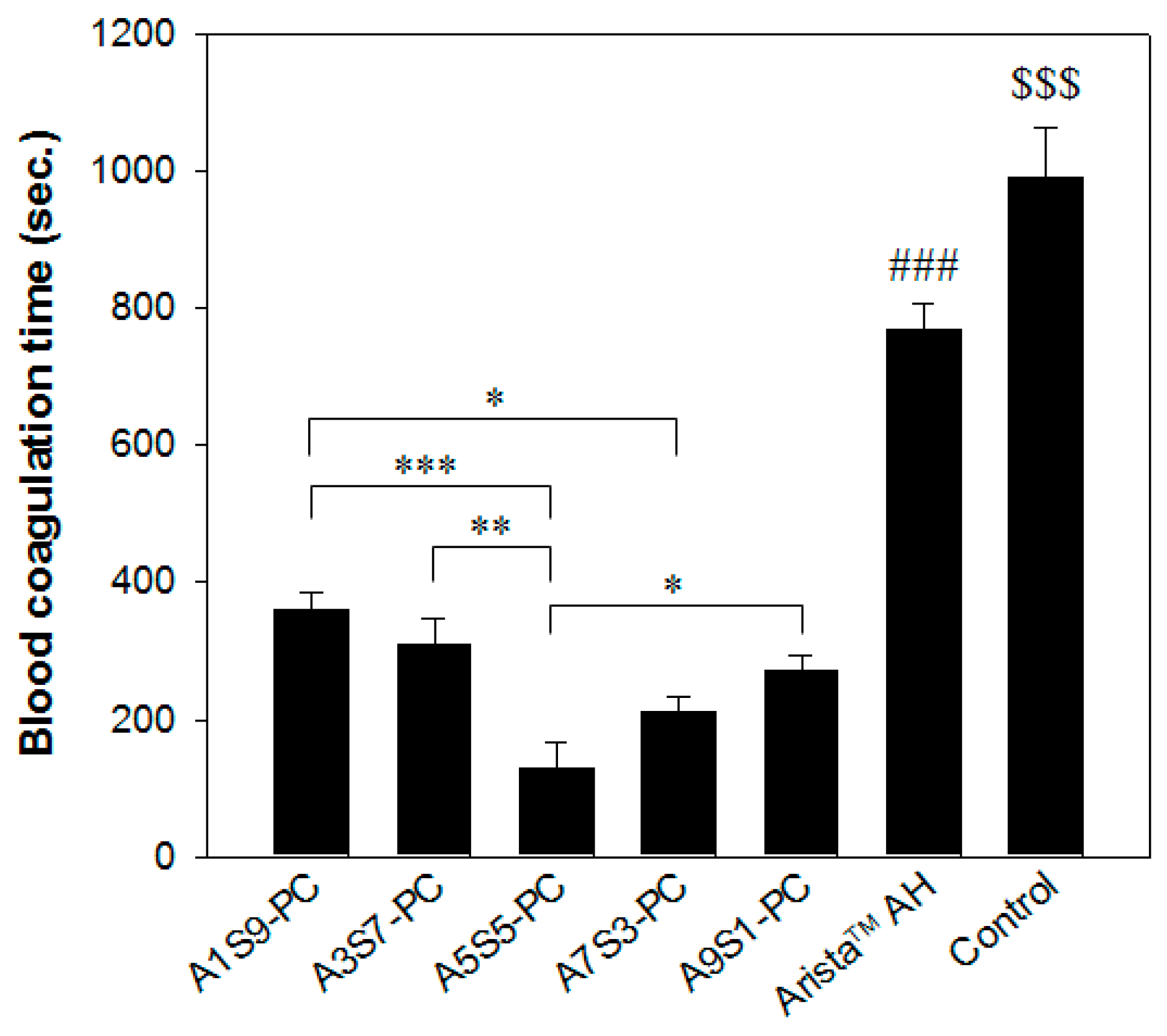
2.6. In Vitro Blood Coagulation Time (Lee–White Method)
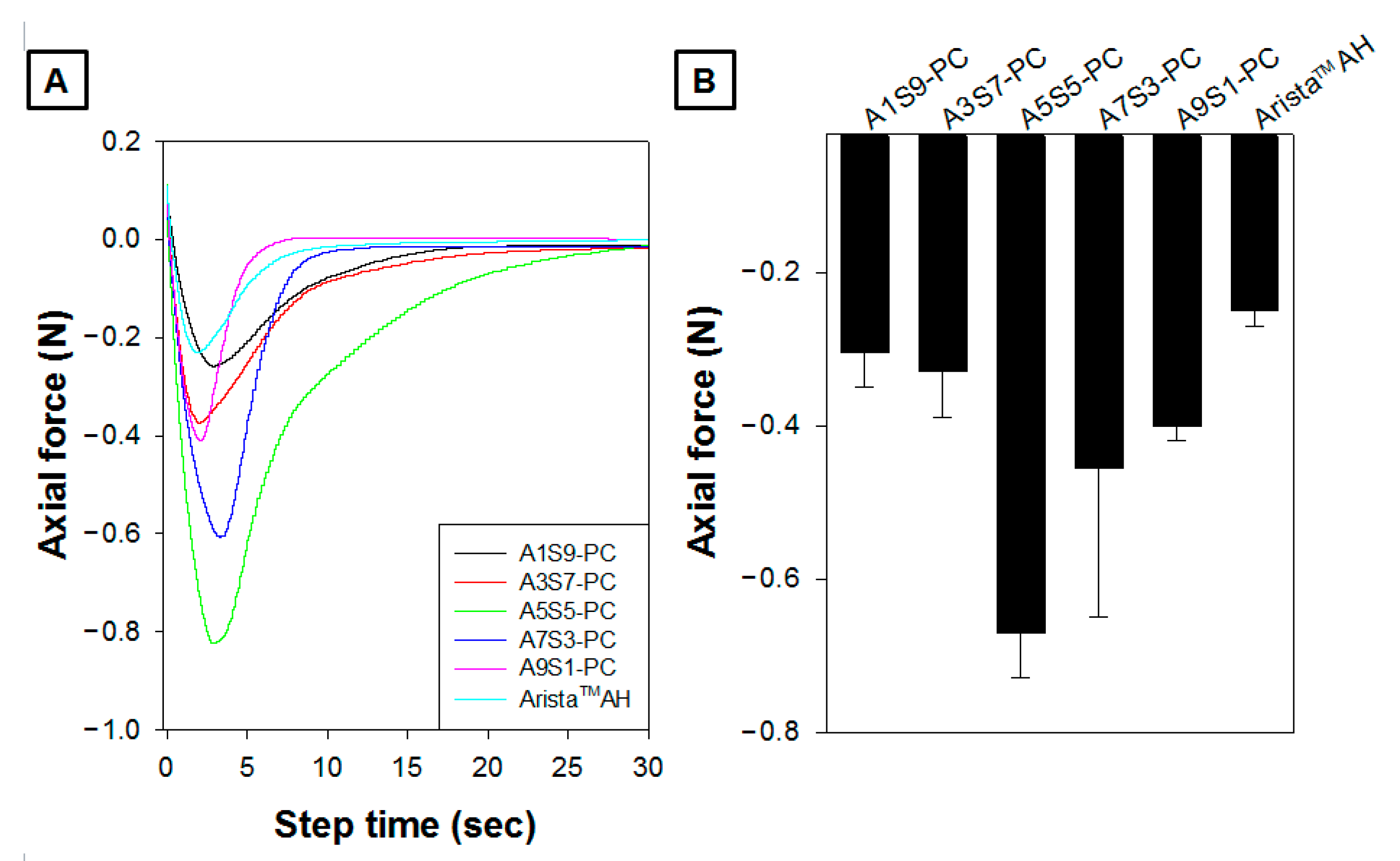
2.7. Tissue Adhesion Ability Test Using Rheometer
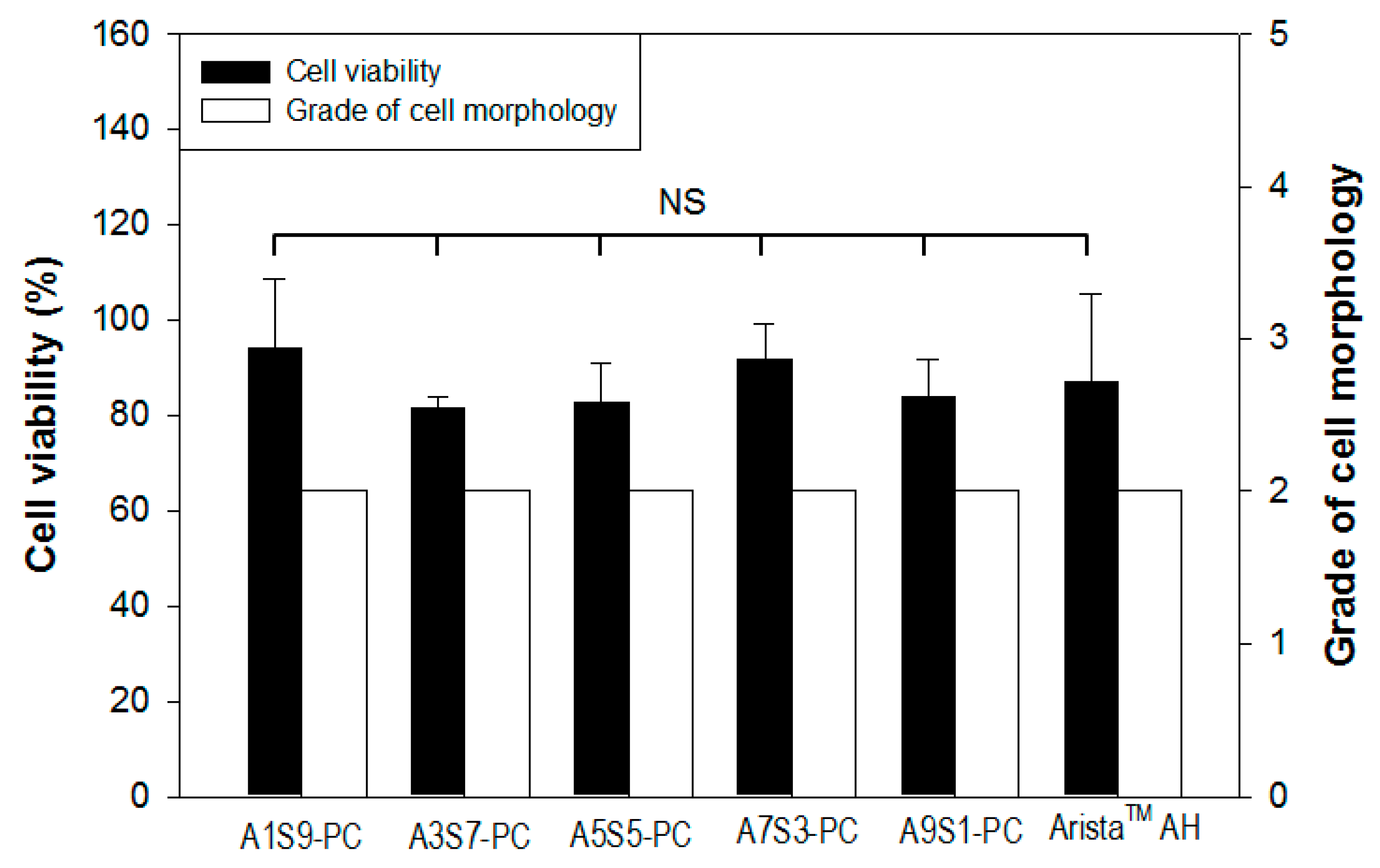
2.8. Cytotoxicity Test
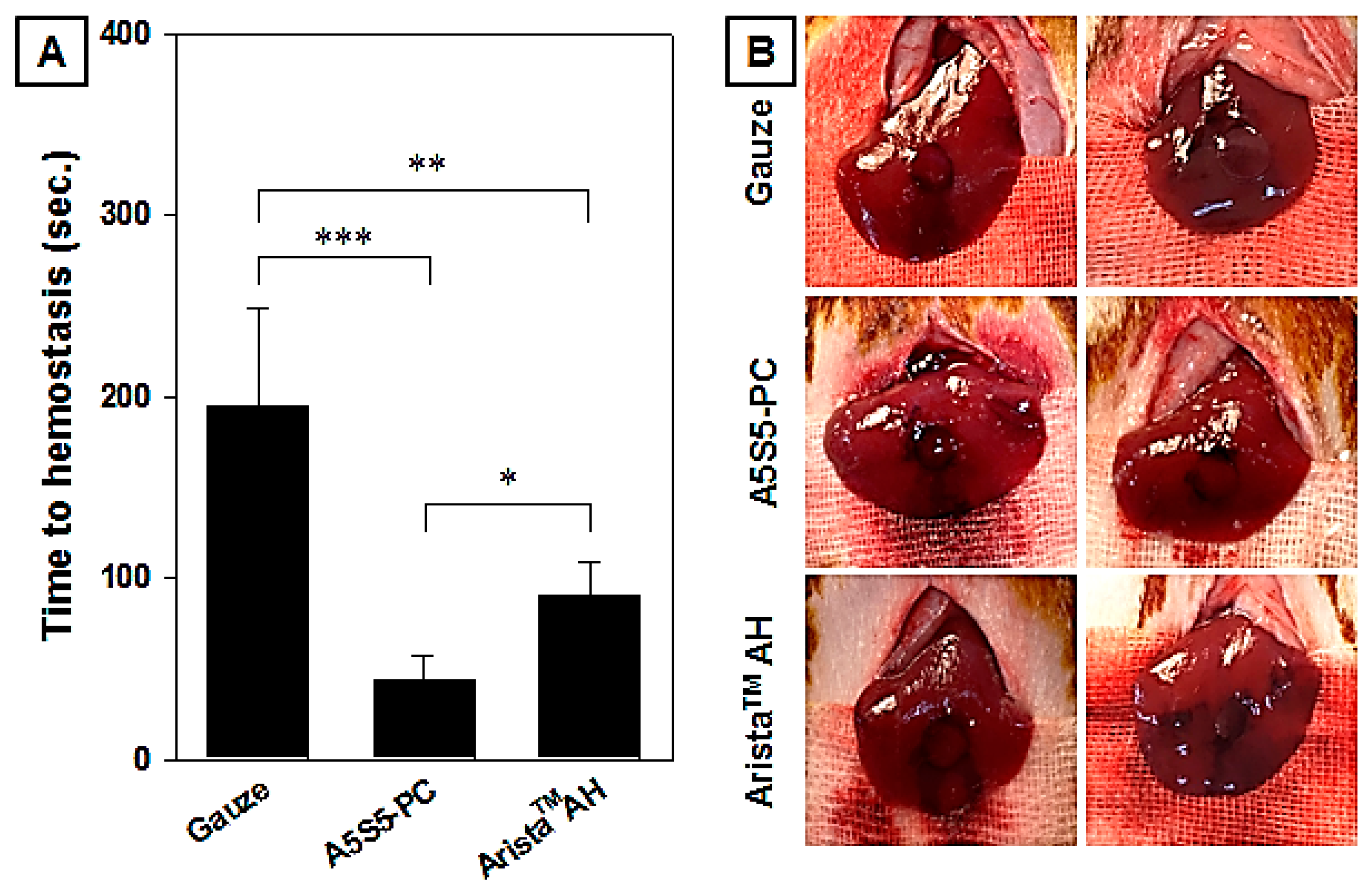
2.9. In Vivo Hemostasis
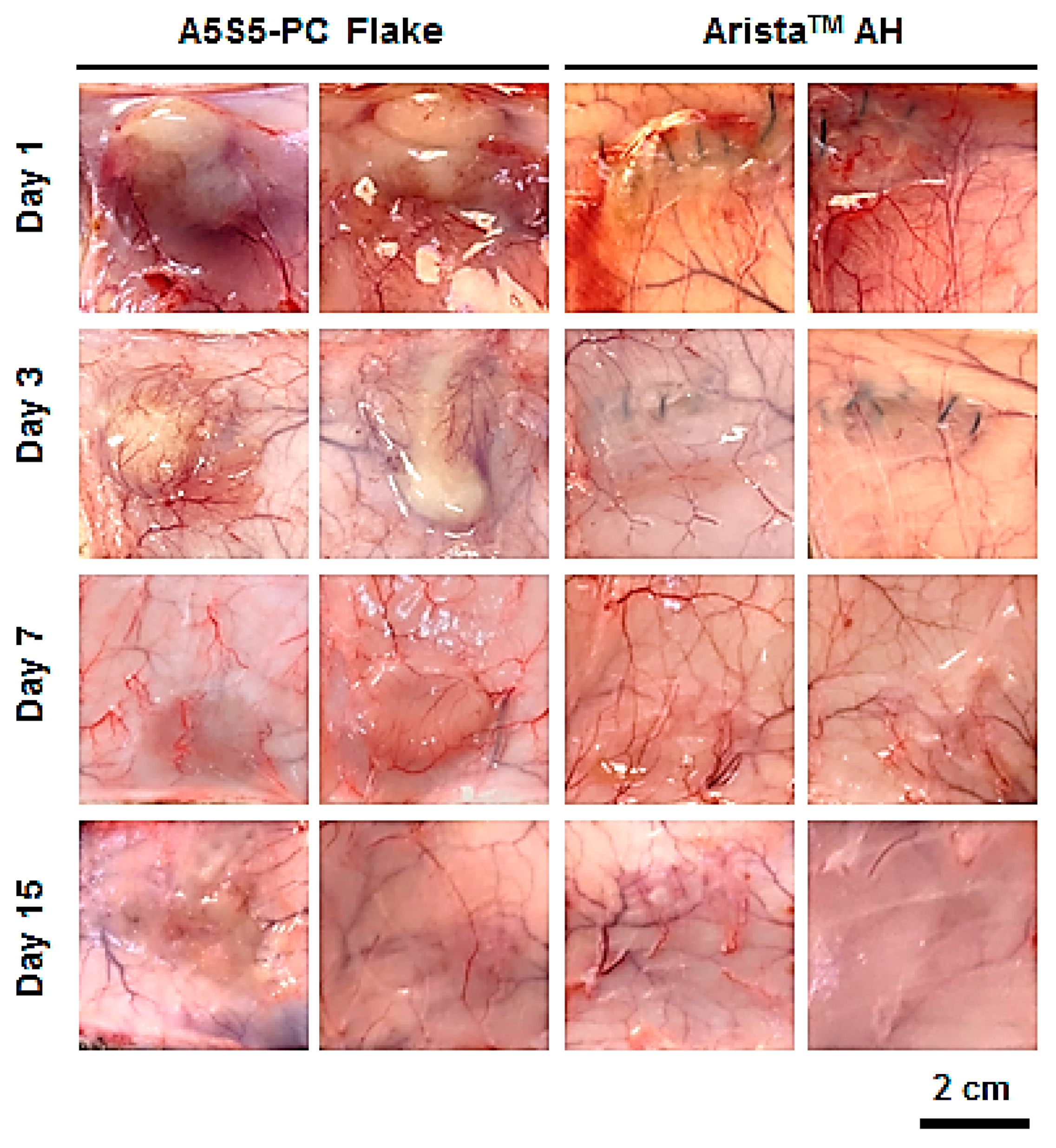
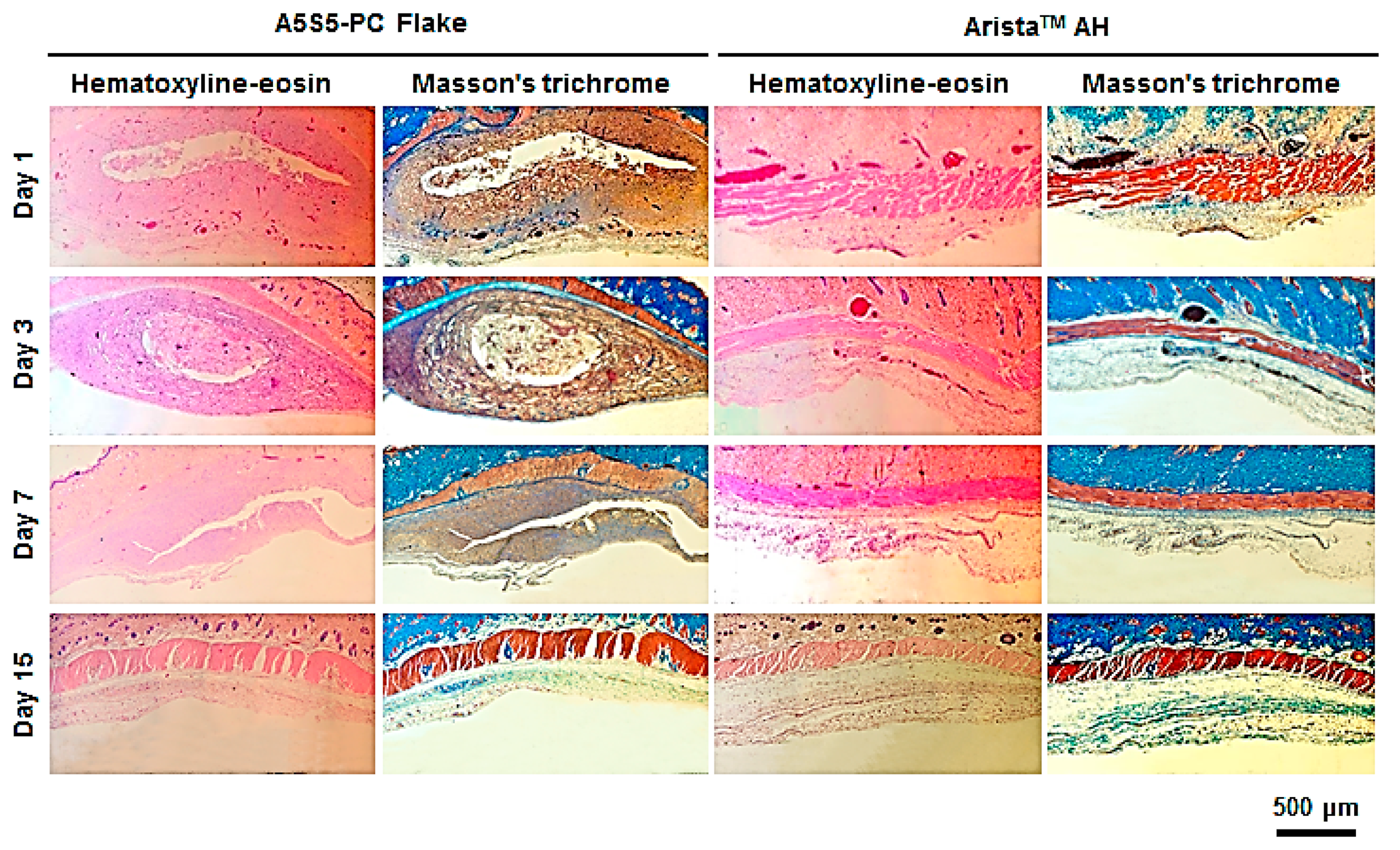
2.10. In Vivo Bioresorbability and Histology
2.11. Statistical Analysis
3. Results and Discussion
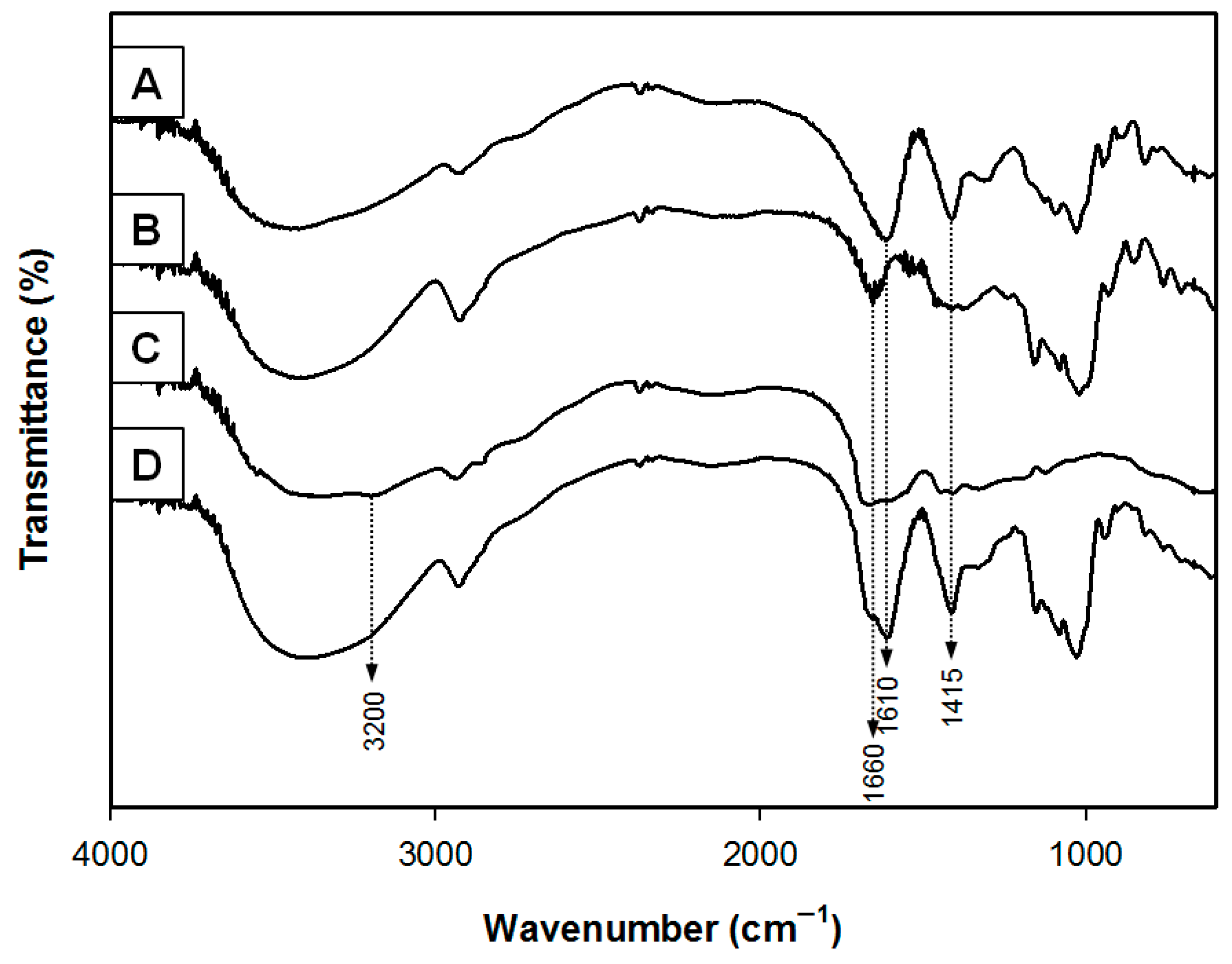
3.1. Chemical Analysis
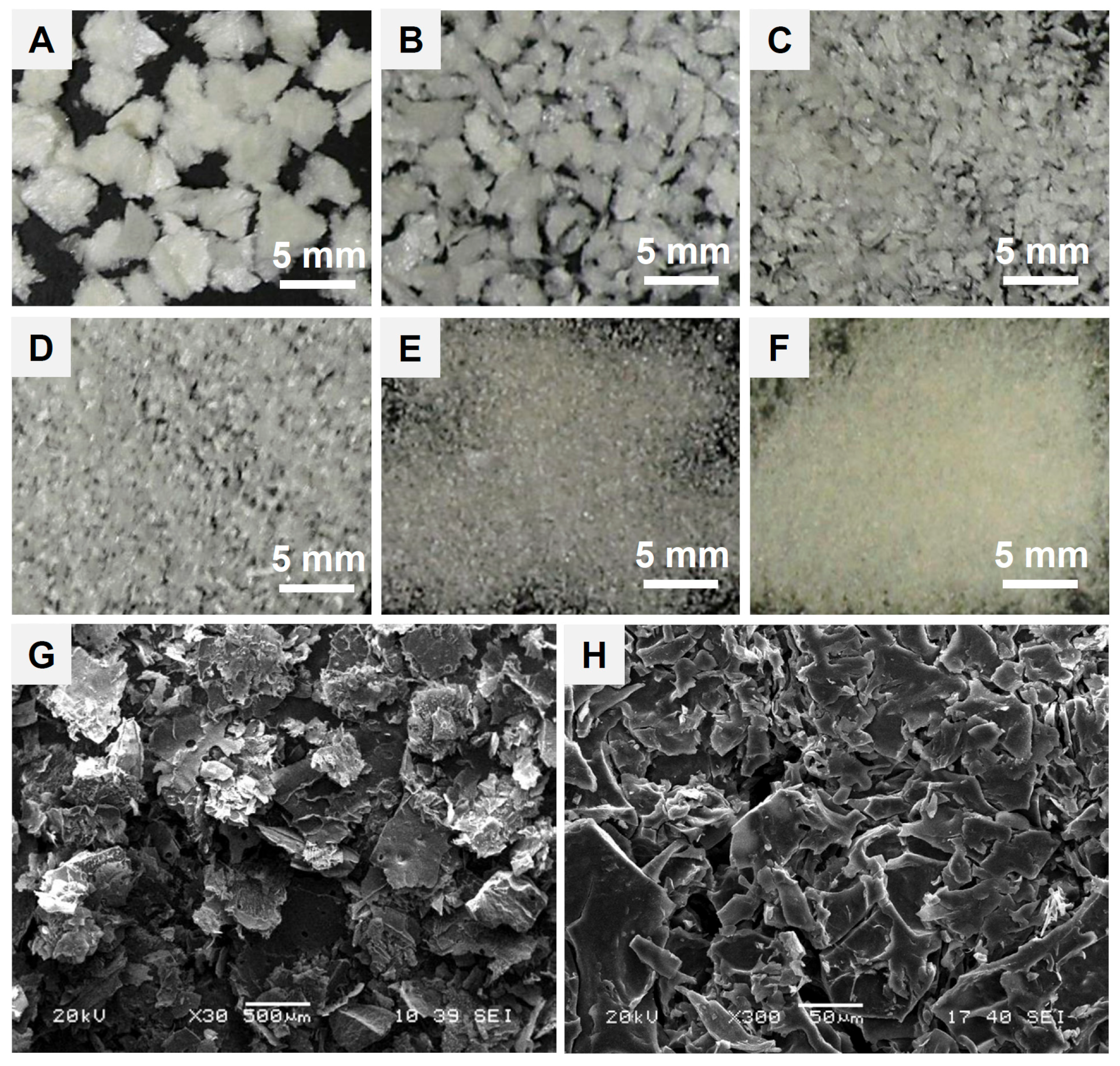
3.2. Microstructure of Hemostatic Flakes
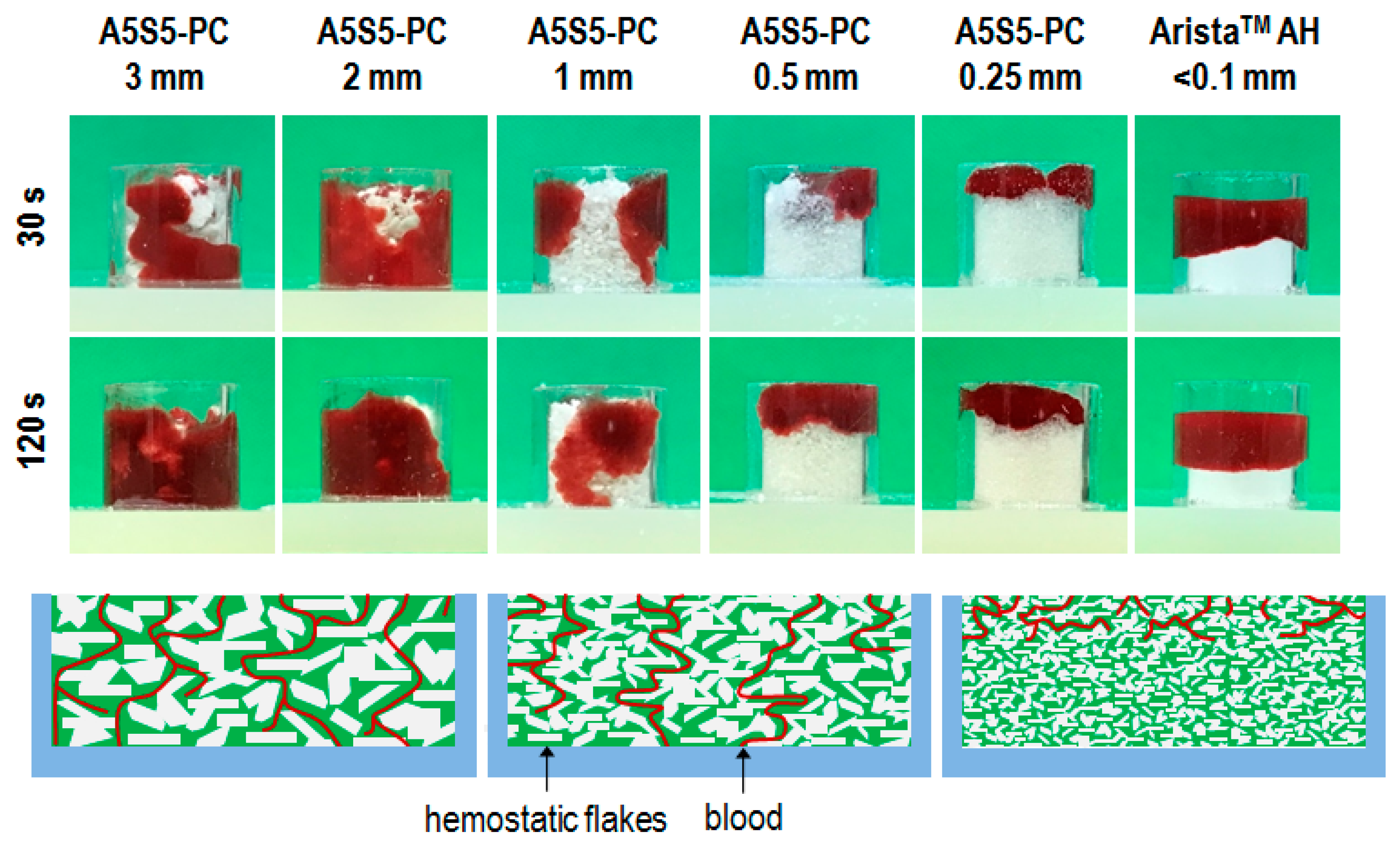
3.3. Blood Infiltration on Flake Dimension
3.4. In Vitro Blood Absorption and Coagulation Time
3.5. Tissue Adhesion Ability
