A Dual-Modified Chitosan-Derived Silica Composite Aerogel with Simultaneous Improvement of Mechanical, Flame Retardancy, and Thermal Insulation Properties
Abstract
1. Introduction
2. Experiment
2.1. Materials
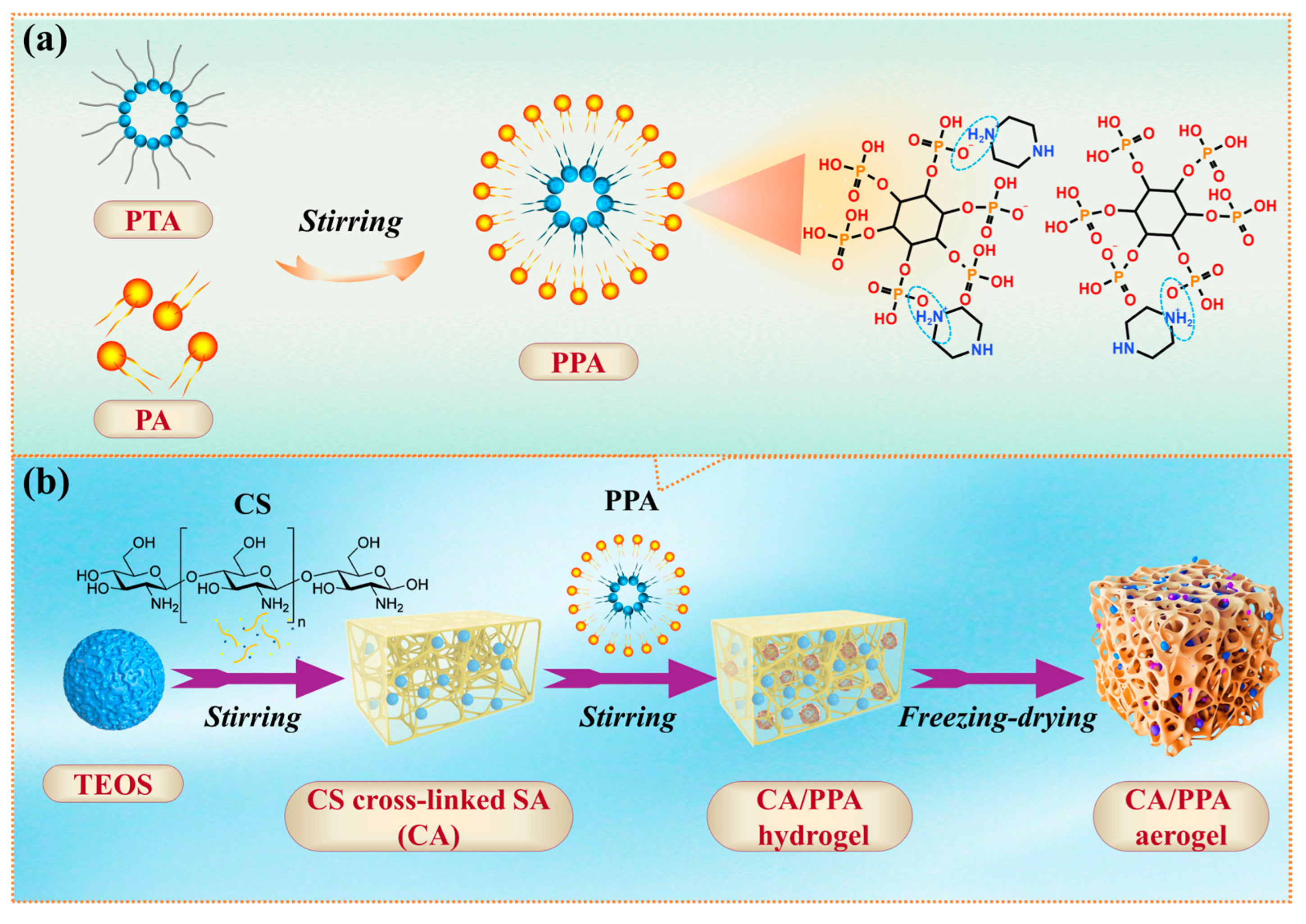
2.2. Preparation of PPA and CA/PPA Composite Aerogel
2.2.1. Designing of CA/PPA Composite Aerogel
2.2.2. Designing of Intumescent Flame Retardant (PPA)
2.3. Characterization
3. Results and Discussion
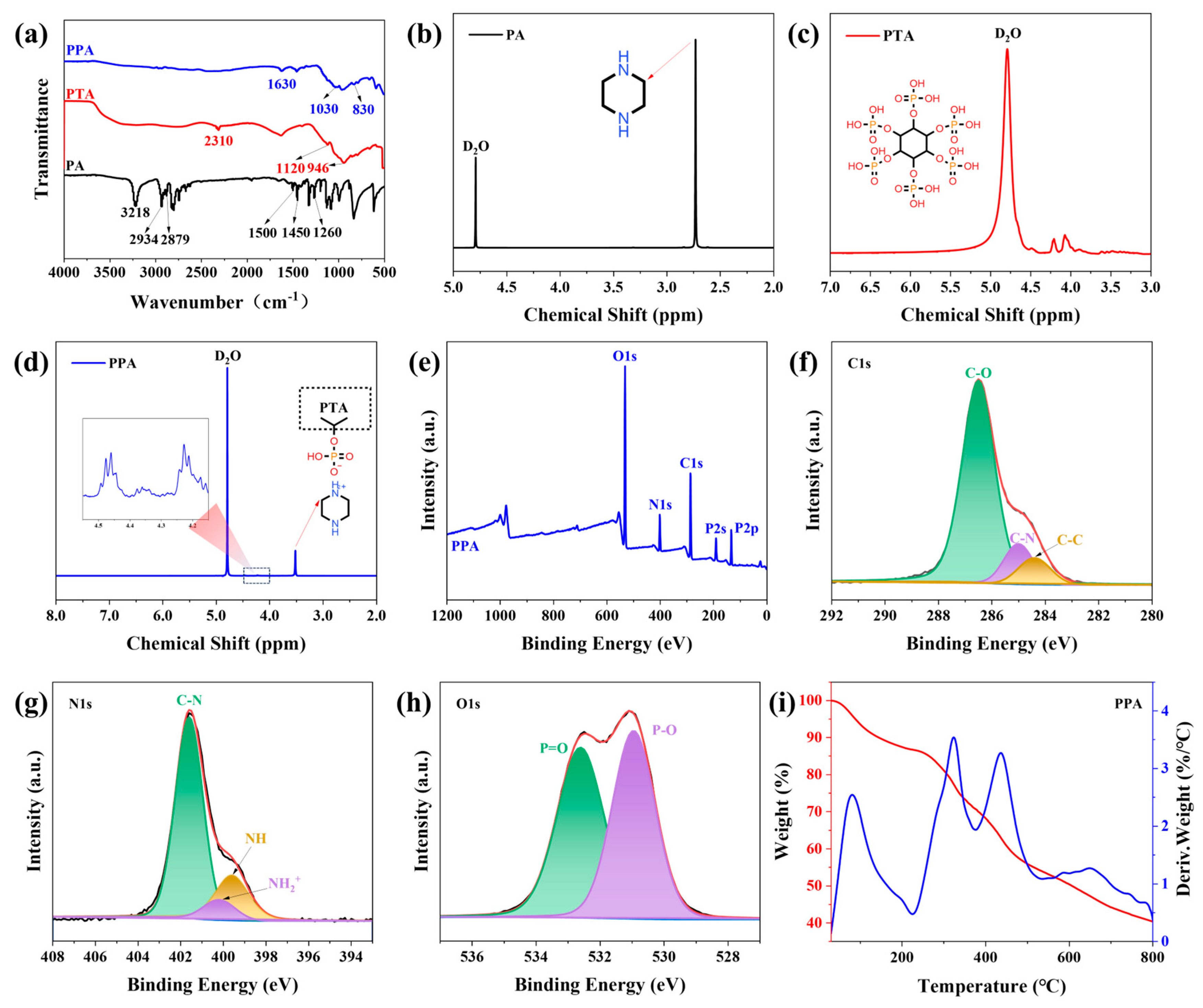
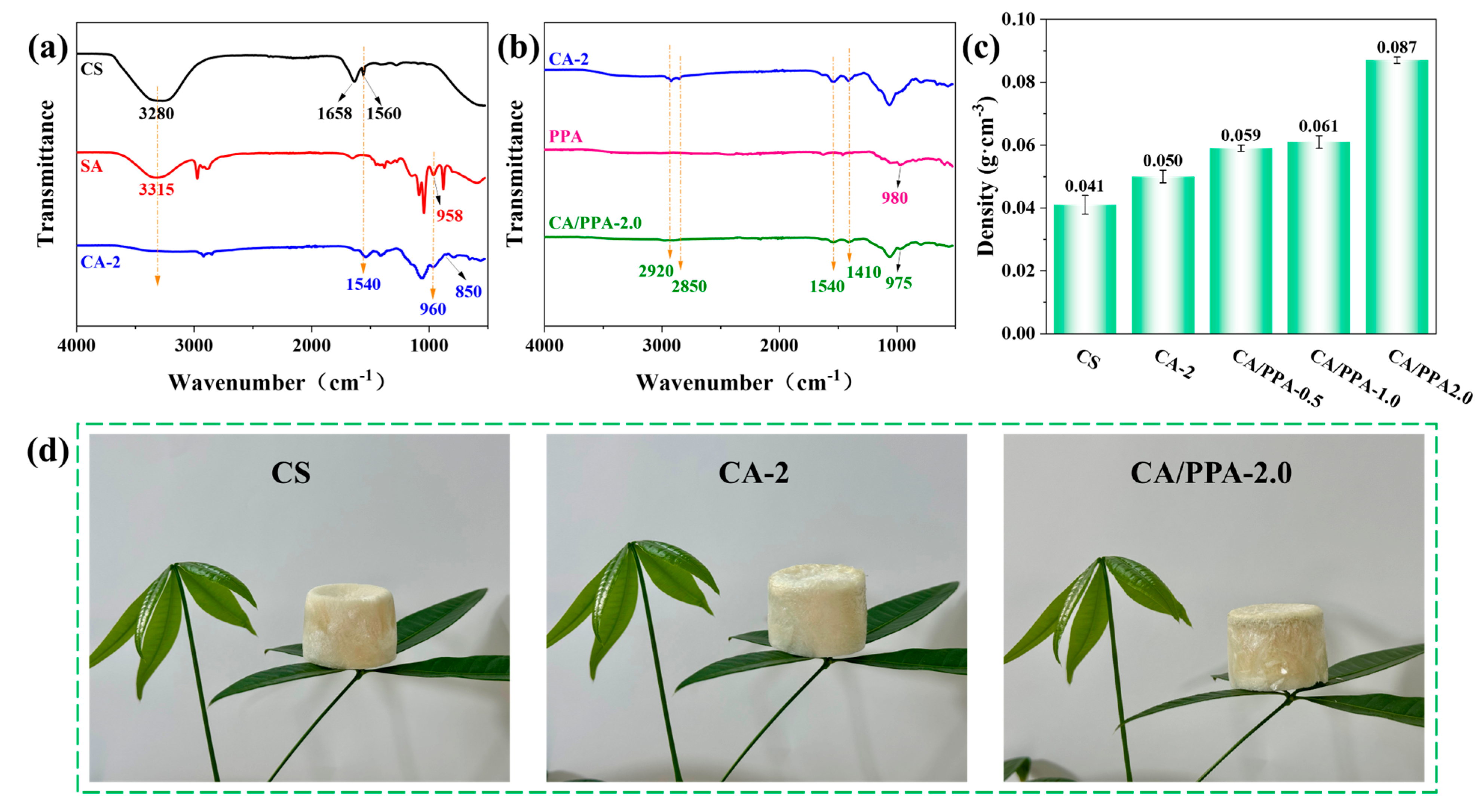
3.1. Characterization of PPA Flame Retardant and Its Composite Aerogel
3.1.1. Characterization of PPA Flame Retardant
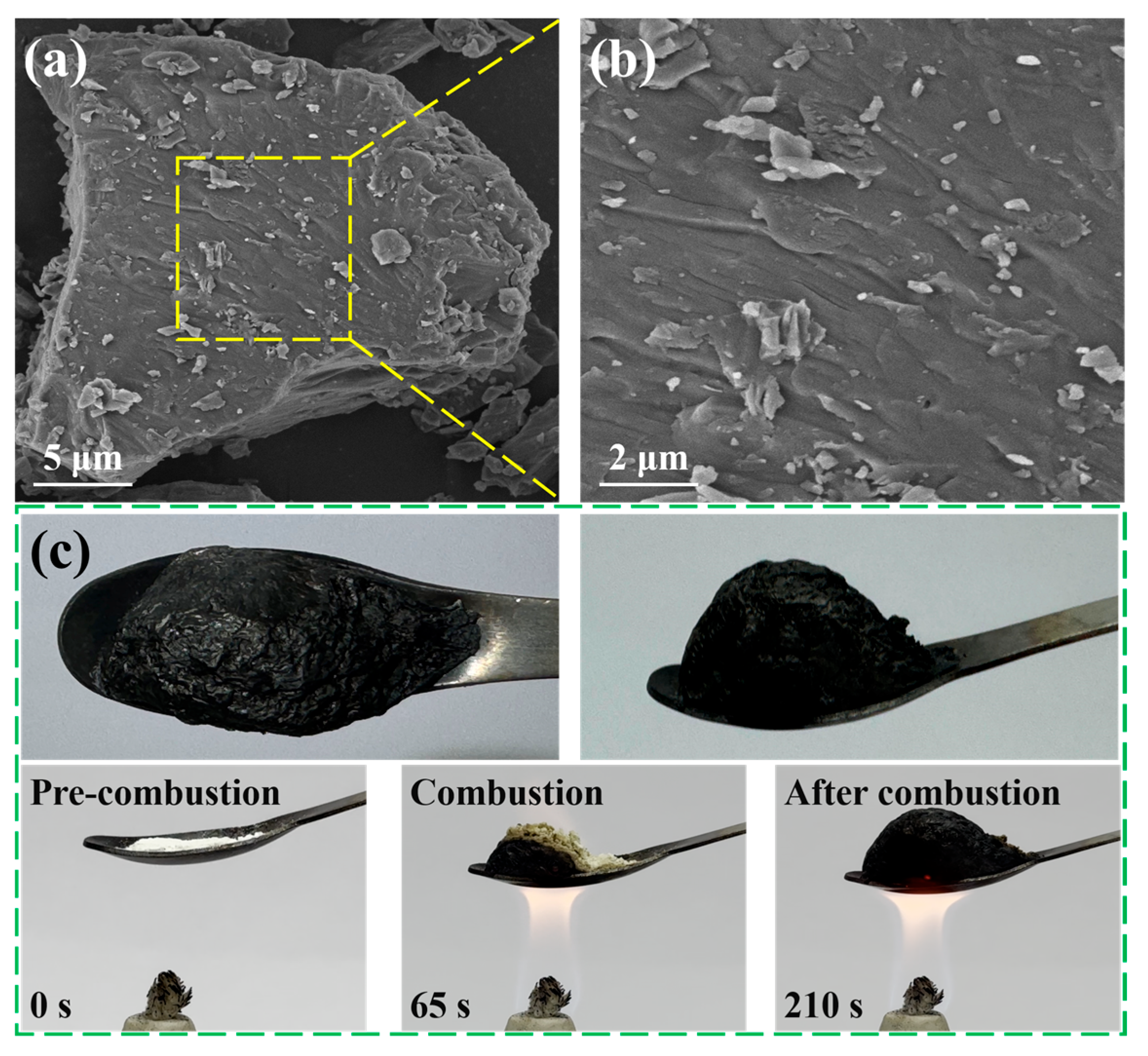
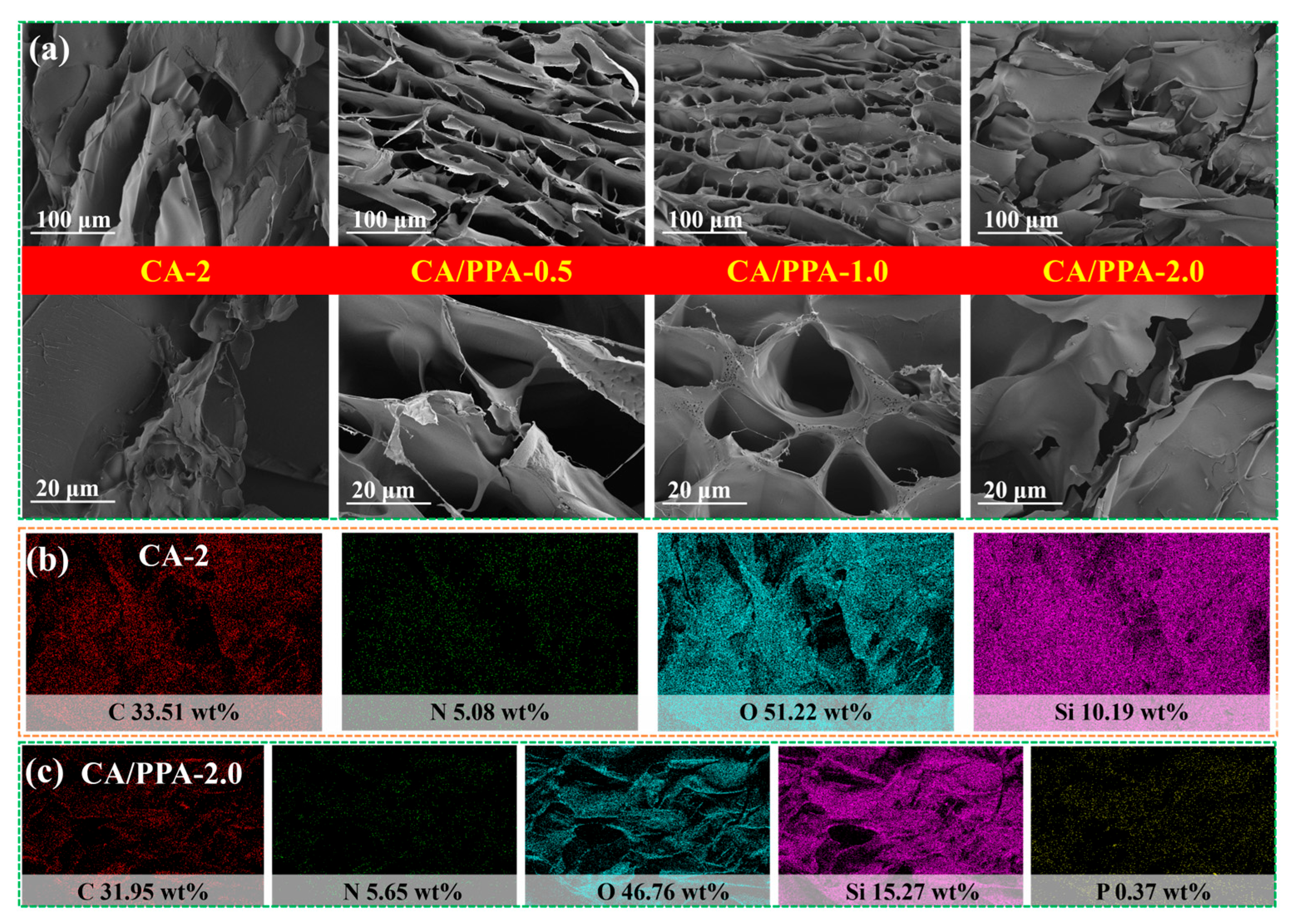
3.1.2. Characterization of Composite Aerogel
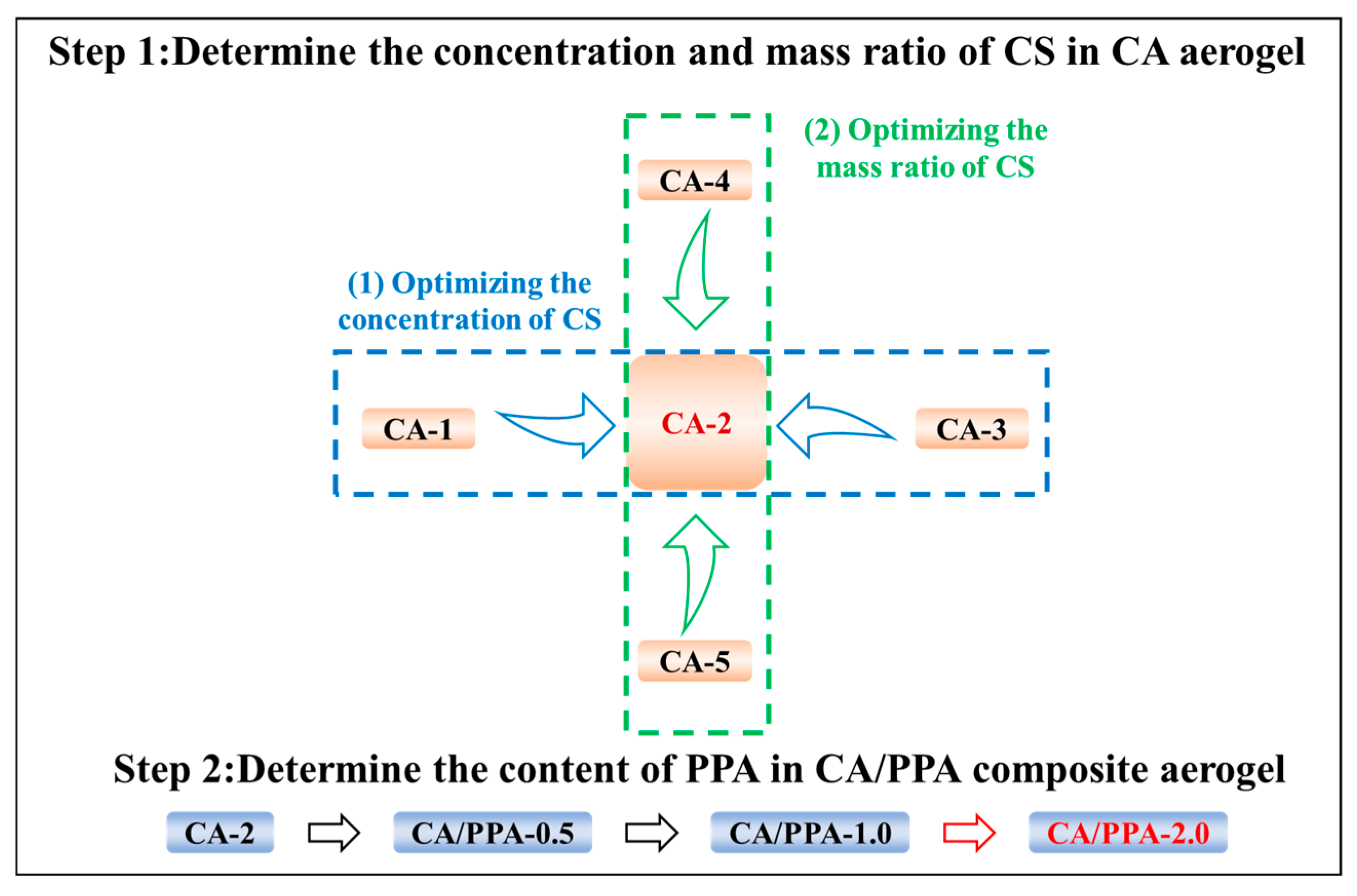
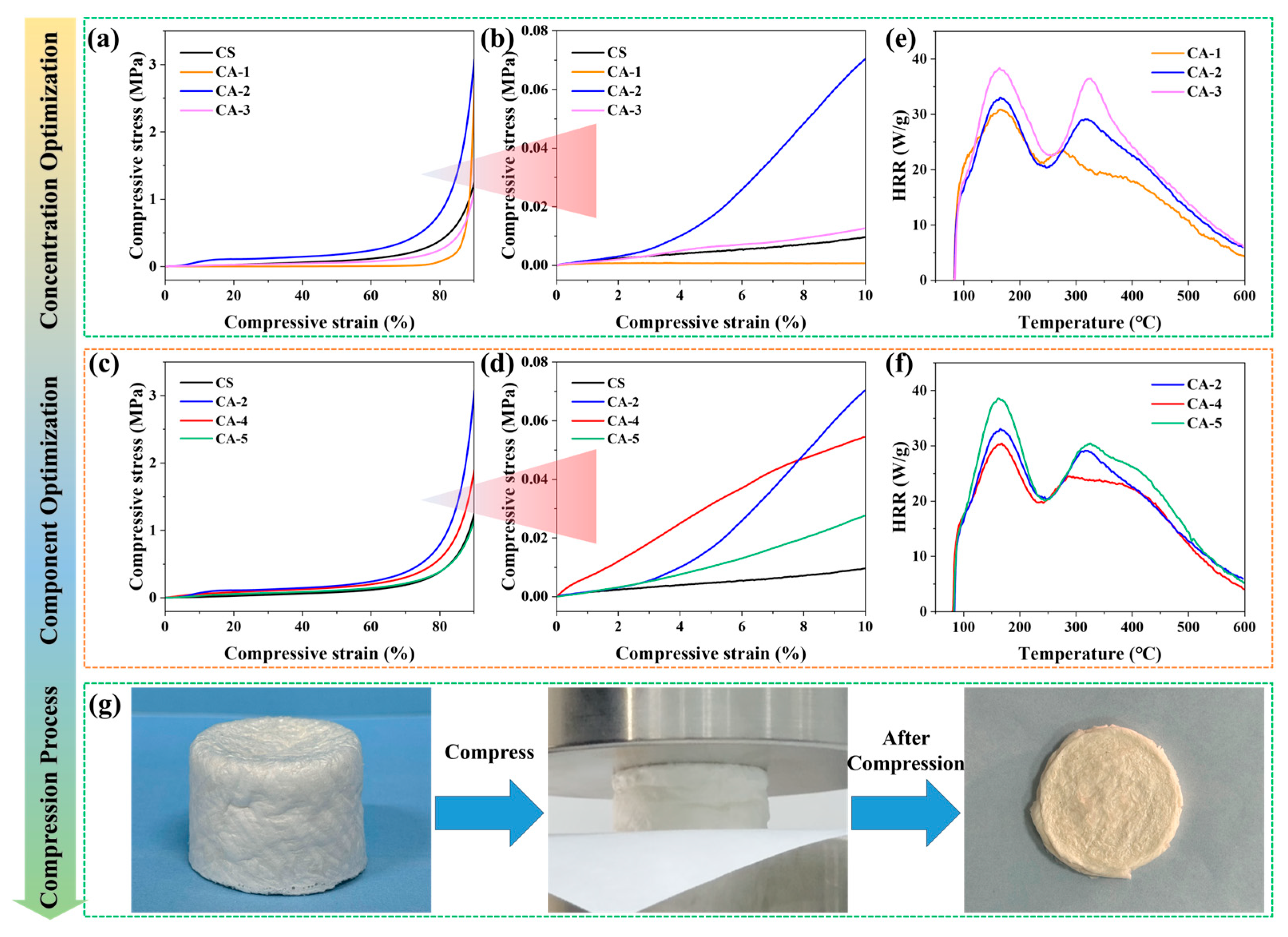
3.2. Optimizing the Compound of CA Based on Its Mechanical and Fire Safety Properties
3.2.1. Determine the Optimal Concentration of CS in CA
3.2.2. Determine the Optimal Ratio of CS in CA
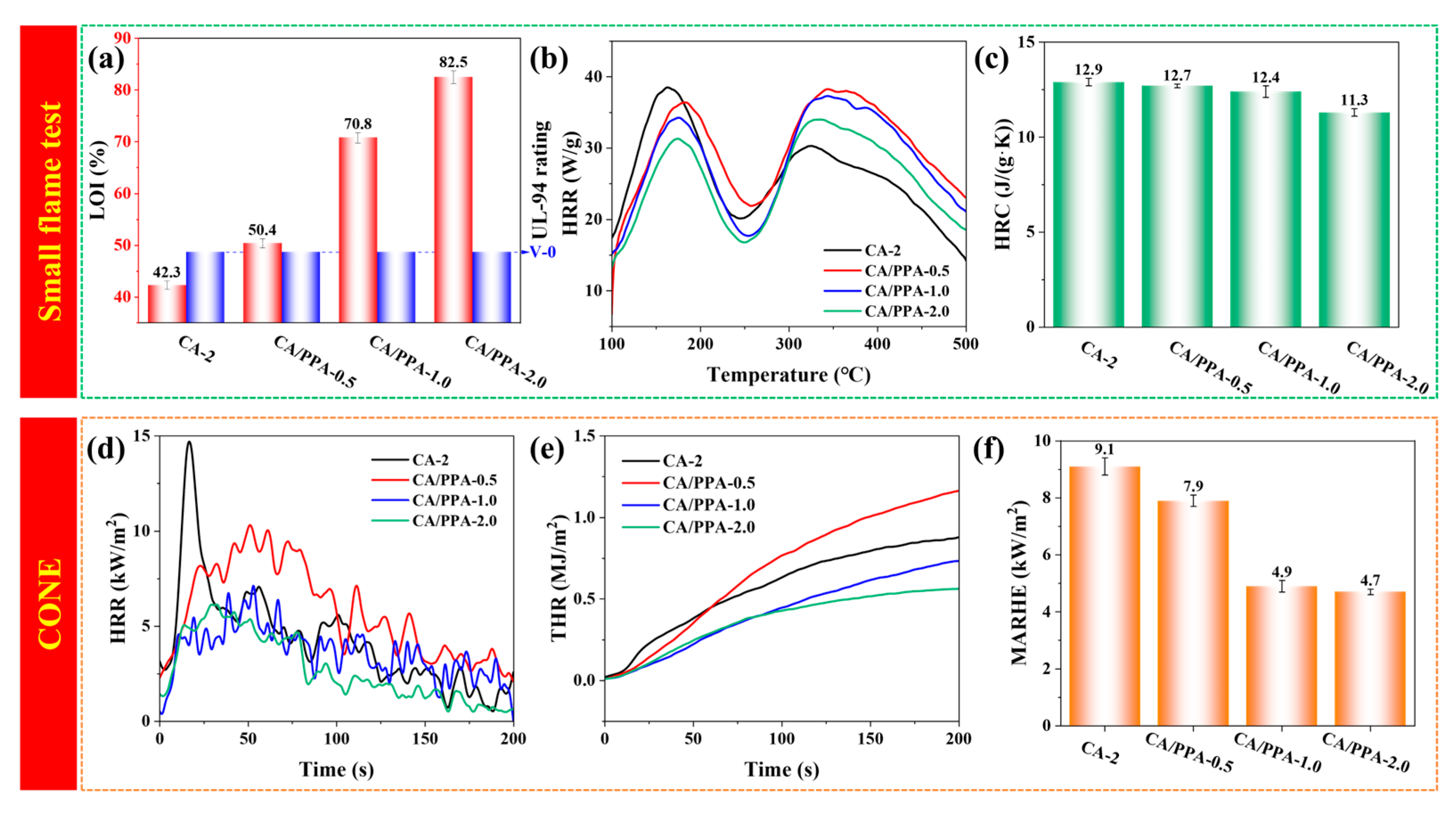
3.3. Fire Behaviors of PPA-Based CA
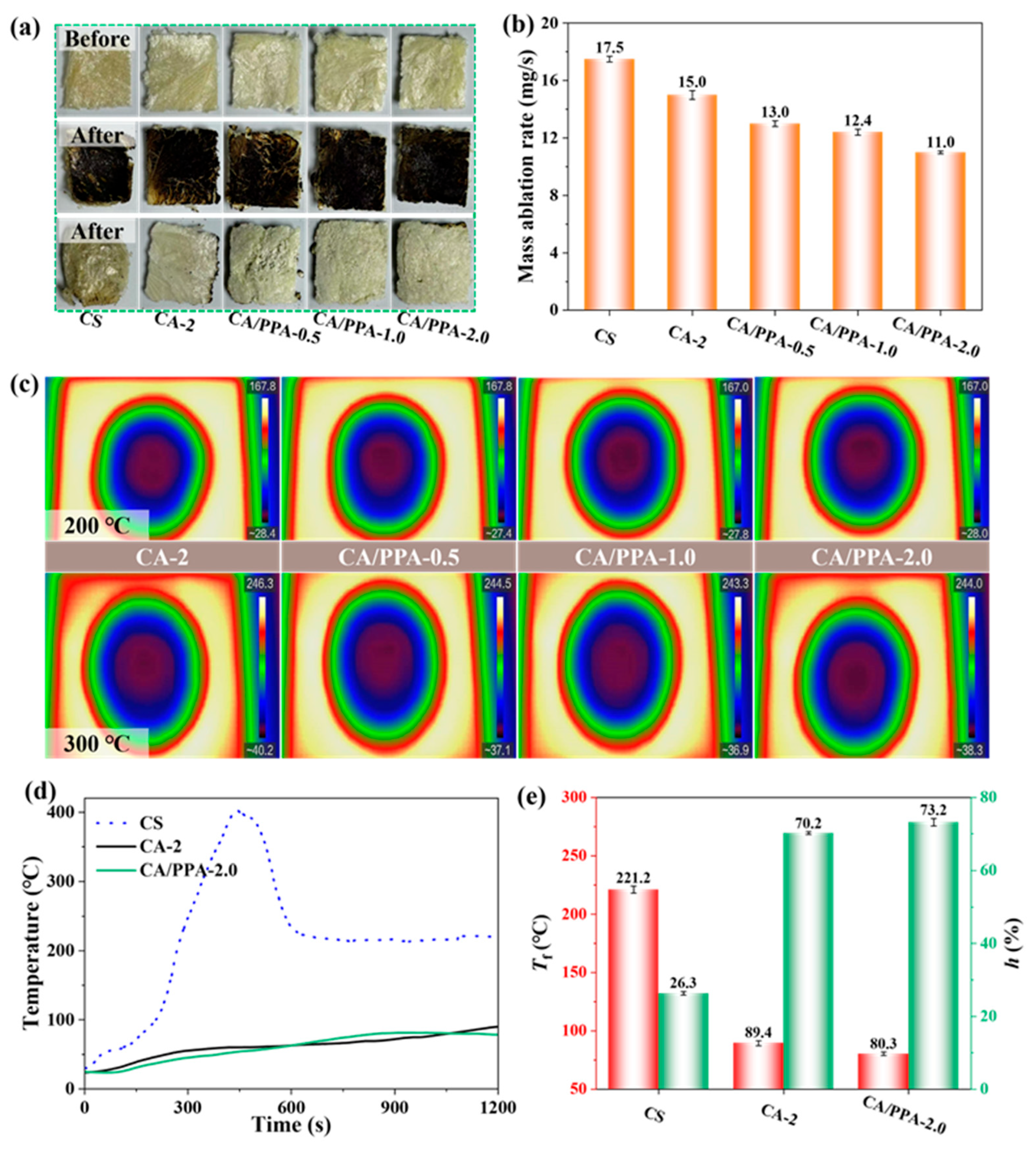
3.3.1. Flame Retardancy Performance of PPA-Based CA
3.3.2. Thermal Insulation and Fire Resistance Properties of PPA-Based CA
