Study on the Mechanical and Thermal Properties of Waterborne Polyurethane-Modified Aluminum Hydroxide and Its Application in LDPE Plastics
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Modification
2.3. Preparation of LDPE-Based Composites
2.4. Characterizations
2.4.1. Contact Angle
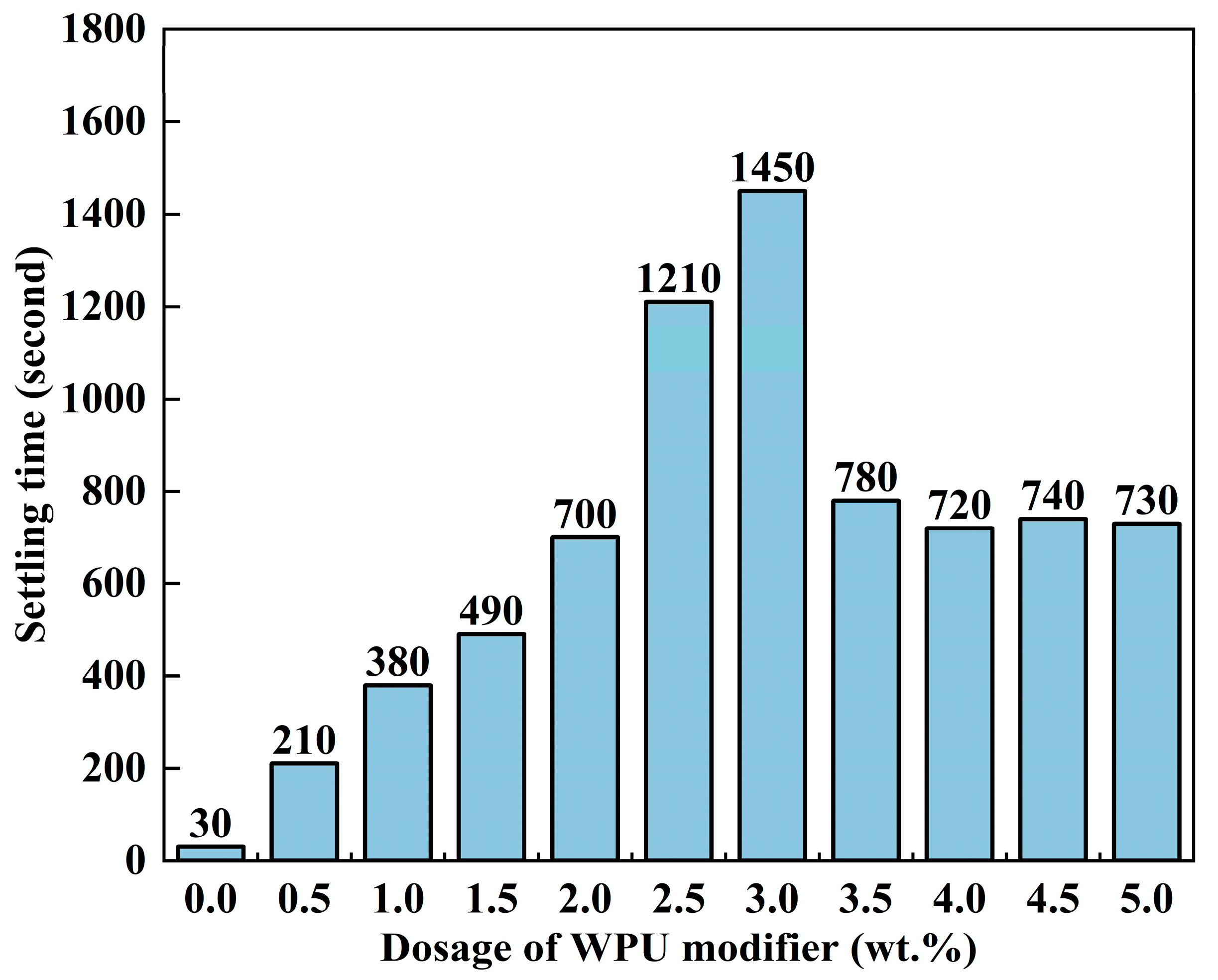
2.4.2. Settling Time
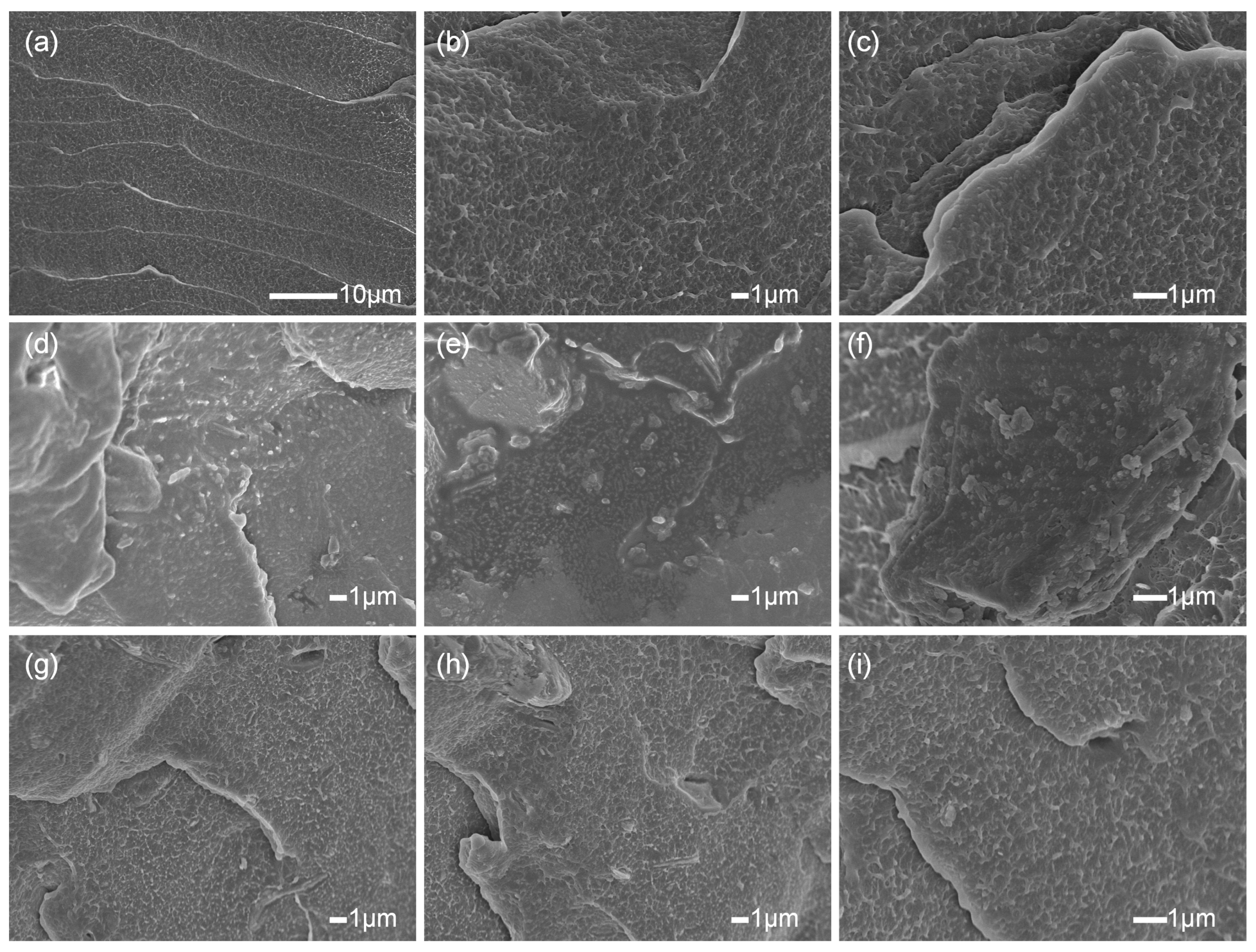
2.4.3. SEM Analysis
2.4.4. XRD Analysis
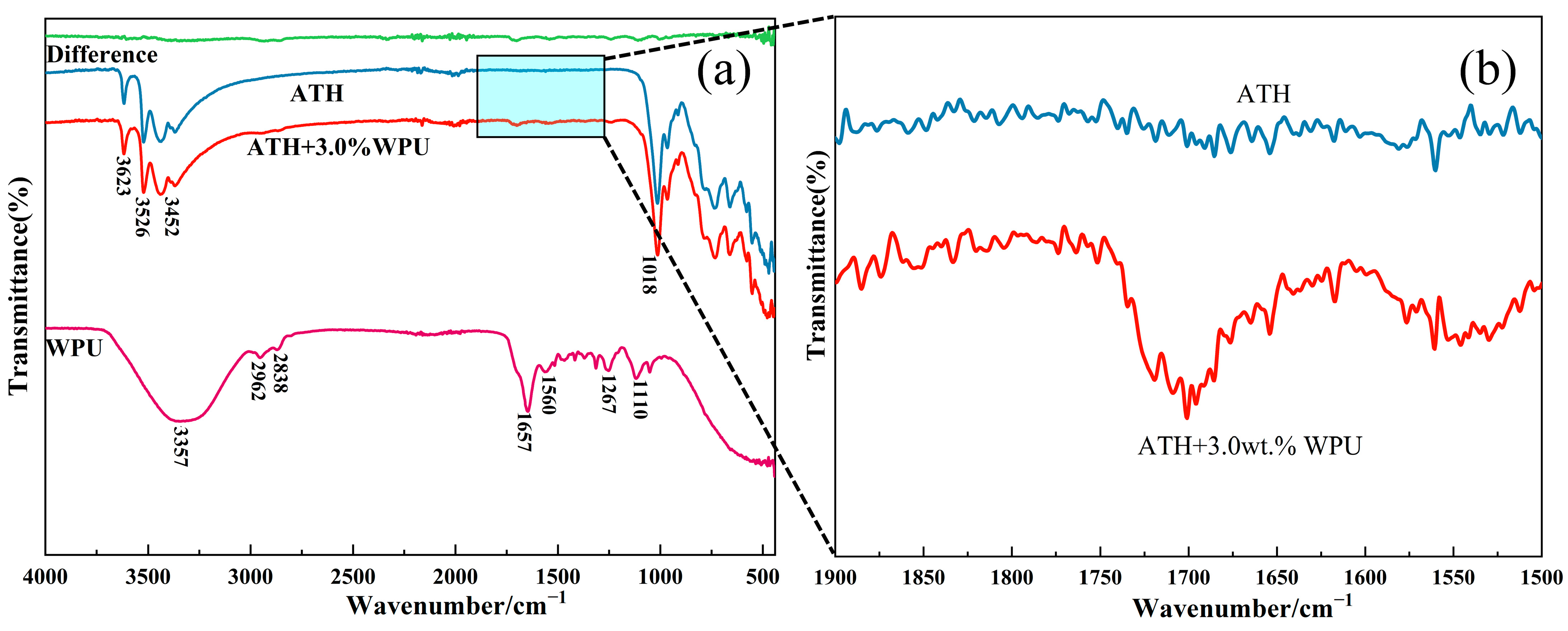
2.4.5. FTIR Analysis
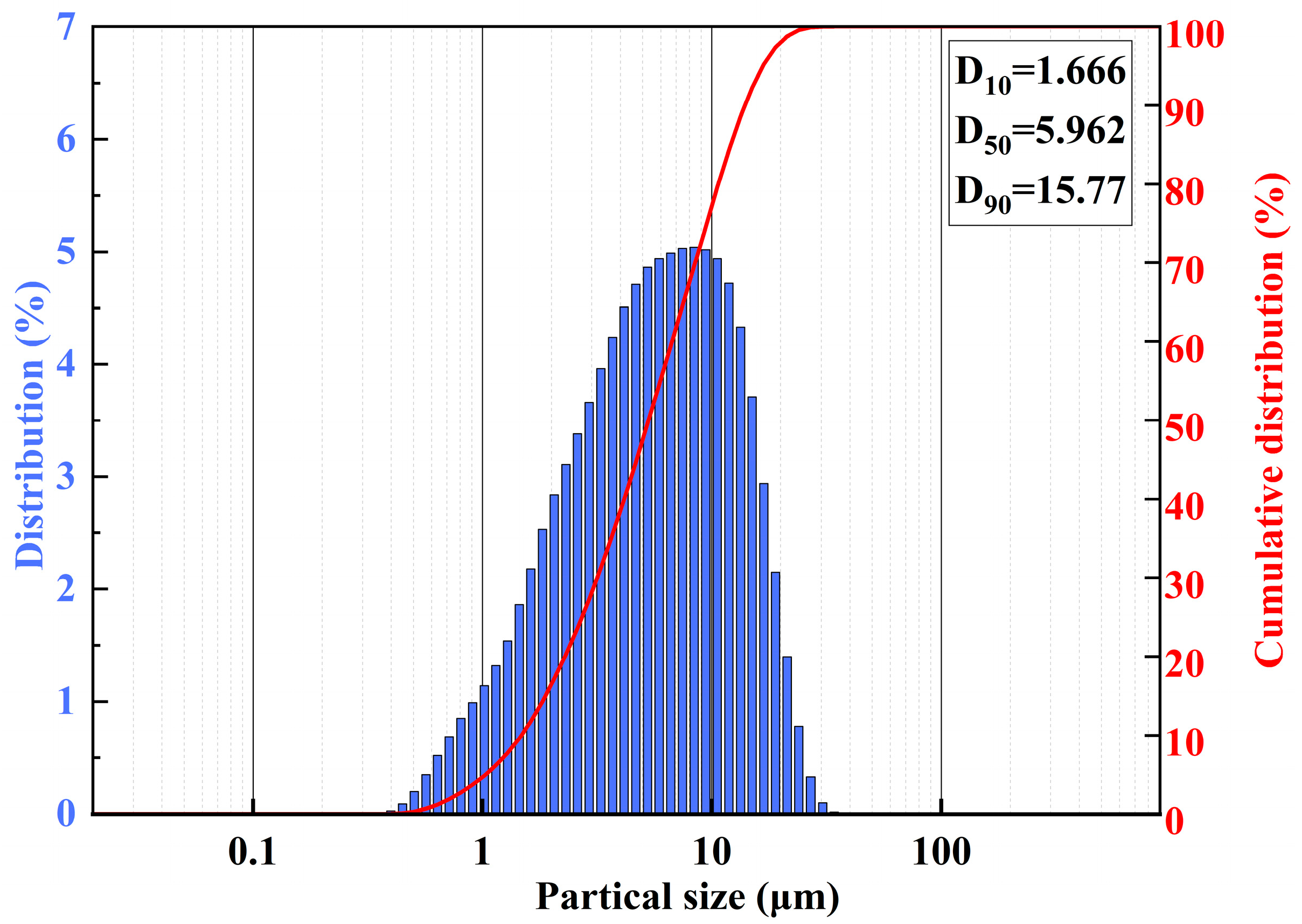
2.4.6. Particle Size Distribution
2.4.7. TGA
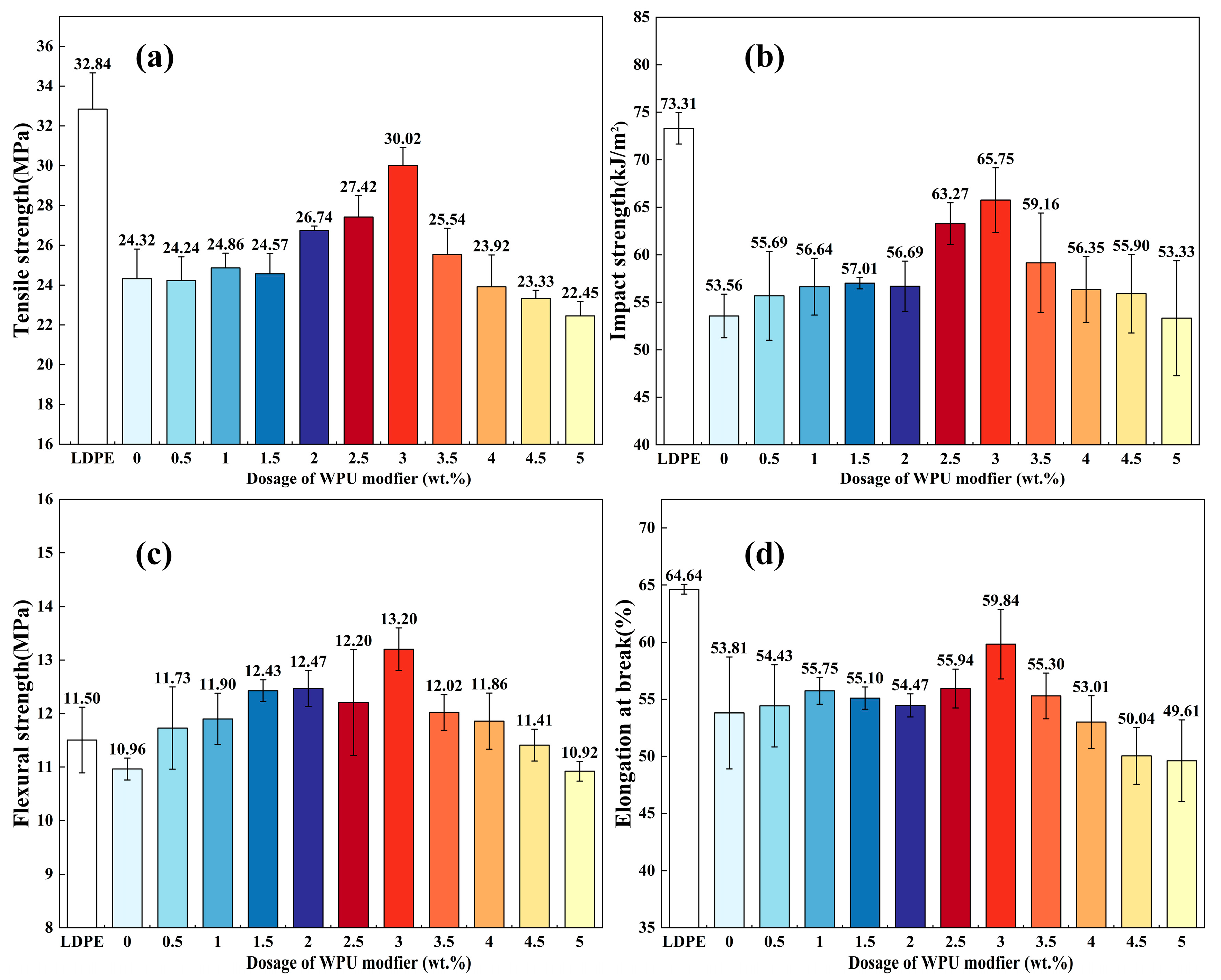
2.4.8. Mechanical Tests
3. Results and Discussion
3.1. Characterization of ATH Particles
3.1.1. Performance Index Test of ATH Particles
3.1.2. Modification Mechanism of ATH
3.2. Characterization of LDPE-Based Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Chen, L.; Dong, L.-P.; Li, L.-J.; Wang, Y.-Z. Organic–Inorganic Hybrid Flame Retardant: Preparation, Characterization and Application in EVA. RSC Adv. 2014, 4, 17812. [Google Scholar] [CrossRef]
- Wenguang, C.; Yanlei, G.; Qing, W.; Wei, M. Influence of the Surface Modification of a Filler on the Properties of High-impact Polystyrene Composites. J. Appl. Polym. Sci. 2009, 112, 359–365. [Google Scholar] [CrossRef]
