Fabrication and In Vitro Evaluation of LL37-Loaded Electrospun PHB/Collagen Nanofibers for Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Method
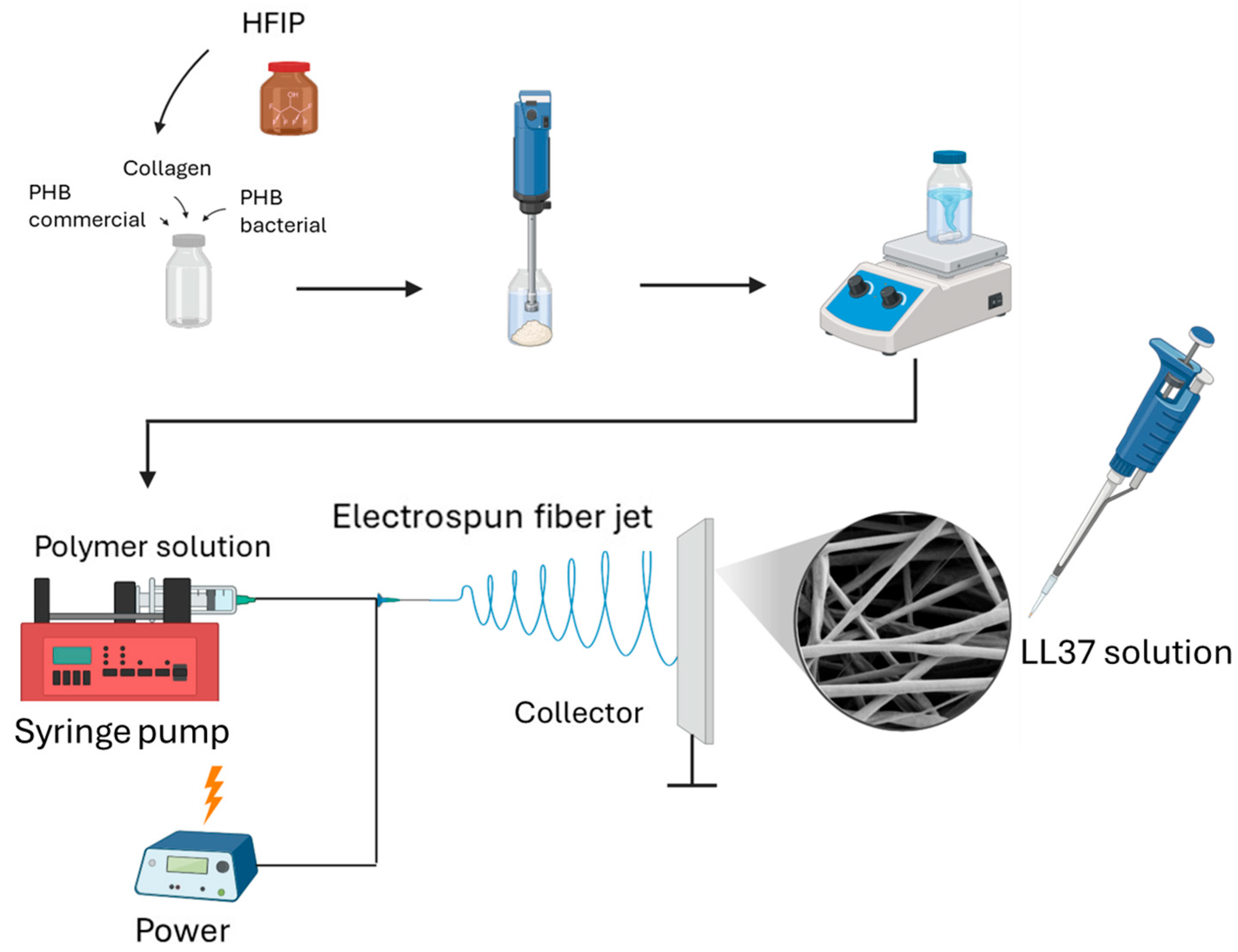
2.2.1. Development of LL37-Loaded PHB/Col Nanofiber Mats
2.2.2. Physicochemical Characterization of LL37/PHB/Col Nanofiber Mats
2.2.3. Degradation of Nanofibers
2.2.4. In Vitro Biocompatibility and Cell Attachment on Nanofibers
2.2.5. Measurement of 2D Wound Healing Activity by Scratch Assay
2.2.6. Evaluation of Antibacterial Activity of Nanofiber Mats
3. Results and Discussion
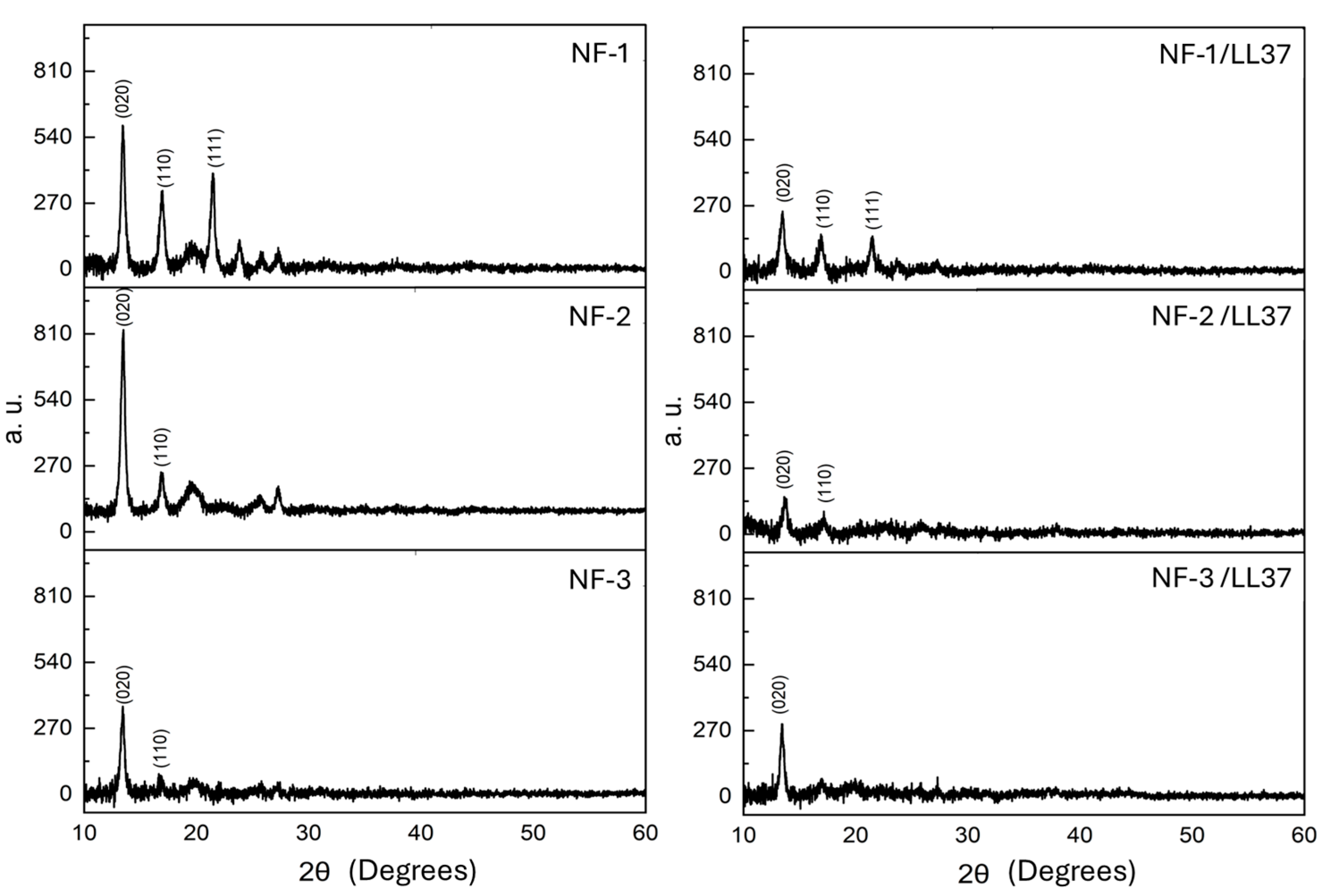
3.1. Physicochemical Characteristics of PHB/Col/LL37 Nanofiber Mats
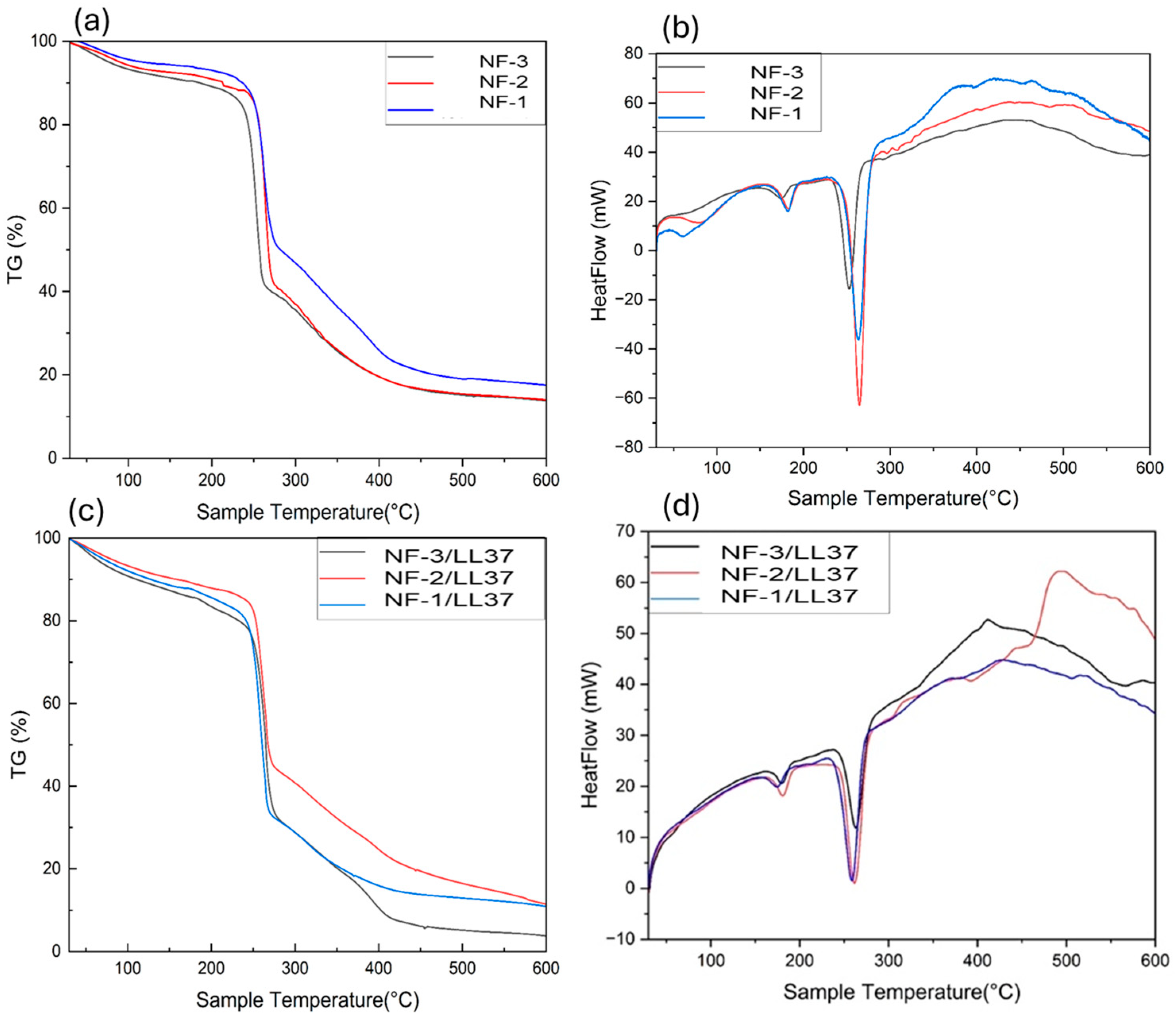
3.2. Degradation of Nanofibers
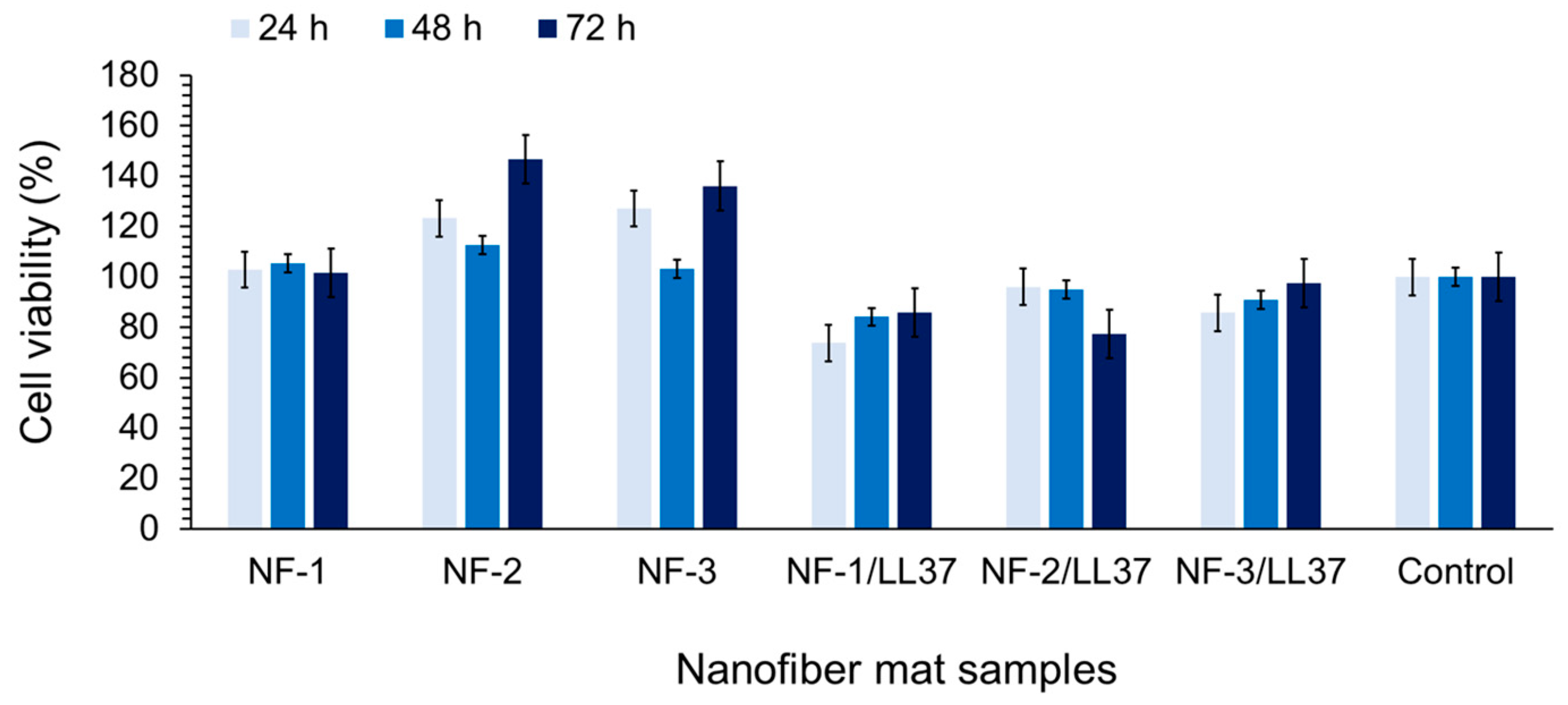
3.3. Biocompatibility and Cell Attachment on Nanofibers
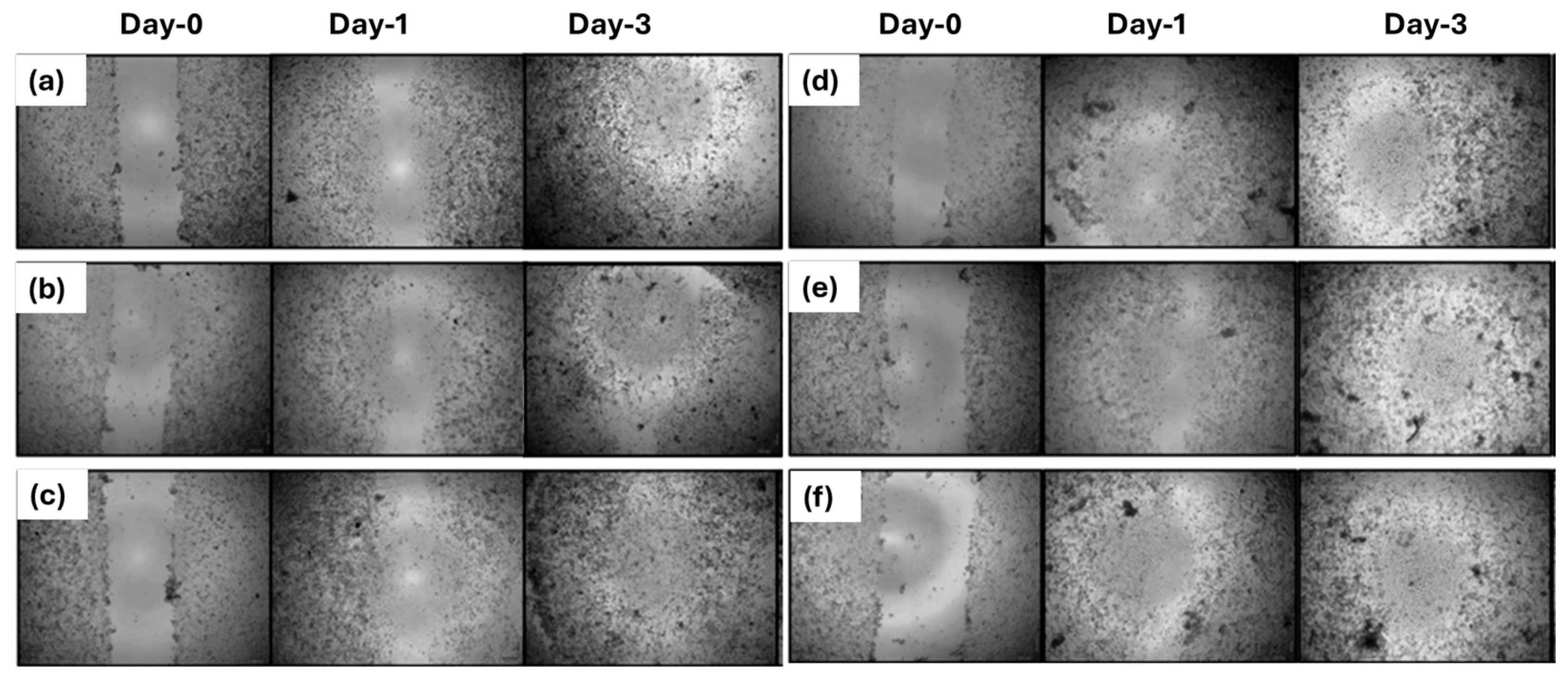
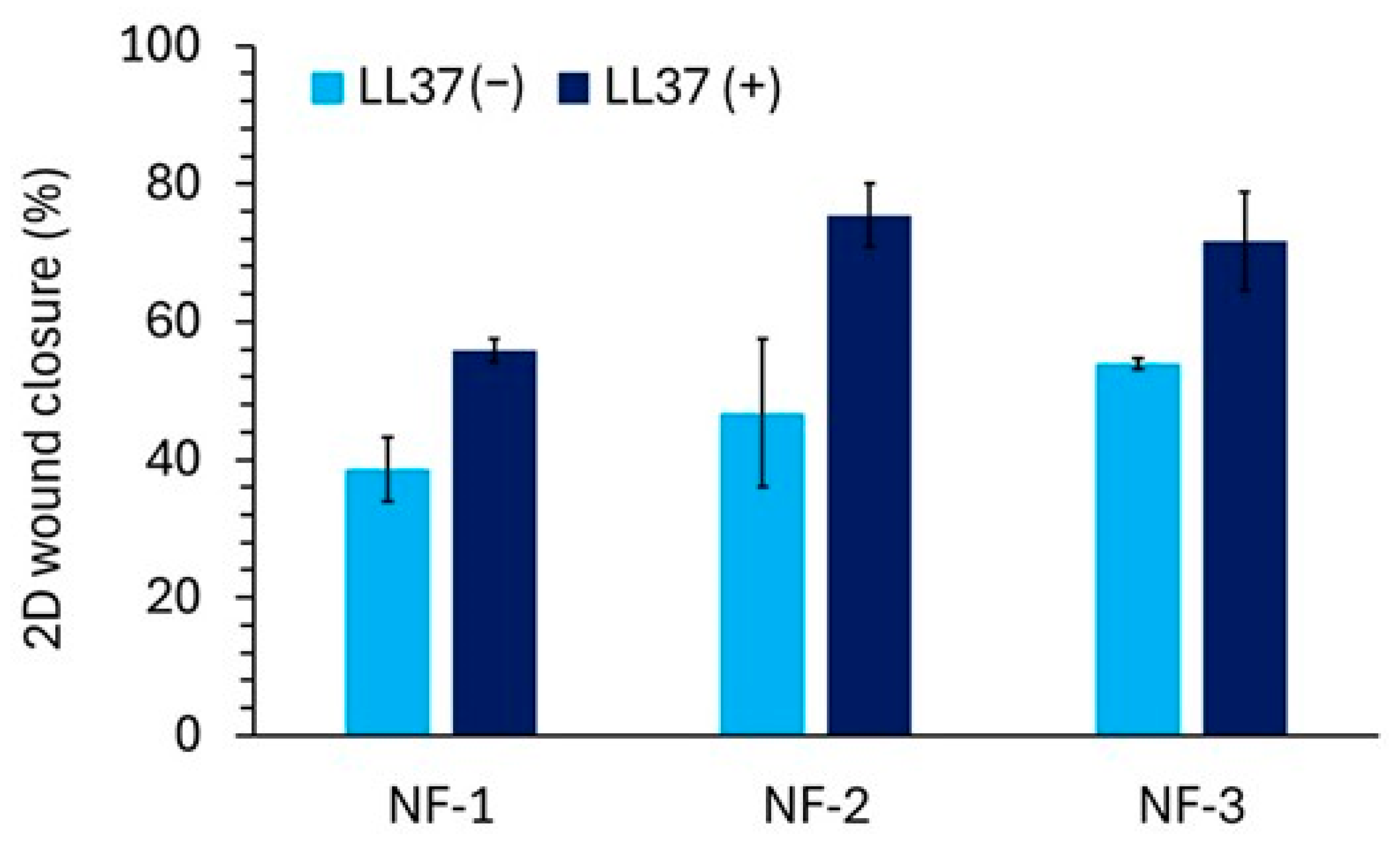
3.4. Two-Dimensional Wound Healing Activity of Nanofiber Mats
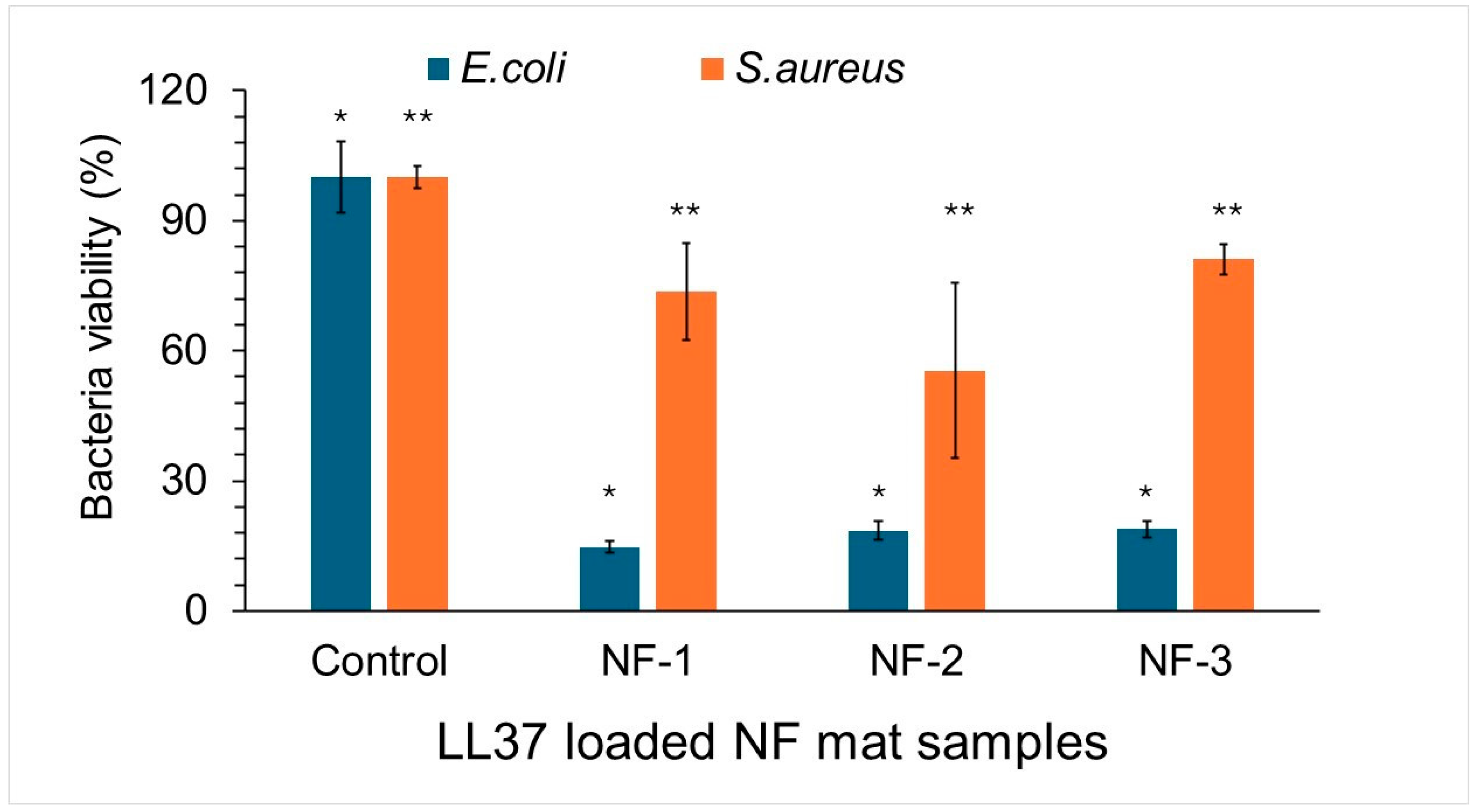