3.3.3. Thermal Stability of PPA-Based CA
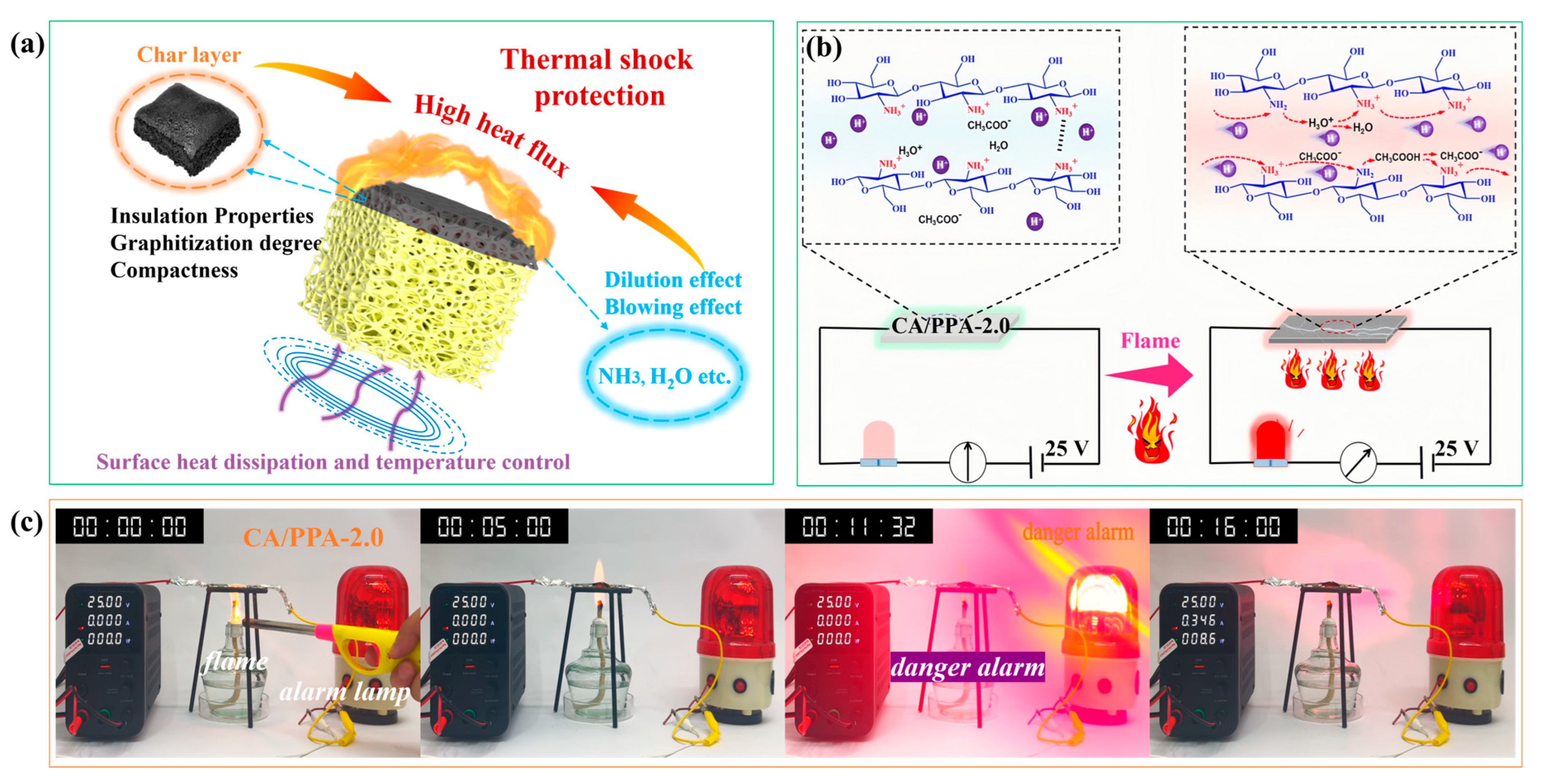
3.4. Flame Retardancy Mechanisms
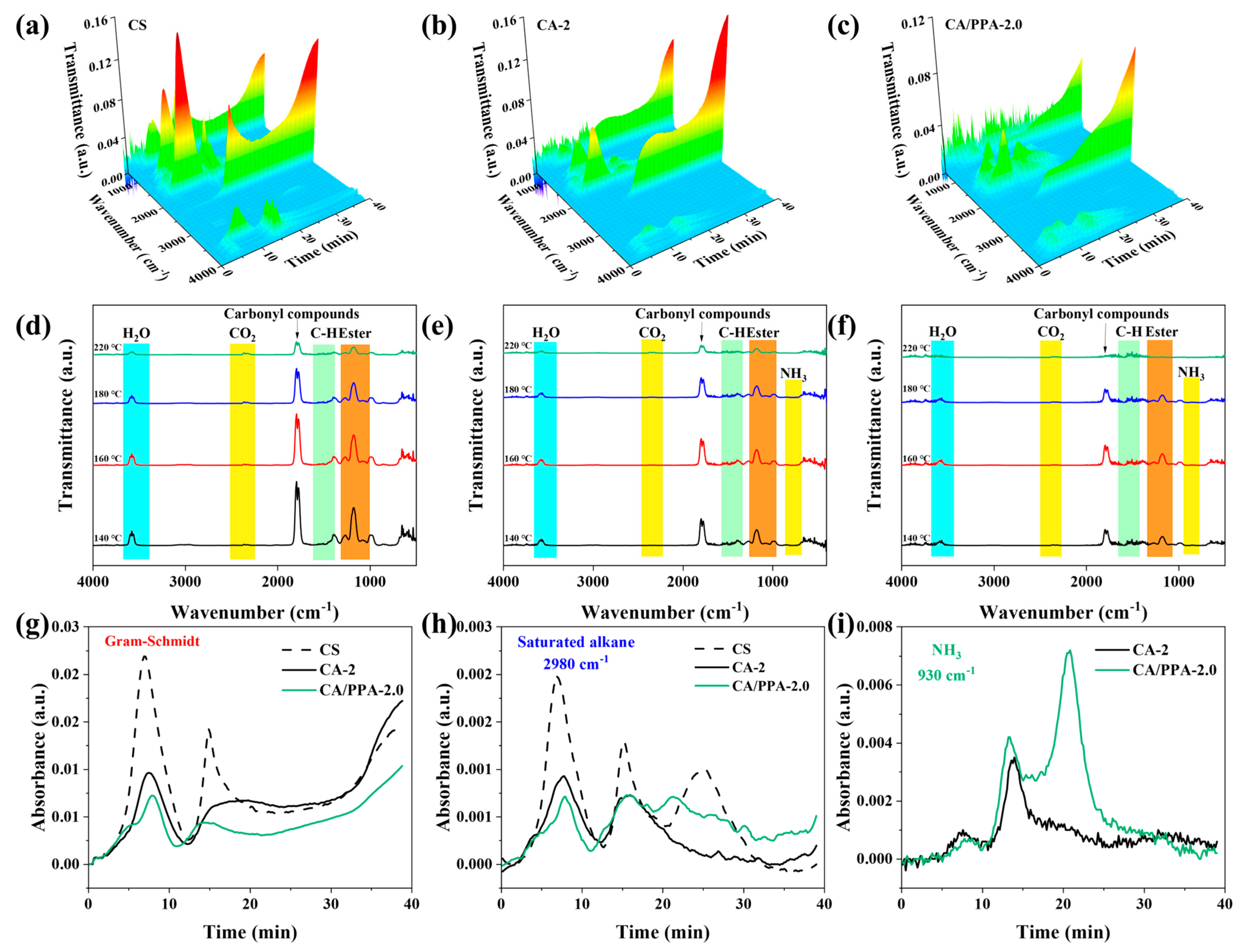
3.4.1. Analysis of Gaseous Phase Product
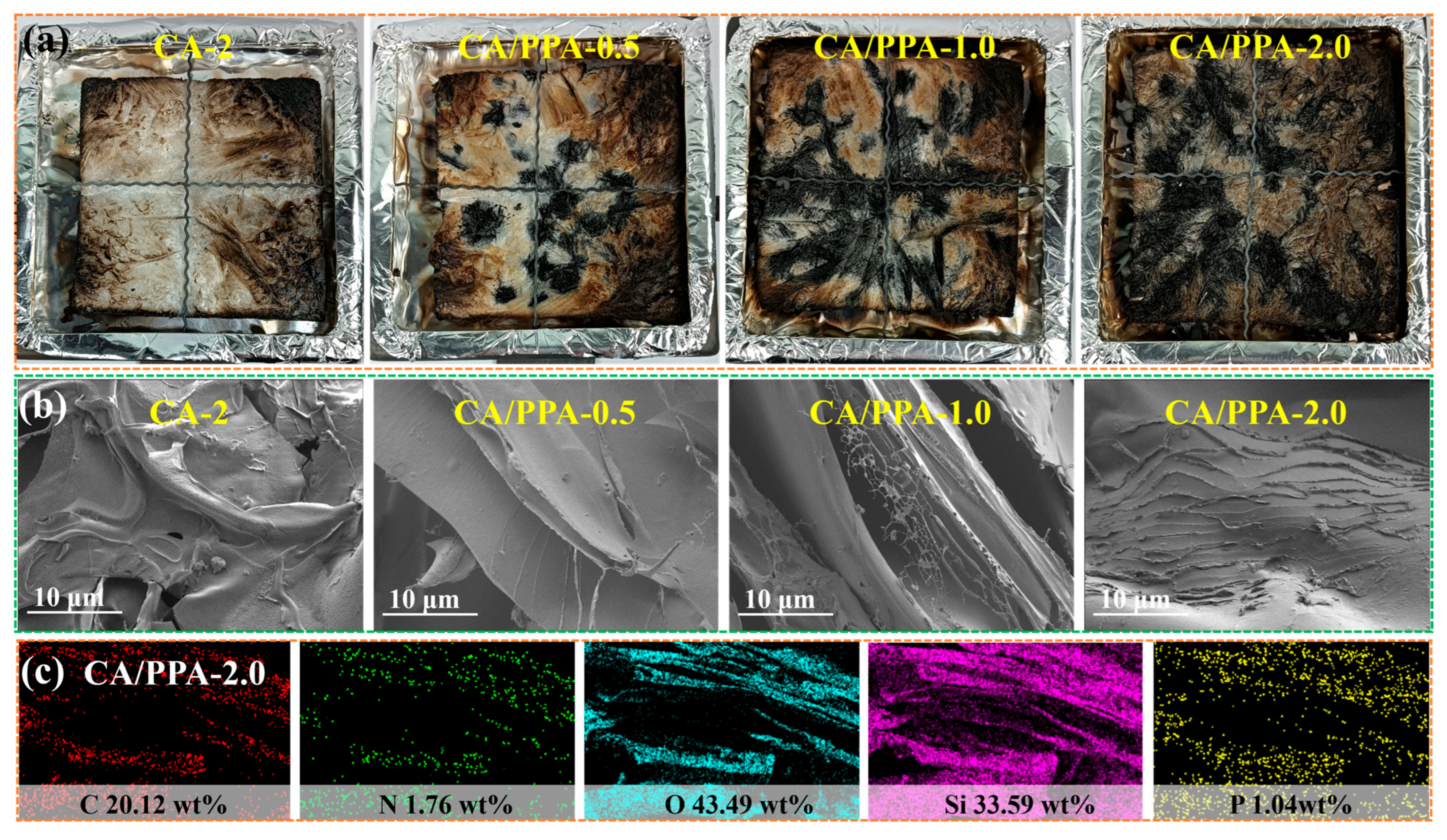
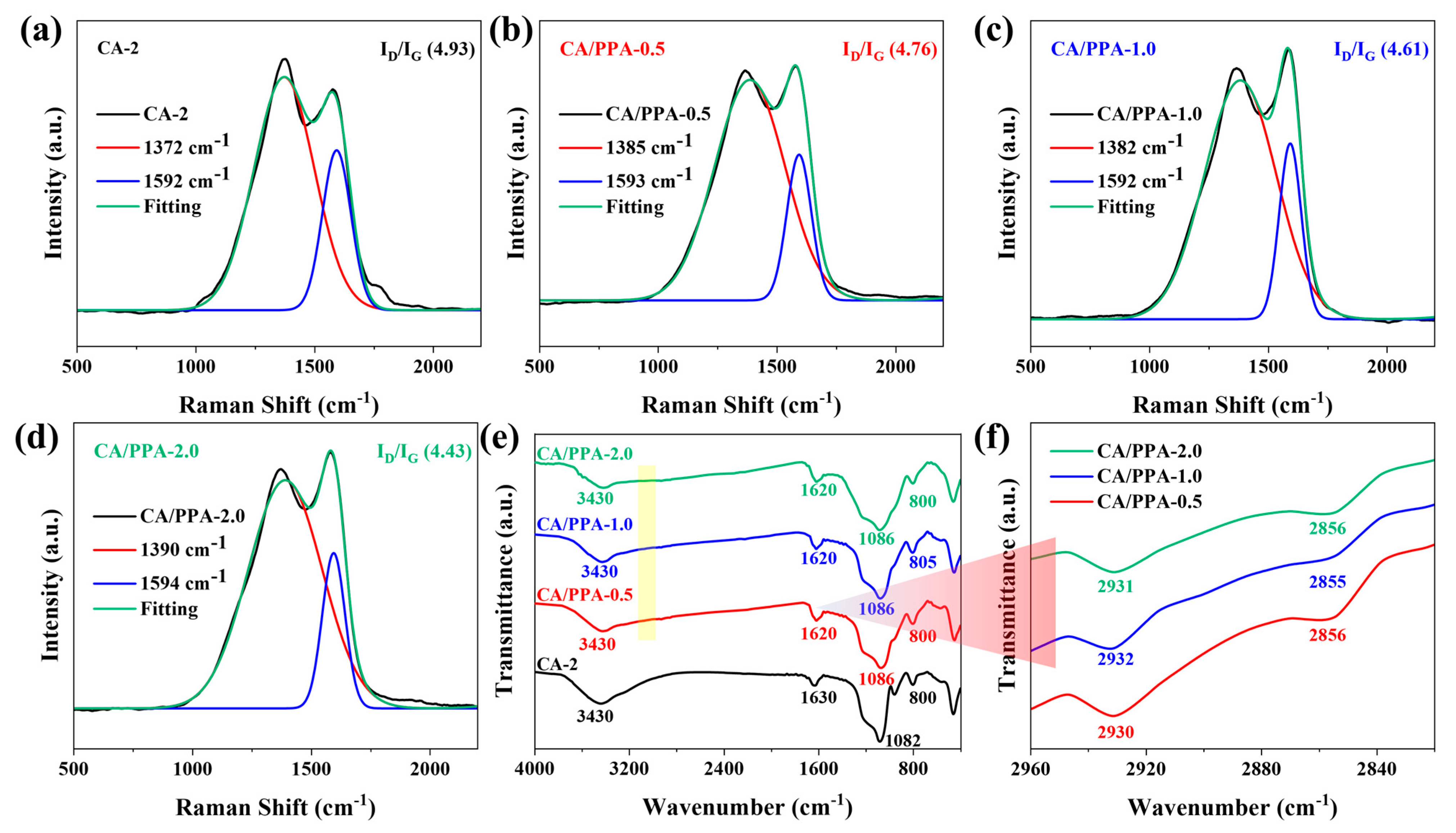
3.4.2. Analysis of Condensed Phase Product
3.4.3. In-Depth Flame-Retardant Mechanisms of PPA
3.5. Fire-Warning Performance and Mechanical Properties of the Composite Aerogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mia, M.H.; Wan, Y.; Jiang, Q.; Huang, L.; Zhou, M.; Xu, J.; Gong, X.; Hu, X.; Yu, Z.; He, H. A sustainable alginate-based multifunctional porous material with integrated thermal barrier and reversible fire warning for enhanced building protection. Carbohydr. Polym. 2025, 358, 123563. [Google Scholar] [CrossRef]
- Li, Z.; Yao, S.; Zhou, F.; Hu, M.; Shen, K.; Liu, M.; Fan, C.; Wu, X. Preparing hydrophobic silica aerogels with superior thermal insulation and fire safety by introducing sepiolite. Constr. Build. Mater. 2025, 461, 139905. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, F.; Li, K.; Yao, J.; Li, X.; Xia, Y. Lightweight, flame retardant Janus carboxymethyl cellulose aerogel with fire-warning properties for smart sensor. Carbohydr. Polym. 