- Shi, J.; Zeng, W.; Yang, Z.; Li, J.; Zhao, P.; Li, H.; Yan, X.; Wen, N.; Lei, Z.; Chen, D.; et al. Effect of Particle Size on Flame Retardancy and Mechanical Properties of Hydroxyethyl Diphosphate Modified Aluminum Hydroxide Intrinsic Polyethylene Terephthalate. J. Appl. Polym. Sci. 2021, 138, 50500. [Google Scholar] [CrossRef]
- Samariha, A.; Bazyar, B. Effect of Nanosilica and Aluminum Hydroxide on Thermal, Flammability, and Morphology Properties of Nanocomposite Made of Recycled High-Density Polyethylene and OCC Flour. BioRes 2020, 15, 3382–3393. [Google Scholar] [CrossRef]
- Scher, J.A.; Foley, B.; Murialdo, M.; Hao, Y.; Heo, T.W.; Weitzner, S.E.; Aubry, S.; Kroonblawd, M.P. Predicted Fracture Tendency of Naturally Occurring Aluminum Surface Coatings under Tensile Loading. ACS Appl. Mater. Interfaces 2024, 16, 25445–25461. [Google Scholar] [CrossRef] [PubMed]
- Gorla, G. New Strategies to Quantify Aluminum Hydroxide in Powder Coatings by Thermogravimetric Analysis and ATR-FT-MIR Spectroscopy Coupled with Chemometrics. Microchem. J. 2022, 172, 107005. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Xing, H.; Wang, K.; Zhang, Y.; Liu, J. Simultaneously Enhancing Fire Retardancy, Acid and Alkali Resistance of Polypropylene/Aluminum Trihydrate Composite by Microencapsulated Red Phosphorus. Vinyl Addit. Technol. 2025, 31, 199–210. [Google Scholar] [CrossRef]
- Silva, F.K. Synthesis of Aluminum Hydroxide Nanoparticles from the Residue of Aluminum Anodization for Application in Polymer Materials as Antiflame Agents. J. Mater. Res. Technol. 2020, 9, 8937–8952. [Google Scholar] [CrossRef]
