Alternative Natural Rubber Cross-Linking Utilizing a Disulfide-Containing Bismaleimide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- NR/BIS-X: formulations without inhibitors, acting as control formulations, with X referring to phr of Bismaleimide introduced; generally called Control formulations;
- NR/BIS-X/CuMA: formulations with CuMA as inhibitor, with X referring to phr of Bismaleimide introduced; CuMA is equimolar to Bismaleimide;
- NR/BIS-X/CuCl2: formulations with CuCl2 as inhibitor, with X referring to phr of Bismaleimide introduced; CuCl2 is equimolar to Bismaleimide;
- Compound: something that has been mixed, but not cured;
- Cured compound: new cured compound;
- Recycled compound: recycled cured compound.
2.2. Preparation of Cystamine
2.3. Synthesis of Bismaleimide Cross-Linker
2.4. Compound Preparation and Curing
2.5. Gel Permeation Chromatography
2.6. Dynamic Differential Scanning Calorimetry (DSC)
2.7. Mechanical Characterization
2.8. Density
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, A.; Fefar, M.M.; Griggs, T.; Akutagawa, K.; Chen, B.; Busfield, J.J.C. Recyclable sulfur cured natural rubber with controlled disulfide metathesis. Commun. Mater. 2024, 5, 212. [Google Scholar] [CrossRef]
- Markl, E.; Lackner, M. Devulcanization Technologies for Recycling of Tire-Derived Rubber: A Review. Materials 2020, 13, 1246. [Google Scholar] [CrossRef] [PubMed]
- Coran, A.Y. Chemistry of the vulcanization and protection of elastomers: A review of the achievements. J. Appl. Polym. Sci. 2003, 87, 24–30. [Google Scholar] [CrossRef]
- Nevejans, S.; Ballard, N.; Miranda, J.I.; Reck, B.; Asua, J.M. The underlying mechanisms for self-healing of poly(disulfide)s. Phys. Chem. Chem. Phys. 2016, 18, 27577–27583. [Google Scholar] [CrossRef] [PubMed]
- Rekondo, A.; Martin, R.; Ruiz de Luzuriaga, A.; Cabañero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horiz. 2014, 1, 237–240. [Google Scholar] [CrossRef]
- Kaur, A.; Gautrot, J.E.; Cavalli, G.; Watson, D.; Bickley, A.; Akutagawa, K.; Busfield, J.J.C. Novel Crosslinking System for Poly-Chloroprene Rubber to Enable Recyclability and Introduce Self-Healing. Polymers 2021, 13, 3347. [Google Scholar] [CrossRef] [PubMed]
- Imbernon, L.; Oikonomou, E.K.; Norvez, S.; Leibler, L. Chemically crosslinked yet reprocessable epoxidized natural rubber via thermo-activated disulfide rearrangements. Polym. Chem. 2015, 6, 4271–4278. [Google Scholar] [CrossRef]
- Xiang, H.P.; Qian, H.J.; Lu, Z.Y.; Rong, M.Z.; Zhang, M.Q. Crack healing and reclaiming of vulcanized rubber by triggering the rearrangement of inherent sulfur crosslinked networks. Green Chem. 2015, 17, 4315–4325. [Google Scholar] [CrossRef]
- Xiang, H.P.; Rong, M.Z.; Zhang, M.Q. Self-healing, Reshaping, and Recycling of Vulcanized Chloroprene Rubber: A Case Study of Multitask Cyclic Utilization of Cross-linked Polymer. ACS Sustain. Chem. Eng. 2016, 4, 2715–2724. [Google Scholar] [CrossRef]
- Kong, Y.; Chen, X.; Li, Z.; Li, G.; Huang, Y. Evolution of crosslinking structure in vulcanized natural rubber during thermal aging in the presence of a constant compressive stress. Polym. Degrad. Stab. 2023, 217, 110513. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Chen, X.; Kong, Y.; Huang, Y.; Lv, Y.; Li, G. Effect of non-rubber components on the crosslinking structure and thermo-oxidative degradation of natural rubber. Polym. Degrad. Stab. 2022, 196, 109845. [Google Scholar] [CrossRef]
- Kitagawa, S.; Ozawa, M.; Shibata, M. Self-healing and reprocessable bismaleimide-diamine thermosets containing disulfide linkages. Polymer 2023, 278, 126008. [Google Scholar] [CrossRef]
- Sim, X.M.; Wang, C.-G.; Liu, X.; Goto, A. Multistimuli Responsive Reversible Cross-Linking–Decross-Linking of Concentrated Polymer Brushes. ACS Appl. Mater. Interfaces 2020, 12, 28711–28719. [Google Scholar] [CrossRef] [PubMed]
- Polgar, L.M.; Cerpentier, R.R.J.; Vermeij, G.H.; Picchioni, F.; Duin, M.v. Influence of the chemical structure of cross-linking agents on properties of thermally reversible networks. Pure Appl. Chem. 2016, 88, 1103–1116. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Sanyal, A. Furan-containing polymeric Materials: Harnessing the Diels-Alder chemistry for biomedical applications. Eur. Polym. J. 2021, 153, 110514. [Google Scholar] [CrossRef]
- Post, C.; van den Tempel, P.; Herrera Sánchez, P.; Maniar, D.; Bose, R.K.; Voet, V.S.D.; Folkersma, R.; Picchioni, F.; Loos, K. Thermoreversible Diels–Alder Cross-Linking of BHMF-Based Polyesters: Synthesis, Characterization and Rheology. ACS Sustain. Chem. Eng. 2025, 13, 3543–3553. [Google Scholar] [CrossRef] [PubMed]
- Hayeemasae, N.; Sensem, Z.; Sahakaro, K.; Ismail, H. Maleated Natural Rubber/Halloysite Nanotubes Composites. Processes 2020, 8, 286. [Google Scholar] [CrossRef]
- Ujianto, O.; Noviyanti, R.; Wijaya, R.; Ramadhoni, B. Effect of maleated natural rubber on tensile strength and compatibility of natural rubber/coconut coir composite. IOP Conf. Ser. Mater. Sci. Eng. 2017, 223, 012014. [Google Scholar] [CrossRef]
- ISO 37:2024(en); Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-strain Properties. International Organization for Standardization: Geneva, Switzerland, 2024.