2024, 328, 121730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, Y.; Zhang, L.; Li, Z.; Yang, H.; Zhang, X.; Zhang, J. Silica aerogel-PVA dough: A high internal phase composite with superior thermal insulation and gas barrier. Compos. Sci. Technol. 2024, 251, 110553. [Google Scholar] [CrossRef]
- Xu, M.; Wei, Y.; Qin, A.; Xu, Y.; Xu, M.; Li, B.; Liu, L. Novel silica hydrogel-based forest fire extinguishing agent: Construction, fire extinguishing performance and mechanism study. J. Clean. Prod. 2025, 486, 144490. [Google Scholar] [CrossRef]
- Yu, Z.-L.; Yang, N.; Apostolopoulou-Kalkavoura, V.; Qin, B.; Ma, Z.-Y.; Xing, W.-Y.; Qiao, C.; Bergström, L.; Antonietti, M.; Yu, S.-H. Fire-Retardant and Thermally Insulating Phenolic-Silica Aerogels. Angew. Chem. Int. Ed. 2018, 57, 4538–4542. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.-Y.; Lu, S.-Y.; Chang, Y.-C. Transparent, Hydrophobic Composite Aerogels with High Mechanical Strength and Low High-Temperature Thermal Conductivities. J. Phys. Chem. B 2008, 112, 11881–11886. [Google Scholar] [CrossRef] [PubMed]
- Kantor, Z.; Wu, T.; Zeng, Z.; Gaan, S.; Lehner, S.; Jovic, M.; Bonnin, A.; Pan, Z.; Mazrouei-Sebdani, Z.; Opris, D.M.; et al. Heterogeneous silica-polyimide aerogel-in-aerogel nanocomposites. Chem. Eng. J. 2022, 443, 136401. [Google Scholar] [CrossRef]