- Shaker, K.; Adnan, M.; Nawab, Y.; Umair, M.; Jabbar, M.; Siddique, A.; Ahmad, A. Mechanical Performance of Glass/Epoxy Composites Loaded with Silane-Treated Aluminum Hydroxide Fillers. Polymers 2023, 15, 3514. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Huo, D.; Li, J.; Wu, S. Thermal Stability and Oil Absorption of Aluminum Hydroxide Treated by Dry Modification with Different Modifiers. Trans. Nonferrous Met. Soc. China 2008, 18, 908–912. [Google Scholar] [CrossRef]
- Ma, T.-K.; Yang, Y.-M.; Jiang, J.-J.; Yang, M.; Jiang, J.-C. Synergistic Flame Retardancy of Microcapsules Based on Ammonium Polyphosphate and Aluminum Hydroxide for Lithium-Ion Batteries. ACS Omega 2021, 6, 21227–21234. [Google Scholar] [CrossRef]
- Thi, N.H.; Nguyen, T.N.; Oanh, H.T.; Trang, N.T.T.; Tham, D.Q.; Nguyen, H.T.; Van Nguyen, T.; Hoang, M.H. Synergistic Effects of Aluminum Hydroxide, Red Phosphorus, and Expandable Graphite on the Flame Retardancy and Thermal Stability of Polyethylene. J. Appl. Polym. Sci. 2021, 138, 50317. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Dong, Q.; Xie, M.; Liu, P.; Ding, Y.; Zhang, S.; Yang, M.; Zheng, G. Core-Shell Expandable Graphite @ Aluminum Hydroxide as a Flame-Retardant for Rigid Polyurethane Foams. Polym. Degrad. Stab. 2017, 146, 267–276. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, H. Design, Preparation and Properties of Polyurethane Dispersions via Prepolymer Method. Molecules 2023, 28, 625. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, P.; Sadowska, E.; Datta, J. Investigation of Thermoplastic Polyurethanes Synthesized via Two Different Prepolymers. J. Polym. Environ. 2019, 27, 2588–2599. [Google Scholar] [CrossRef]
- Li, G.; Tan, Y.; Li, Z.; Zhou, G.; Yu, X.; Nie, Q.; Chen, J.; Yong, Q.; Xie, Z. Advances in Waterborne Polyurethane Matting Resins: A Review. Appl. Surf. Sci. Adv. 2024, 19, 100557. [Google Scholar] [CrossRef]
- Hong, C.; Li, J.; Zhang, H.; Zhang, H.; Liu, M.; Wang, Y.; Song, Y.; Zhou, C. Facile Fabrication of Waterborne Polyurethane Coatings with Good Hydrophobicity and Antifouling Properties by Leveraging Fluorinated Polysiloxane. Prog. Org. Coat. 2024, 186, 108077. [Google Scholar] [CrossRef]
- Song, X.; Yu, M.; Niu, H.; Zhu, Y.; Zhao, K.; Zhou, C.; Liu, L.; Wu, G. Polyester/Polyether Polyurethane Sizing Coatings with Poly(Diaminosiloxane) Branched Dual Interpenetrating Crosslinked Networks for Enhancing Carbon Fibre Wettability and Interfacial Properties. Eur. Polym. J. 2023, 200, 112525. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hosseini, D.; Isfahani, A.P.; Dehghani, Z.; Shams, E. Waterborne Polyurethane Nanocomposite Incorporated with Phytic Acid Intercalated Layered Double Hydroxides: A Highly Stable Aqueous Dispersion with Desired Corrosion Protection Capability. Polym. Adv. Techs. 2021, 32, 4014–4028. [Google Scholar] [CrossRef]