3.6. Cytotoxicity
3.7. In Vivo Bioresorption and Histology
3.8. In Vivo Hemostasis Evaluation in Rat Hepatic Hemorrhage Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Turrentine, F.E.; Wang, H.; Simpson, V.B.; Jones, R.S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 2006, 203, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.B. Estimating allowable blood loss: Corrected for dilution. J. Am. Soc. Anesthesiol. 1983, 58, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.L.; Fauci, A.S.; Kasper, D.L.; Hauser, S.L.; Longo, D.L.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 20th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Vine, A.K. Recent advances in haemostasis and thrombosis. Retina 2009, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, C.P.; Keenan, A.C. Evolution of hemostatic agents in surgical practice. Indian J. Urol. 2010, 26, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Pogorielov, M.; Kalinkevich, O.; Deineka, V.; Garbuzova, V.; Solodovnik, A.; Kalinkevich, A.; Kalinichenko, T.; Gapchenko, A.; Sklyar, A.; Danilchenko, S. Haemostatic chitosan coated gauze: In vitro interaction with human blood and in-vivo effectiveness. Biomater. Res. 2015, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Oshima, G. Inhibition by calcium ions of thrombin. Thromb. Res. 1990, 58, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Montán, C.; Wannberg, M.; Holst, J.; Wahlgren, C.M. Perioperative haemorrhage in endovascular abdominal aneurysm repair affects outcome. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Hachem, L.D.; Ghanekar, A.; Selzner, M.; Famure, O.; Li, Y.; Kim, S.J. Postoperative surgical-site hemorrhage after kidney transplantation: Incidence, risk factors, and outcomes. Transpl. Int. 2017, 30, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.A.; Molenaar, I.Q.; Porte, R.J.; de Boer, M.T. Topical haemostatic agents in liver surgery: Do we need them? HPB 2009, 11, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Brustia, R.; Granger, B.; Scatton, O. An update on topical haemostatic agents in liver surgery: Systematic review and meta analysis. J. Hepatobiliary Pancreat. Sci. 2016, 23, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Antisdel, J.L.; Janney, C.G.; Long, J.P.; Sindwani, R. Hemostatic agent microporous polysaccharide hemospheres (MPH) does not affect healing or intact sinus mucosa. Laryngoscope 2008, 118, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-G.; Kim, B.N.; Kim, E.J.; Chung, H.Y.; Park, S.Y.; Kim, Y.-J.; Kwon, O.H. Bioabsorbable carboxymethyl starch–calcium ionic assembly powder as a hemostatic agent. Polymers 2022, 13, 3909. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Nose, Y. A new method for evalution of antithrombogenicity of materials. J. Biomed. Mater. Res. 1972, 6, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sezer, U.A.; Kocer, Z.; Aru, B.; Demirel, G.Y.; Gulmez, M.; Aktekin, A.; Ozkara, S.; Sezer, S. Combination of gelatin and tranexamic acid offers improved haemostasis and safe use on internal hemorrhage control. RSC Adv. 2016, 6, 95189–95198. [Google Scholar] [CrossRef]