- Chen, Y.X.; Sepahvand, S.; Gauvin, F.; Schollbach, K.; Brouwers, H.J.H.; Yu, Q. One-pot synthesis of monolithic silica-cellulose aerogel applying a sustainable sodium silicate precursor. Constr. Build. Mater. 2021, 293, 123289. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, Y.; Lu, K.; Wang, Z.; Wang, J.; Lai, L.; Xin, W.; Xiao, Y.; Xiong, S.; Ding, F. Chitosan/silica hybrid aerogels with synergistic capability for superior hydrophobicity and mechanical robustness. Carbohydr. Polym. 2023, 320, 121245. [Google Scholar] [CrossRef]
- Sun, Y.; Zou, Y.; Liu, Y.; Cheng, B.; Hou, Y.; Huang, F. A self-cross-linked core-shell Fe3O4/polyimide composite aerogel: High-mechanical, intelligent fire protection and electromagnetic wave absorption performance. Compos. Sci. Technol. 2025, 270, 111256. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Yang, F.; Luo, Z.; Yuan, B.; Chen, X.; Wang, L. Serendipity discovery of fire early warning function of chitosan film. Carbohydr. Polym. 2022, 277, 118884. [Google Scholar] [CrossRef]
- Cao, J.; Tao, J.; Yang, M.; Liu, C.; Yan, C.; Zhao, Y.; Yu, C.; Zhao, H.-B.; Rao, W. A novel phosphorus-modified silica aerogel for simultaneously improvement of flame retardancy, mechanical and thermal insulation properties in rigid polyurethane foam. Chem. Eng. J. 2024, 485, 149909. [Google Scholar] [CrossRef]
- Chen, B.; Wu, D.; Wang, T.; Liu, Q.; Jia, D. Porous carbon generation by burning starch-based intumescent flame retardants for supercapacitors. Chem. Eng. J. 2024, 486, 150353. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, H.; Tao, B.; Yin, R.; Liu, L.; Li, B. Safe and economical preparation of amino acid-derived bio-based triazine char-forming agent for efficient intumescent flame retardant polypropylene. Constr. Build. Mater. 2025, 484, 141876. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Ni, A.; Ding, A.; Sun, Z.; Han, X. Effect of novel intumescent flame retardant on mechanical and flame retardant properties of continuous glass fibre reinforced polypropylene composites. Compos. Struct. 2018, 203, 894–902. [Google Scholar] [CrossRef]
- Xiao, F.; Peng, W.; Li, K.; Yuan, B.; Fontaine, G.; Bourbigot, S. A highly fire-safe wood hybrid based on delignification and multi-component co-impregnation strategies. Constr. Build. Mater. 2025, 489, 142360. [Google Scholar] [CrossRef]