- Lee, J.; Morita, K.; Tanaka, T. Determination of Macro-Contact Angle and Line Tension at High Temperatures for Au/Al2O3 System at 1373 K Using a Micro-Scale Wetting Method. Mater. Trans. 2003, 44, 2659–2663. [Google Scholar] [CrossRef][Green Version]
- Gai, W.-Z.; Zhang, S.-H.; Yang, Y.; Sun, K.; Jia, H.; Deng, Z.-Y. Defluoridation Performance Comparison of Aluminum Hydroxides with Different Crystalline Phases. Water Supply 2022, 22, 3673–3684. [Google Scholar] [CrossRef]
- Shen, S.; Chow, P.S.; Chen, F.; Feng, S.; Tan, R.B.H. Synthesis of Submicron Gibbsite Platelets by Organic-Free Hydrothermal Crystallization Process. J. Cryst. Growth 2006, 292, 136–142. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, S.; Chen, Z.; Wei, S.; Sun, Z.; Li, C. Study on Surface Modification of Ground Calcium Carbonate with Novel Modifier and Its PVC Filling Performance. Powder Technol. 2022, 412, 118028. [Google Scholar] [CrossRef]
- Yang, X.; Shuai, H.; Du, G.; Wang, J.; Shen, J. Investigation into the Mechanism of Waterborne Polyurethane Modification of Ultrafine Talc and Its Impact on the Properties of Polypropylene Plastics. Polymers 2025, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shuai, H.; Du, G.; Wang, J.; Shen, J. Study on the Mechanism of Waterborne Polyurethane Modified Ultrafine Ground Calcium Carbonate and Its Properties in Polypropylene Plastics. Polym. Compos. 2024; in press. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Xiao, K.; Zhang, Z. Research on the Thermal Aging Behaviors of LDPE/TiO2 Nanocomposites. J. Nanomater. 2017, 2017, 5048382. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Wu, L.; Zhang, Y.; Mai, K. β-Crystallization and Mechanical Properties of Aluminum Hydroxide-filled Polypropylene Composites. Polym. Compos. 2019, 40, E194–E201. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, T.T.L.; Pham, T.D.; Le, T.S. Removal of Lindane from Aqueous Solution Using Aluminum Hydroxide Nanoparticles with Surface Modification by Anionic Surfactant. Polymers 2020, 12, 960. [Google Scholar] [CrossRef]
- Mashkovtsev, M.; Tarasova, N.; Baksheev, E.; Rychkov, V.; Zhuravlev, N.; Solodovnikova, P.; Galiaskarova, M. Spectroscopic Study of Five-Coordinated Thermal Treated Alumina Formation: FTIR and NMR Applying. Int. J. Mol. Sci. 2023, 24, 5151. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.; Prado, M.; Le Roux, C.; Poirier, M.; Micoud, P.; Ligabue, R.; Martin, F.; Einloft, S. Synthetic Talc as Catalyst and Filler for Waterborne Polyurethane-Based Nanocomposite Synthesis. Polym. Bull. 2020, 77, 975–987. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Duong, L.V.; Wood, B.J.; Frost, R.L. XPS Study of the Major Minerals in Bauxite: Gibbsite, Bayerite and (Pseudo-) Boehmite. J. Colloid. Interface Sci. 2006, 296, 572–576. [Google Scholar] [CrossRef]