3.5. Antibacterial Activity of Nanofiber Mats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Seidi, F.; Azarfam, M.Y.; Yazdi, M.K.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J.; et al. Biopolymer-based composites for tissue engineering applications: A basis for future opportunities. Compos. Part B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Chen, W.-C.; Wu, H.-S.; Janarthanan, O.-M. Biodegradable and Biocompatible Biomaterial, Polyhydroxybutyrate, Produced by an Indigenous Vibrio sp. BM-1 Isolated from Marine Environment. Mar. Drugs 2011, 9, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Roohi; Zaheer, M.R.; Kuddus, M. PHB (poly-β-hydroxybutyrate) and its enzymatic degradation. Polym. Adv. Technol. 2018, 29, 30–40. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Ren, K.; Xu, T.; Zhang, Z.; Xu, M.; Rao, Z.; Zhang, X. A Review of Antimicrobial Peptides: Structure, Mechanism of Action, and Molecular Optimization Strategies. Fermentation 2024, 10, 540. [Google Scholar] [CrossRef]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Akpınar, B.N. Production of Polyhydroxybutyrate (PHB) by Cereibacter sphaeroides O.U. 001, Rhodopseudomonas palustris 7850 and Cupriavidus necator H16 Grown in Acetate Medium and Characterization of Produced Polymers. Master’s Thesis, Necmettin Erbakan University, Konya, Türkiye, 2023. [Google Scholar]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Ng, V.W.L.; Ono, R.J.; Chan, J.M.W.; Krishnamurthy, S.; Wang, Y.; Hedrick, J.L.; Yang, Y.Y. Role of non-covalent and covalent interactions in cargo loading capacity and stability of polymeric micelles. J. Control. Release 2014, 193, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Carofiglio, V.E.; Stufano, P.; Bonfrate, V.; Calò, E.; Scarlino, S.; Nitti, P.; Centrone, D.; Cascione, M.; Leporatti, S.; et al. Potential of Electrospun Poly(3-hydroxybutyrate)/Collagen Blends for Tissue Engineering Applications. J. Healthc. Eng. 2018, 2018, 6573947. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Vatankhah, E.; Ramakrishna, S. Electrospun aligned PHBV/collagen nanofibers as substrates for nerve tissue engineering. Biotechnol. Bioeng. 2013, 110, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Rizvi, S.F.; Asif, M.; Ahmad, A.; Alshammari, M.B.; Gupta, A.; Zaheer, M.R.; Roohi, R. Polyhydroxybutyrate (PHB) bioplastic characterization from the isolate Pseudomonas stutzeri PSB1 synthesized using potato peel feedstock to combat solid waste management. Biocatal. Agric. Biotechnol. 