- Wu, Q.; Cui, X.; Mu, C.; Sun, J.; Gu, X.; Li, H.; Zhang, S. Toward a new approach to synchronously improve the fire performance and toughness of polylactic acid by the incorporation of facilely synthesized ammonium polyphosphate derivatives. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106595. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, B.; Liu, Y.; Cheng, B.; Wang, J.; Yan, J.; Huang, F. Novel bio-based nanosheets: Improving the fire safety, electromagnetic shielding and mechanical properties of polylactic acid. Compos. Part A Appl. Sci. Manuf. 2024, 179, 108044. [Google Scholar] [CrossRef]
- Yuan, B.; Sun, Y.; Chen, X.; Shi, Y.; Dai, H.; He, S. Poorly-/well-dispersed graphene: Abnormal influence on flammability and fire behavior of intumescent flame retardant. Compos. Part A Appl. Sci. Manuf. 2018, 109, 345–354. [Google Scholar] [CrossRef]
- Paul, J.; Qamar, A.; Ahankari, S.S.; Thomas, S.; Dufresne, A. Chitosan-based aerogels: A new paradigm of advanced green materials for remediation of contaminated water. Carbohydr. Polym. 2024, 338, 122198. [Google Scholar] [CrossRef]
- ASTM D6641/D6641M-16e1; Standard Test Method for Compressive Properties of Polymer Matrix Composite Materials Using a Combined Loading Compression (CLC) Test Fixture. ASTM: West Conshohocken, PA, USA, 2021.