- Chen, L.; Ye, G.; Xu, D.; Zhu, L.; Lu, Z.; Dong, L.; Liu, Y. Chemical Bond Change of Gibbsite and Fumed Silica Mixture during Mechanical Activation. Mater. Lett. 2012, 85, 91–94. [Google Scholar] [CrossRef]
- McGarry, K.; Zilberman, J.; Hull, T.R.; Woolley, W.D. Decomposition and Combustion of EVA and LDPE Alone and When Fire Retarded with ATH. Polym. Int. 2000, 49, 1193–1198. [Google Scholar] [CrossRef]
- Sabet, M.; Soleimani, H.; Hassan, A.; Ratnam, C.T. Electron Beam Irradiation of LDPE Filled with Calcium Carbonate and Metal Hydroxides. Polym.-Plast. Technol. Eng. 2014, 53, 1362–1366. [Google Scholar] [CrossRef]
- Ramírez-Vargas, E.; Sánchez-Valdes, S.; Parra-Tabla, O.; Castañeda-Gutiérrez, S.; Méndez-Nonell, J.; Francisco Ramos-deValle, L.; López-León, A.; Lujan-Acosta, R. Structural Characterization of LDPE/EVA Blends Containing Nanoclay-flame Retardant Combinations. J. Appl. Polym. Sci. 2012, 123, 1125–1136. [Google Scholar] [CrossRef]
- Bahattab, M.A.; Mosnáček, J.; Basfar, A.A.; Shukri, T.M. Cross-Linked Poly (Ethylene Vinyl Acetate) (EVA)/Low Density Polyethylene (LDPE)/Metal Hydroxides Composites for Wire and Cable Applications. Polym. Bull. 2010, 64, 569–580. [Google Scholar] [CrossRef]





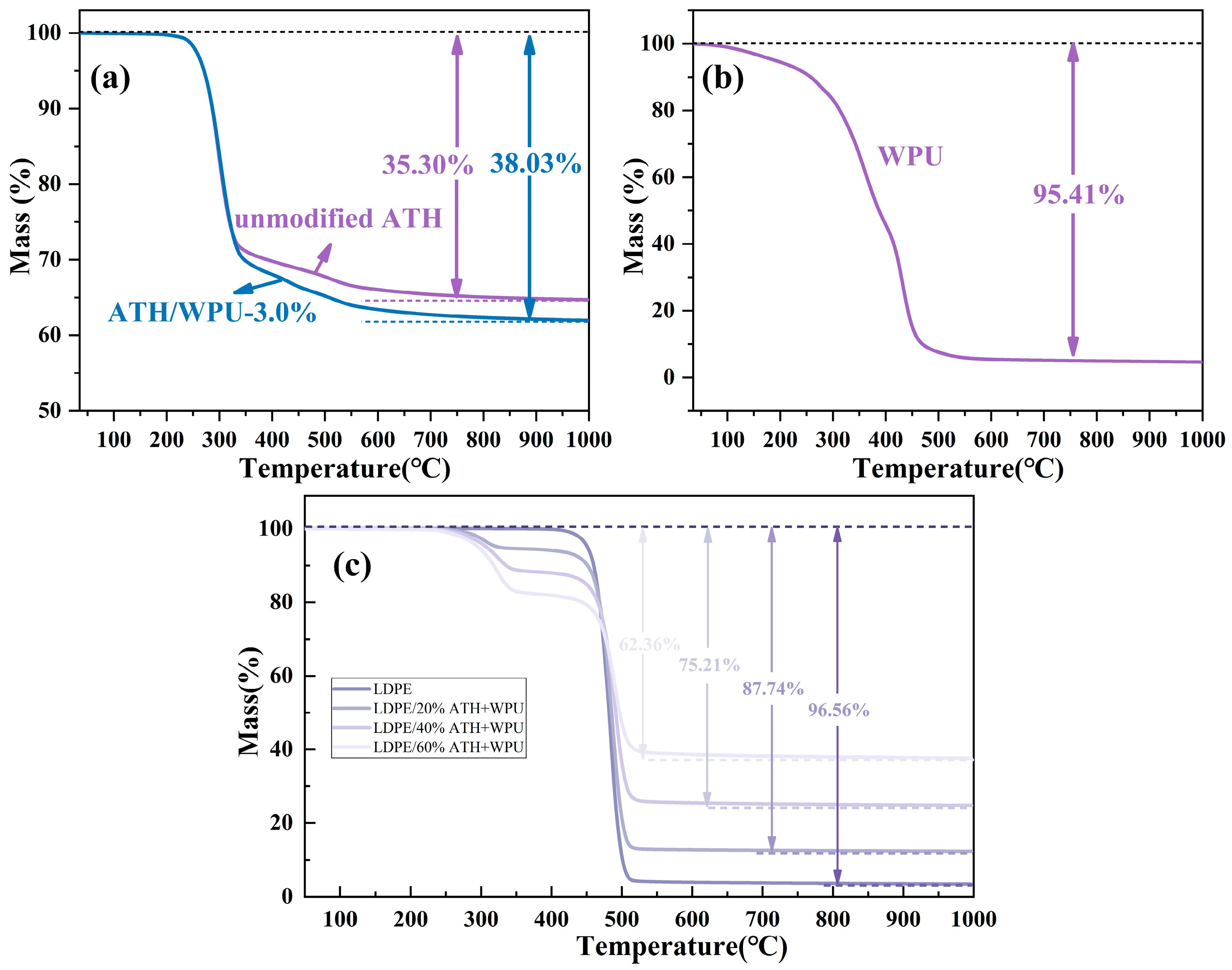
| Sample | LDPE (wt.%) | ATH (wt.%) | WPU on ATH (%) |
|---|---|---|---|
| LDPE | 100 | 0 | 0 |
| LDPE/20% ATH | 80 | 20 | 0 |
| LDPE/20% ATH + WPU | 80 | 20 | 3.0 |
| LDPE/40% ATH + WPU | 60 | 40 | 3.0 |