2024, 57, 103097. [Google Scholar] [CrossRef]
- Frone, A.N.; Nicolae, C.A.; Eremia, M.C.; Tofan, V.; Ghiurea, M.; Chiulan, I.; Radu, E.; Damian, C.M.; Panaitescu, D.M. Low molecular weight and polymeric modifiers as toughening agents in poly(3-hydroxybutyrate) films. Polymers 2020, 12, 2446. [Google Scholar] [CrossRef]
- Pradhan, S.; Dikshit, P.K.; Moholkar, V.S. Production, ultrasonic extraction, and characterization of poly(3-hydroxybutyrate) (PHB) using Bacillus megaterium and Cupriavidus necator. Polym. Adv. Technol. 2018, 29, 2392–2400. [Google Scholar] [CrossRef]
- de Sousa Junior, R.R.; Dos Santos, C.A.S.; Ito, N.M.; Suqueira, A.N.; Lackner, M.; Dos Santos, D.J. PHB processability and property improvement with linear-chain polyester oligomers used as plasticizers. Polymers 2022, 14, 4197. [Google Scholar] [CrossRef]
- Farrag, Y.; Barral, L.; Gualillo, O.; Moncada, D.; Montero, B.; Rico, M.; Bouza, R. Effect of different plasticizers on thermal, crystalline, and permeability properties of poly(3–hydroxybutyrate–co−3–hydroxyhexanoate) films. Polymers 2022, 14, 3503. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In vivo and post-synthesis strategies to enhance the properties of PHB-based materials: A review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef]
- Sarıipek, F.B. Biopolymeric nanofibrous scaffolds of poly(3-hydroxybutyrate)/chitosan loaded with biogenic silver nanoparticle synthesized using curcumin and their antibacterial activities. Int. J. Biol. Macromol. 2024, 256, 128330. [Google Scholar] [CrossRef]
- Gharahgheshlagh, S.N.; Ghadimi, T.; Latifi, N.; Forghani, S.F.; Milan, P.B.; Hivechi, A.; Sarmadi, V.H.; Arabsorkhi-Mishabi, A.; Amini, N.; Saboury, M.; et al. Fabricating modified cotton wound dressing via exopolysaccharide-incorporated marine collagen nanofibers. Mater. Today Commun. 2024, 39, 108706. [Google Scholar] [CrossRef]
- Li, C.; Du, L.; Xiao, Y.; Fan, L.; Li, Q.; Cao, C.Y. Multi-active phlorotannins boost antimicrobial peptide LL-37 to promote periodontal tissue regeneration in diabetic periodontitis. Mater. Today Bio 2025, 31, 101535. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, M.; Behzad, T.; Karbasi, S.; Khorzoghi, M.B.; Mohammadalipour, Z. Osteogenic potential of PHB-lignin/cellulose nanofiber electrospun scaffold as a novel bone regeneration construct. Int. J. Biol. Macromol. 2023, 250, 126076. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Khaki, M.; Ghaznavi-Rad, E.; Abtahi, H. Investigation of a novel bilayered PCL/PVA electrospun nanofiber incorporated chitosan-LL37 and chitosan-VEGF nanoparticles as an advanced antibacterial cell growth-promoting wound dressing. Int. J. Pharm. 2024, 661, 124341. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, X.; Gao, F.; Zang, M.; Huang, L.; Liu, W.; Xu, J.; Yu, S.; Wang, T.; Sun, H.; et al. Fighting bacteria with bacteria: A biocompatible living hydrogel patch for combating bacterial infections and promoting wound healing. Acta Biomater. 2024, 181, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Hu, C.; Liu, W.; Wu, C.; Lu, L.; Yang, L.; Wang, Y. Injectable multifunctional hyaluronic acid/methylcellulose hydrogels for chronic wounds repairing. Carbohydr. Polym. 2022, 289, 119456. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of specialized biomolecules against Gram-positive and Gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Dong, P.; Wang, J.; Wang, L.; Zhao, A.; Qu, G.; Li, H.; Gunarathne, K.D.M.; Zhang, W.; et al. Thermosensitive-based synergistic antibacterial effects of novel LL37@ZPF-2 loaded poloxamer hydrogel for infected skin wound healing. Int. J. Pharm. 2025, 670, 125210. [Google Scholar] [CrossRef]




| Nanofiber Types | PHB(b) */PHB(c) ** (w/w) | Collagen (w) | Solvent |
|---|---|---|---|
| NF-1: %0 bacterial PHB/Collagen | 0 g PHB(b)/0.2 g PHB(c) | 0.2 g | HFIP |
| NF-2: %25 bacterial PHB/Collagen | 0.05 g PHB(b)/0.15 g PHB(c) | 0.2 g | HFIP |
| NF-3: %50 bacterial PHB/Collagen | 0.1 g PHB(b)/0.1 g PHB(c) | 0.2 g | HFIP |
| NF-4: %75 bacterial PHB/Collagen | 0.15 g PHB(b)/0.05 g PHB(c) | 0.2 g | HFIP |
| NF-5: %100 bacterial PHB/Collagen | 0.2 g PHB(b)/0 g PHB(c) | 0.2 g | HFIP |
| Functional Group | Wavenumbers (cm−1) |
|---|---|
| CH3 | 1380 |
| CH2 | 1450 |
| C-H | 2976–2932 |
| C=O | 1724–1719 |
| C-O | 1280–1275 |