- ASTM D2863-19; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-like Combustion of Plastics (Oxygen Index). ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D3801-20a; Standard Test Method for Measuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM D7309-20; Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry. ASTM: West Conshohocken, PA, USA, 2021.
- ISO5660-3:2012; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 3: Guidance on Measurement. International Organization for Standardization: Geneva, Switzerland, 2012.
- Xu, M.-J.; Xia, S.-Y.; Liu, C.; Li, B. Preparation of Poly(phosphoric acid piperazine) and Its Application as an Effective Flame Retardant for Epoxy Resin. Chin. J. Polym. Sci. 2018, 36, 655–664. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Zou, Y.; Wang, J.; Bai, F.; Yan, J.; Huang, F. Poorly-/well-dispersed Fe3O4: Abnormal influence on electromagnetic wave absorption behavior of high-mechanical performance polyurea. Chem. Eng. J. 2024, 493, 152833. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, S.; Zhai, J.; Chen, T.; Mai, Y. Thermal properties and combustion behaviors of flame-retarded glass fiber-reinforced polyamide 6 with piperazine pyrophosphate and aluminum hypophosphite. J. Therm. Anal. Calorim. 2016, 125, 175–185. [Google Scholar] [CrossRef]
- Huang, N.-J.; Cao, C.-F.; Li, Y.; Zhao, L.; Zhang, G.-D.; Gao, J.-F.; Guan, L.-Z.; Jiang, J.-X.; Tang, L.-C. Silane grafted graphene oxide papers for improved flame resistance and fast fire alarm response. Compos. Part B Eng. 2019, 168, 413–420. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Cai, W.; Qiu, S.; Hu, Y.; Liew, K.M. Studies on Synthesis of Electrochemically Exfoliated Functionalized Graphene and Polylactic Acid/Ferric Phytate Functionalized Graphene Nanocomposites as New Fire Hazard Suppression Materials. ACS Appl. Mater. Interfaces 2016, 8, 25552–25562. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z. Synthesis of a bio-based piperazine phytate flame retardant for epoxy resin with improved flame retardancy and smoke suppression. Polym. Adv. Technol. 2021, 32, 4282–4295. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Deng, C.; Du, Y.-Y.; Huang, S.-C.; Wang, Y.-Z. A novel bio-based flame retardant for polypropylene from phytic acid. Polym. Degrad. Stab. 2019, 161, 298–308. [Google Scholar] [CrossRef]
- Yao, C.; Xing, W.; Ma, C.; Song, L.; Hu, Y.; Zhuang, Z. Synthesis of phytic acid-based monomer for UV-Cured coating to improve fire safety of PMMA. Prog. Org. Coat. 2020, 140, 105497. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Wang, W.; Li, H.; Liu, X.; Gu, X.; Bourbigot, S.; Wang, Z.; Sun, J.; Zhang, S. The flammability and mechanical properties of poly (lactic acid) composites containing Ni-MOF nanosheets with polyhydroxy groups. Compos. Part B Eng. 2020, 183, 107568. [Google Scholar] [CrossRef]
- Lecouvet, B.; Sclavons, M.; Bailly, C.; Bourbigot, S. A comprehensive study of the synergistic flame retardant mechanisms of halloysite in intumescent polypropylene. Polym. Degrad. Stab. 2013, 98, 2268–2281. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. An Efficient Mono-Component Polymeric Intumescent Flame Retardant for Polypropylene: Preparation and Application. Acs Appl. Mater. Interfaces 2014, 6, 7363–7370. [Google Scholar] [CrossRef]
- Deng, C.-L.; Du, S.-L.; Zhao, J.; Shen, Z.-Q.; Deng, C.; Wang, Y.-Z. An intumescent flame retardant polypropylene system with simultaneously improved flame retardancy and water resistance. Polym. Degrad. Stab. 2014, 108, 97–107. [Google Scholar] [CrossRef]
- Sai, T.; Su, Y.; Shen, H.; Ran, S.; Huo, S.; Guo, Z.; Fang, Z. Fabrication and Mechanism Study of Cerium-Based P, N-Containing Complexes for Reducing Fire Hazards of Polycarbonate with Superior Thermostability and Toughness. ACS Appl. Mater. Interfaces 2021, 13, 30061–30075. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, J.; Li, Y.; Zhu, T.; Li, H.; Gu, X.; Fei, B.; Zhang, S. Life cycle design of fully bio-based poly(lactic acid) composites with high flame retardancy, UV resistance, and degradation capacity. J. Clean. Prod. 2022, 360, 132165. [Google Scholar] [CrossRef]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable Self-Healing Adhesive Chitosan Hydrogel with Antioxidative, Antibacterial, and Hemostatic Activities for Rapid Hemostasis and Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Tian, J.; Yang, Y.; Xue, T.; Chao, G.; Fan, W.; Liu, T. Highly flexible and compressible polyimide/silica aerogels with integrated double network for thermal insulation and fire-retardancy. J. Mater. Sci. Technol. 2022, 105, 194–202. [Google Scholar] [CrossRef]
- Yang, N.; Yuan, R.; You, D.; Zhang, Q.; Yang, R.; Wang, J.; Cheng, Q.; Ge, L. Dual fire-alarm LBL safeguarding coatings with flame-retardant, EMI shielding and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128763. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.W. Flammability and thermal stability studies of polymer layered-silicate (clay) nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Ren, Q.-R.; Gu, S.; Wang, Y.-Z.; Chen, L. A phosphonate-derived epoxy vitrimer with intrinsic flame retardancy and catalyst-free reprocessability. J. Polym. Sci. 2024, 62, 3195–3205. [Google Scholar] [CrossRef]
- Niu, H.; Xiao, Z.; Zhang, P.; Guo, W.; Hu, Y.; Wang, X. Flame retardant, heat insulating and hydrophobic chitosan-derived aerogels for the clean-up of hazardous chemicals. Sci. Total Environ. 2024, 908, 168261. [Google Scholar] [CrossRef]
- Chen, J.; Xie, H.; Lai, X.; Li, H.; Gao, J.; Zeng, X. An ultrasensitive fire-warning chitosan/montmorillonite/carbon nanotube composite aerogel with high fire-resistance. Chem. Eng. J. 2020, 399, 125729. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Niu, G.; Wang, J.; Zhu, D. Poly(vinylalcohol)/chitosan-based high-strength, fire-retardant and smoke-suppressant composite aerogels incorporating aluminum species via freeze drying. Compos. Part B Eng. 2021, 219, 108919. [Google Scholar] [CrossRef]
- Cui, H.; Wu, N.; Ma, X.; Niu, F. Superior intrinsic flame-retardant phosphorylated chitosan aerogel as fully sustainable thermal insulation bio-based material. Polym. Degrad. Stab. 2023, 207, 110213. [Google Scholar] [CrossRef]
- Zhu, Z.; Niu, Y.; Wang, S.; Su, M.; Long, Y.; Sun, H.; Liang, W.; Li, A. Magnesium hydroxide coated hollow glass microspheres/chitosan composite aerogels with excellent thermal insulation and flame retardancy. J. Colloid Interface Sci. 2022, 612, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Varamesh, A.; Zhu, Y.; Hu, G.; Wang, H.; Rezania, H.; Li, Y.; Lu, Q.; Ren, X.; Jiang, F.; Bryant, S.L.; et al. Fully biobased thermal insulating aerogels with superior fire-retardant and mechanical properties. Chem. Eng. J. 2024, 495, 153587. [Google Scholar] [CrossRef]
- Tao, J.; Yang, F.; Wu, T.; Shi, J.; Zhao, H.-B.; Rao, W. Thermal insulation, flame retardancy, smoke suppression, and reinforcement of rigid polyurethane foam enabled by incorporating a P/Cu-hybrid silica aerogel. Chem. Eng. J. 2023, 461, 142061. [Google Scholar] [CrossRef]
- GB 50264-2013; Code for Design of Industrial Equipment and Piping Insulation Engineering. National Standards of China: Beijing, China, 2013.