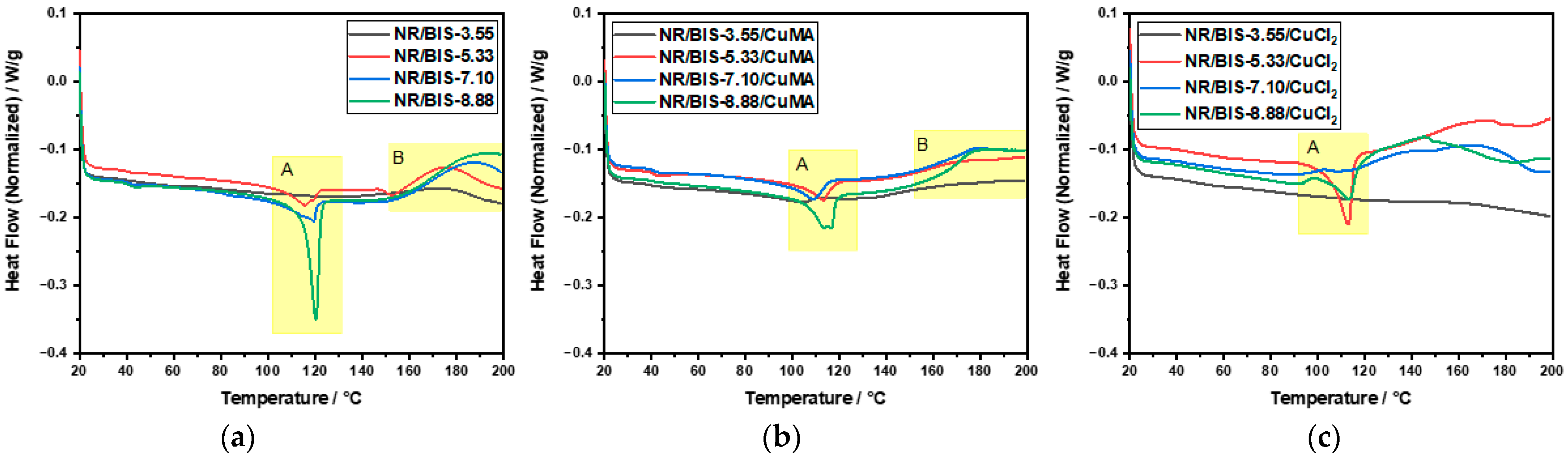
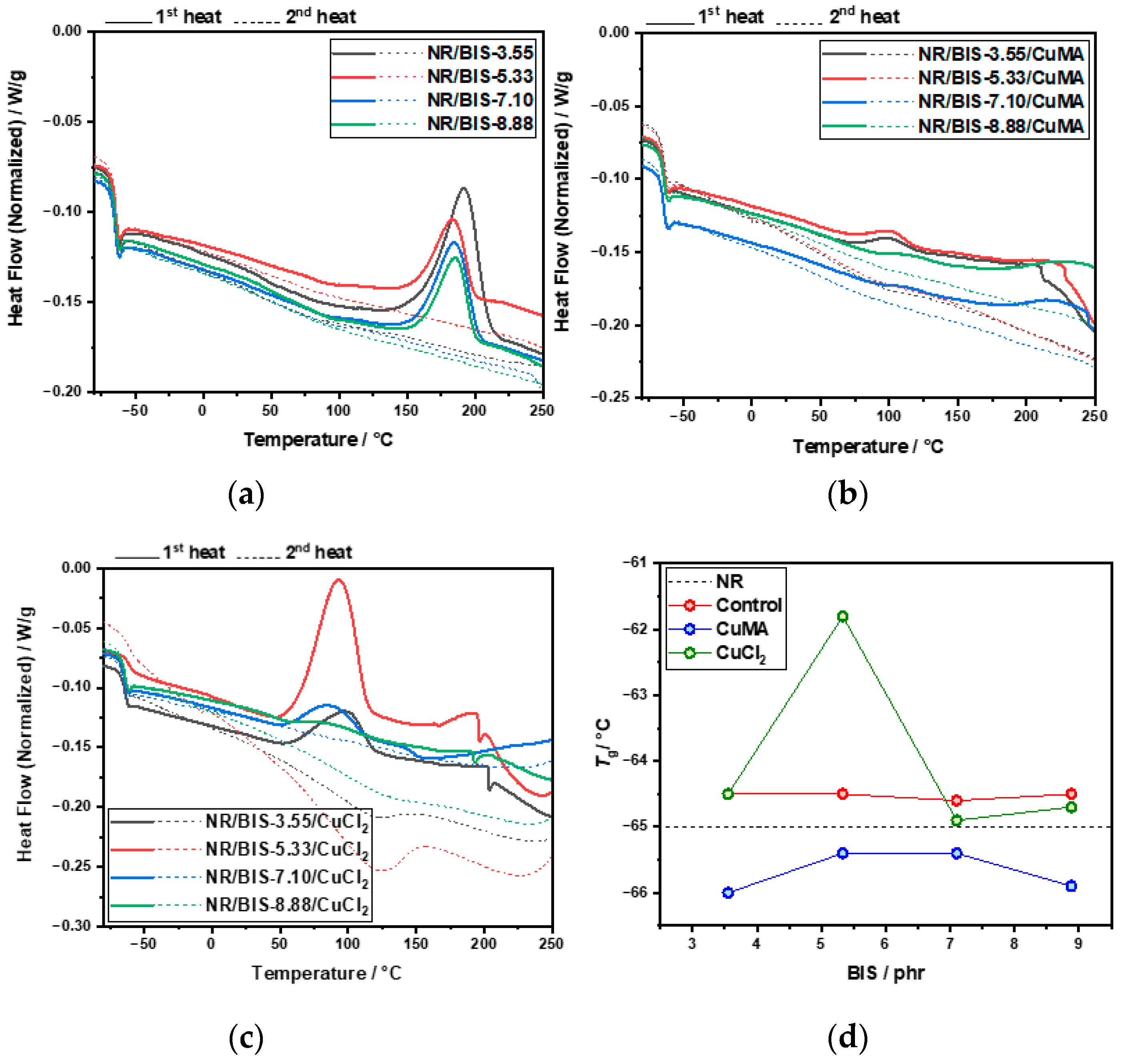



| Formulation | NR/phr | BIS/phr | CuMA/phr | CuCl2/phr | t90/min |
|---|---|---|---|---|---|
| NR/BIS-3.55 | 100 | 3.55 | 42 | ||
| NR/BIS-3.55/CuMA | 100 | 3.55 | 2.38 | 42 | |
| NR/BIS-3.55/CuCl2 | 100 | 3.55 | 1.37 | 49 | |
| NR/BIS-5.33 | 100 | 5.33 | 43 | ||
| NR/BIS-5.33/CuMA | 100 | 5.33 | 3.58 | 44 | |
| NR/BIS-5.33/CuCl2 | 100 | 5.33 | 2.06 | 45 | |
| NR/BIS-7.10 | 100 | 7.10 | 46 | ||
| NR/BIS-7.10/CuMA | 100 | 7.10 | 4.77 | 45 | |
| NR/BIS-7.10/CuCl2 | 100 | 7.10 | 2.74 | 43 | |
| NR/BIS-8.88 | 100 | 8.88 | 46 | ||
| NR/BIS-8.88/CuMA | 100 | 8.88 | 5.96 | 45 | |
| NR/BIS-8.88/CuCl2 | 100 | 8.88 | 3.43 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, A.; Tucker, M.; Akutagawa, K.; Chen, B.; Busfield, J.J.C. Alternative Natural Rubber Cross-Linking Utilizing a Disulfide-Containing Bismaleimide. Polymers 2025, 17, 3302. https://doi.org/10.3390/polym17243302
Kaur A, Tucker M, Akutagawa K, Chen B, Busfield JJC. Alternative Natural Rubber Cross-Linking Utilizing a Disulfide-Containing Bismaleimide. Polymers. 2025; 17(24):3302. https://doi.org/10.3390/polym17243302
Chicago/Turabian StyleKaur, Anureet, Maria Tucker, Keizo Akutagawa, Biqiong Chen, and James J. C. Busfield. 2025. "Alternative Natural Rubber Cross-Linking Utilizing a Disulfide-Containing Bismaleimide" Polymers 17, no. 24: 3302. https://doi.org/10.3390/polym17243302
APA StyleKaur, A., Tucker, M., Akutagawa, K., Chen, B., & Busfield, J. J. C. (2025). Alternative Natural Rubber Cross-Linking Utilizing a Disulfide-Containing Bismaleimide. Polymers, 17(24), 3302. https://doi.org/10.3390/polym17243302