| O-H | 3436 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayaner Taşçı, B.N.; Kozan, S.; Demirel Kars, M.; Çetin, K.; Karslıoğlu, S.; Kars, G. Fabrication and In Vitro Evaluation of LL37-Loaded Electrospun PHB/Collagen Nanofibers for Wound Healing. Polymers 2025, 17, 2486. https://doi.org/10.3390/polym17182486
Sayaner Taşçı BN, Kozan S, Demirel Kars M, Çetin K, Karslıoğlu S, Kars G. Fabrication and In Vitro Evaluation of LL37-Loaded Electrospun PHB/Collagen Nanofibers for Wound Healing. Polymers. 2025; 17(18):2486. https://doi.org/10.3390/polym17182486
Chicago/Turabian StyleSayaner Taşçı, Beyza Nur, Sümeyye Kozan, Meltem Demirel Kars, Kemal Çetin, Sema Karslıoğlu, and Gökhan Kars. 2025. "Fabrication and In Vitro Evaluation of LL37-Loaded Electrospun PHB/Collagen Nanofibers for Wound Healing" Polymers 17, no. 18: 2486. https://doi.org/10.3390/polym17182486
APA StyleSayaner Taşçı, B. N., Kozan, S., Demirel Kars, M., Çetin, K., Karslıoğlu, S., & Kars, G. (2025). Fabrication and In Vitro Evaluation of LL37-Loaded Electrospun PHB/Collagen Nanofibers for Wound Healing. Polymers, 17(18), 2486. https://doi.org/10.3390/polym17182486