- Panwar, V.; Sharma, A.; Thomas, J.; Chopra, V.; Kaushik, S.; Kumar, A.; Ghosh, D. In-vitro and In-vivo evaluation of biocompatible and biodegradable calcium-modified carboxymethyl starch as a topical hemostat. Materialia 2019, 7, 100373. [Google Scholar] [CrossRef]
- Xi, C.; Zhu, L.; Zhuang, Y.; Wang, S.; Sun, G.; Liu, Y.; Wang, D. Experimental evaluation of tranexamic acid–loaded porous starch as a hemostatic powder. Clin. Appl. Thromb./Hemost. 2018, 24, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Sim, E.J.; Ko, Y.-G.; Kim, E.J.; Kwon, O.K.; Kwon, O.H. Fabrication of Bioabsorbable Poly (vinyl pyrrolidone) Nanofibrous Sheets Containing Blood Coagulants for Hemostatic Application. Polymer Korea 2019, 43, 629–639. [Google Scholar] [CrossRef]
- Singh, R.K.; Baumgartner, B.; Mantei, J.R.; Parreno, R.N.; Sanders, P.J.; Lewis, K.M.; Barry, J.J. Hemostatic comparison of a polysaccharide powder and a gelatin powder. J. Investig. Surg. 2019, 32, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Behrens, A.M.; Sikorski, M.J.; Li, T.; Wu, Z.J.; Griffith, B.P.; Kofinas, P. Blood-aggregating hydrogel particles for use as a hemostatic agent. Acta Biomater. 2014, 10, 701–708. [Google Scholar] [CrossRef] [PubMed]

| Samples | Alginate (wt%) | Starch (wt%) | PAMID (wt%) | CaCl2 in Alginate (wt%) | Water (wt%) |
|---|---|---|---|---|---|
| A1S9-PC | 0.5 | 4.5 | 0.5 | 0.05 | 94.5 |
| A3S7-PC | 1.5 | 3.5 | 0.5 | 0.05 | 94.5 |
| A5S5-PC | 2.5 | 2.5 | 0.5 | 0.05 | 94.5 |
| A7S3-PC | 3.5 | 1.5 | 0.5 | 0.05 | 94.5 |
| A9S1-PC | 4.5 | 0.5 | 0.5 | 0.05 | 94.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, Y.; Kim, E.J.; Kwon, O.H. Adhesive Hemostatic Flake Particulates Composed of Calcium Alginate–Starch–Polyacrylamide/Poly(Acrylic Acid) Ionic Networks. Polymers 2025, 17, 568. https://doi.org/10.3390/polym17050568
Ko Y, Kim EJ, Kwon OH. Adhesive Hemostatic Flake Particulates Composed of Calcium Alginate–Starch–Polyacrylamide/Poly(Acrylic Acid) Ionic Networks. Polymers. 2025; 17(5):568. https://doi.org/10.3390/polym17050568
Chicago/Turabian StyleKo, Yunjeh, Eun Jin Kim, and Oh Hyeong Kwon. 2025. "Adhesive Hemostatic Flake Particulates Composed of Calcium Alginate–Starch–Polyacrylamide/Poly(Acrylic Acid) Ionic Networks" Polymers 17, no. 5: 568. https://doi.org/10.3390/polym17050568
APA StyleKo, Y., Kim, E. J., & Kwon, O. H. (2025). Adhesive Hemostatic Flake Particulates Composed of Calcium Alginate–Starch–Polyacrylamide/Poly(Acrylic Acid) Ionic Networks. Polymers, 17(5), 568. https://doi.org/10.3390/polym17050568