- Qin, Z.-Q.; Xiao, Z.-T.; Lan, X.-Y.; Wang, X.; Hu, Y.; Song, L. Cardanol-derived polybenzoxazine networks integrated with high glass transition temperatures, flame retardancy and toughness. Polym. Degrad. Stab. 2025, 234, 111232. [Google Scholar] [CrossRef]
- Wang, D.; Feng, X.; Zhang, L.; Li, M.; Liu, M.; Tian, A.; Fu, S. Cyclotriphosphazene-bridged periodic mesoporous organosilica-integrated cellulose nanofiber anisotropic foam with highly flame-retardant and thermally insulating properties. Chem. Eng. J. 2019, 375, 121933. [Google Scholar] [CrossRef]
- Cheng, B.; Sun, Y.; Zhang, W.; Zhang, W.; Li, D.; Yang, R. A bio-based flame retardant functionalized boron nitride nanosheet to improve flame retardancy and thermal conductivity of EVA composites. J. Mater. Sci. Technol. 2026, 252, 135–146. [Google Scholar] [CrossRef]
- Ding, H.; Wang, J.; Yu, P.; He, H.; Wang, H.; Zhang, W.; Wang, L.; Lei, Y.; Yu, B. Rapidly recyclable, monomer recovery and flame-retardant bio-based polyimine networks. Chem. Eng. J. 2024, 481, 148024. [Google Scholar] [CrossRef]
- Shang, S.; Ma, X.; Yuan, B.; Chen, G.; Sun, Y.; Huang, C.; He, S.; Dai, H.; Chen, X. Modification of halloysite nanotubes with supramolecular self-assembly aggregates for reducing smoke release and fire hazard of polypropylene. Compos. Part B Eng. 2019, 177, 107371. [Google Scholar] [CrossRef]
- Qi, P.; Chen, F.; Li, Y.; Li, H.; Gu, X.; Sun, J.; Zhang, S. A Review of Durable Flame-Retardant Fabrics by Finishing: Fabrication Strategies and Challenges. Adv. Fiber Mater. 2023, 5, 731–763. [Google Scholar] [CrossRef]
- Yan, J.; Xu, M.; Hu, X.; Liu, L.; Xiao, X.; Li, B. A multifunctional epoxy composites based on cellulose nanofiber/carbon nanotube aerogels: Simultaneously enhancing fire-safety, thermal conductive and photothermal performance. Compos. Part A Appl. Sci. Manuf. 2024, 187, 108514. [Google Scholar] [CrossRef]
- Pinilla-Peñalver, E.; Del Fresno, Ó.; Cantero, D.; Moreira, A.; Gomes, F.; Miranda, F.; Oliveira, M.; Ornelas, M.; Sánchez-Silva, L.; Romero, A. Enhancing Flame-Retardant Properties of Polyurethane Aerogels Doped with Silica-Based Particles. Gels 2024, 10, 465. [Google Scholar] [CrossRef]
- Li, Z.; Fu, T.; Guo, D.-M.; Lu, J.-H.; He, J.-H.; Chen, L.; Li, W.-D.; Wang, Y.-Z. Trinity flame retardant with benzimidazole structure towards unsaturated polyester possessing high thermal stability, fire-safety and smoke suppression with in-depth insight into the smoke suppression mechanism. Polymer 2023, 275, 125928. [Google Scholar] [CrossRef]
- Wang, C.; Huo, S.; Ye, G.; Song, P.; Wang, H.; Liu, Z. A P/Si-containing polyethylenimine curing agent towards transparent, durable fire-safe, mechanically-robust and tough epoxy resins. Chem. Eng. J. 2023, 451, 138768. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, B.; Shang, S.; Zhang, H.; Shi, Y.; Yu, B.; Qi, C.; Dong, H.; Chen, X.; Yang, X. Surface modification of ammonium polyphosphate by supramolecular assembly for enhancing fire safety properties of polypropylene. Compos. Part B Eng. 2020, 181, 107588. [Google Scholar] [CrossRef]
- Gao, S.; Wang, R.; Xu, Y.; Zhang, H.; Zhang, S.; Xu, M.; Liu, L.; Tao, B.; Xue, H.; Li, S.; et al. Facile construction of a micro-nano structure of green polyvinyl alcohol-based composite aerogel with superior mechanical properties, thermal insulation, and fire safety. Polym. Degrad. Stab. 2025, 241, 111522. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, Y.; Xu, K.; Yan, C.; Qin, A.; Du, C.; Xu, M.; Wang, C.; Li, B.; Liu, L. Fire-resistant and thermal-insulating alginate aerogel with intelligent bionic armor for exceptional mechanical and fire early-warning performance. Chem. Eng. J. 2024, 498, 155181. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. Synthesis of mechanically reinforced silica aerogels via surface-initiated reversible addition-fragmentation chain transfer (RAFT) polymerization. J. Mater. Chem. A 2015, 3, 1594–1600. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Liebig, W.V.; Mecklenburg, M.; Fiedler, B.; Smazna, D.; Adelung, R.; Schulte, K. Fracture, failure and compression behaviour of a 3D interconnected carbon aerogel (Aerographite) epoxy composite. Compos. Sci. Technol. 2016, 122, 50–58. [Google Scholar] [CrossRef]
- Woignier, T.; Primera, J.; Alaoui, A.; Despetis, F.; Calas-Etienne, S.; Faivre, A.; Duffours, L.; Levelut, C.; Etienne, P. Techniques for characterizing the mechanical properties of aerogels. J. Sol-Gel Sci. Technol. 2020, 93, 6–27. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Xu, H.; Li, B.; Wei, G.; Zhang, X.; Zhang, J. Ionic liquid-mediated facile synthesis of porous cellulose/silica aerogel composites with improved thermal insulation. Int. J. Biol. Macromol. 2025, 307, 142418. [Google Scholar] [CrossRef]
- Su, L.; Jia, S.; Ren, J.; Lu, X.; Guo, S.-W.; Guo, P.; Cai, Z.; Lu, D.; Niu, M.; Zhuang, L.; et al. Strong yet flexible ceramic aerogel. Nat. Commun. 2023, 14, 7057. [Google Scholar] [CrossRef]
- Guo, Y.; Gu, S.; Li, R.; Xue, B.; Zhou, Q.; Yuan, R.; Cong, L. Silicon-modified boron nitride aerogels with enhanced thermal stability for thermal insulation applications in high-temperature. J. Alloys Compd. 2025, 1036, 181759. [Google Scholar] [CrossRef]
| Sample | Composition (wt%) | CS Concentration a (g/100 mL) | ||
|---|---|---|---|---|
| SA Solution | CS Solution | PPA | ||
| CS | - | 100 | - | 2.0 |
| CA-1 | 14.3 | 85.7 | - | 1.0 |
| CA-2 | 14.3 | 85.7 | - | 2.0 |
| CA-3 | 14.3 | 85.7 | - | 3.0 |
| CA-4 | 20.0 | 80.0 | - | 2.0 |
| CA-5 | 11.1 | 88.9 | - | 2.0 |
| CA/PPA-0.5 | 14.2 | 85.3 | 0.5 | 2.0 |
| CA/PPA-1.0 | 14.1 | 84.9 | 1.0 | 2.0 |
| CA/PPA-2.0 | 14.0 | 84.0 | 2.0 | 2.0 |
| Sample | MCC | CCT | |||||
|---|---|---|---|---|---|---|---|
| PHRRm (W/g) | TPHRR (°C) | tign (s) | PHRRc (kW/m2) | tPHRR (s) | THRc (MJ/m2) | FGI (kW/m2s) | |
| CS | 234.3 ± 0.6 | 317.0 ± 0.6 | 3 | 146.2 ± 0.5 | 14 ± 1 | 3.3 ± 0.3 | 10.44 |
| CA-2 | 38.7 ± 0.1 | 161.9 ± 0.2 | 12 | 15.3 ± 0.4 | 17 ± 1 | 1.8 ± 0.2 | 0.90 |
| CA/PPA-0.5 | 36.4 ± 0.2 | 184.9 ± 0.3 | SF a | 10.5 ± 0.3 | 51 ± 1 | 1.6 ± 0.1 | 0.21 |
| CA/PPA-1.0 | 34.3 ± 0.1 | 174.7 ± 0.3 | SF | 7.6 ± 0.6 | 49 ± 2 | 1.1 ± 0.1 | 0.16 |
| CA/PPA-2.0 | 31.4 ± 0.1 | 172.9 ± 0.5 | SF | 5.8 ± 0.3 | 40 ± 1 | 0.8 ± 0.1 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Hou, Y.; Xiang, G.; Hu, C.; Hu, B.; Ji, Y.; Zhang, W.; Zhang, S. A Dual-Modified Chitosan-Derived Silica Composite Aerogel with Simultaneous Improvement of Mechanical, Flame Retardancy, and Thermal Insulation Properties. Polymers 2025, 17, 3162. https://doi.org/10.3390/polym17233162
Zhou S, Hou Y, Xiang G, Hu C, Hu B, Ji Y, Zhang W, Zhang S. A Dual-Modified Chitosan-Derived Silica Composite Aerogel with Simultaneous Improvement of Mechanical, Flame Retardancy, and Thermal Insulation Properties. Polymers. 2025; 17(23):3162. https://doi.org/10.3390/polym17233162
Chicago/Turabian StyleZhou, Sicong, Ying Hou, Guifeng Xiang, Chuang Hu, Baisong Hu, Yingxi Ji, Wei Zhang, and Shaofeng Zhang. 2025. "A Dual-Modified Chitosan-Derived Silica Composite Aerogel with Simultaneous Improvement of Mechanical, Flame Retardancy, and Thermal Insulation Properties" Polymers 17, no. 23: 3162. https://doi.org/10.3390/polym17233162
APA StyleZhou, S., Hou, Y., Xiang, G., Hu, C., Hu, B., Ji, Y., Zhang, W., & Zhang, S. (2025). A Dual-Modified Chitosan-Derived Silica Composite Aerogel with Simultaneous Improvement of Mechanical, Flame Retardancy, and Thermal Insulation Properties. Polymers, 17(23), 3162. https://doi.org/10.3390/polym17233162