| LDPE/60% ATH + WPU | 40 | 60 | 3.0 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Flexural Strength (MPa) |
|---|---|---|---|
| LDPE | 32.84 | 63.43 | 11.50 |
| LDPE/20%ATH | 24.32 | 53.81 | 10.96 |
| LDPE/20%ATH + WPU | 30.02 | 59.84 | 13.20 |
| LDPE/40%ATH + WPU | 32.87 | 50.75 | 13.43 |
| LDPE/60%ATH + WPU | 39.90 | 14.03 | 20.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Du, G.; Shuai, H.; Xu, X.; Wang, J. Study on the Mechanical and Thermal Properties of Waterborne Polyurethane-Modified Aluminum Hydroxide and Its Application in LDPE Plastics. Polymers 2025, 17, 556. https://doi.org/10.3390/polym17050556
Yang X, Du G, Shuai H, Xu X, Wang J. Study on the Mechanical and Thermal Properties of Waterborne Polyurethane-Modified Aluminum Hydroxide and Its Application in LDPE Plastics. Polymers. 2025; 17(5):556. https://doi.org/10.3390/polym17050556
Chicago/Turabian StyleYang, Xianrong, Gaoxiang Du, Huan Shuai, Xi Xu, and Jiao Wang. 2025. "Study on the Mechanical and Thermal Properties of Waterborne Polyurethane-Modified Aluminum Hydroxide and Its Application in LDPE Plastics" Polymers 17, no. 5: 556. https://doi.org/10.3390/polym17050556
APA StyleYang, X., Du, G., Shuai, H., Xu, X., & Wang, J. (2025). Study on the Mechanical and Thermal Properties of Waterborne Polyurethane-Modified Aluminum Hydroxide and Its Application in LDPE Plastics. Polymers, 17(5), 556. https://doi.org/10.3390/polym17050556






